2024 年 72 巻 1 号 p. 127-134
2024 年 72 巻 1 号 p. 127-134
Although curcumin and its analogs exhibit anticancer activity, they are still not used as anticancer drugs because of their water insolubility and extremely poor bioavailability. This study describes the development of water-soluble prodrugs of GO-Y030, a potent antitumor C5-curcuminoid, in an attempt to enhance its bioavailability. These prodrugs release the parent compound via a retro-thia-Michael reaction. To endow sufficient hydrophilicity onto GO-Y030 via a single thia-Michael reaction of an aqueous entity, we used a modified glycoconjugate with a thiol group. The water-solubilizing motif was installed on GO-Y030 by the thia-Michael reaction of propargyl-polyethylene glycol (PEG)-thiol and subsequent click chemistry (CuAAC) reaction with 1-glycosyl azide. Turbidity measurements revealed a significantly improved water solubility of the prodrugs, demonstrating that disaccharide conjugates were completely dissolved in water at 100 µM. Their cytotoxicity was comparable to that of the parent compound GO-Y030, indicating the gradual in situ release of GO-Y030. The release of GO-Y030 from GO-Y199 via the retro-thia-Michael reaction was demonstrated through a degradation study in water. Our retro-thia-Michael reaction-based prodrug system can be used for targeting cancer cells.
Curcumin, a polyphenol, is abundantly found in the edible plant Curcuma longa L. Curcumin has long been known to exhibit a wide range of pharmacological activities, including antitumor, antioxidant, anti-inflammatory, and antiviral activities1) (Fig. 1). The antitumor activity of curcumin has, in particular, received extensive attention, resulting in several clinical trials to understand its properties and role in the human body.2,3) However, even though no therapeutically relevant toxicity has been observed, even at as high a dose as 8 g/d, curcumin has not been approved as an anticancer drug, mainly because of its disappointingly low bioavailability. To overcome these shortcomings, various curcumin analogs are being designed and synthesized in an attempt to increase their bioavailability and improve their biological activity. The presence of a 1,3-dicarbonyl group4) is chiefly responsible for the instability of curcumin. To overcome this hurdle, various molecular designs have been suggested that involve replacing the 1,3-dicarbonyl group with another moiety, resulting in the formation of the following two major strains: C7-tethered bis(aryl) derivatives with 1,3-dicarbonyl modifications (Type-1: substituted or cyclic) and bis(aryl) derivatives with monocarbonyl analogs (Type-2). C5-curcuminoids containing a 1,5-diaryl-3-oxo-1,4-pentadiene motif (two aromatic rings linked by five carbon atoms) were originally designed and synthesized as curcumin analogs based on the intuition of medicinal chemists.5,6) Some of these analogs were isolated from turmeric, such as 1,5-bis(4-hydroxy-3-methoxyphenyl)-(1E,4E)-1,4-pentadien-3-one (hereafter, C5-curcumin), which has the same aromatic ring substitution pattern as that of curcumin.7)

In 2006, we developed GO-Y030, a C5-curcuminoid. GO-Y030 exhibits approximately 30 times more enhanced cancer-selective cytotoxicity than that of curcumin.8) At approximately the same time, however, curcumin was widely considered to be a PAINS (pan-assay interference compounds)9,10) and an IMPS (invalid metabolic panaceas)11,12) candidate. Consequently, it is generally not studied as a lead antitumor agent.13) Unfortunately, GO-Y030 is also regarded as a PAINS because of its reactive crossed-dienone functionality (covalent-bonding modifier). Nevertheless, GO-Y030 exhibits cancer chemopreventive activity in familial adenomatous polyposis mice in vivo,14) suggesting that GO-Y030 is a promising lead antitumor agent. Several C5-curcuminoids, including GO-Y030, with enhanced antitumor activities are now commercially available as curcumin derivatives.15–17)
We are interested in conducting the in vivo studies of GO-Y030 with various administration methods such as intraperitoneal administration with the maximum concentration at 100 µM. However, similar to curcumin, GO-Y030 exhibits poor water solubility, which is predicted to be only 0.26 mg/L as determined using ADME profiles (approximately two times lower than that of curcumin (0.54 mg/L)).18) This critical issue prompted us to develop a water-soluble prodrug of GO-Y030. We have previously developed the first-generation prodrugs of GO-Y030 with the release mode of the parent compound via a retro-thia-Michael reaction, based on the finding that the crossed-dienone functionality of C5-curcuminoids undergoes a reversible thia-Michael reaction with several intracellular thiols, such as glutathione (GSH).19) The inherent reversibility of GO-Y030 distinguishes it from the traditional PAINS with undesirable characteristics. However, the synthesis of this prodrug is still riddled with various problems, such as difficulty in controlling the number of glutathione moieties that can be added. In addition, it is not easy to separate the mono-adduct from the bis-adduct. As part of our ongoing research exploring the medicinal potential of GO-Y030, our group is investigating the preparation of water-soluble prodrugs by controlling the number of water-soluble structural units such as sugars that can be introduced in such prodrugs. Herein, we describe the development of retro-thia-Michael reaction-based glycoconjugates of GO-Y030 as novel water-soluble prodrugs.
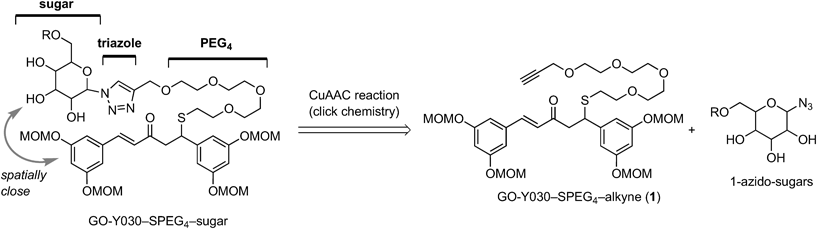
The logic underlying the design and synthesis of GO-Y030–SPEG4–sugar is described in Chart 1. As mono-thia-Michael adducts exhibit a higher cytotoxicity than their corresponding bis-thia-Michael adducts,19–21) we chose the former as the prodrug structure. In addition, the larger size of the bis-adduct is expected to decrease its membrane permeability, although these concerns might not be relevant for sustained-release prodrugs. From the viewpoint of synthetic chemistry, it has been suggested that the sugar moiety should be introduced at the last stage of the synthesis by adding an appropriate hydrophilic linker to avoid the destabilizing steric effect of the GO-Y030 moiety. The hydrophilic linker may facilitate the crucial retro-thia-Michael reaction.19) The introduction of a linker moiety without the water-soluble sugar moiety facilitates the purification of the mono-adduct. However, subsequent attachment of sugars to the chemically unstable thiol adducts is a challenging task, primarily because of the concerns associated with retro-thia-Michael reactions.
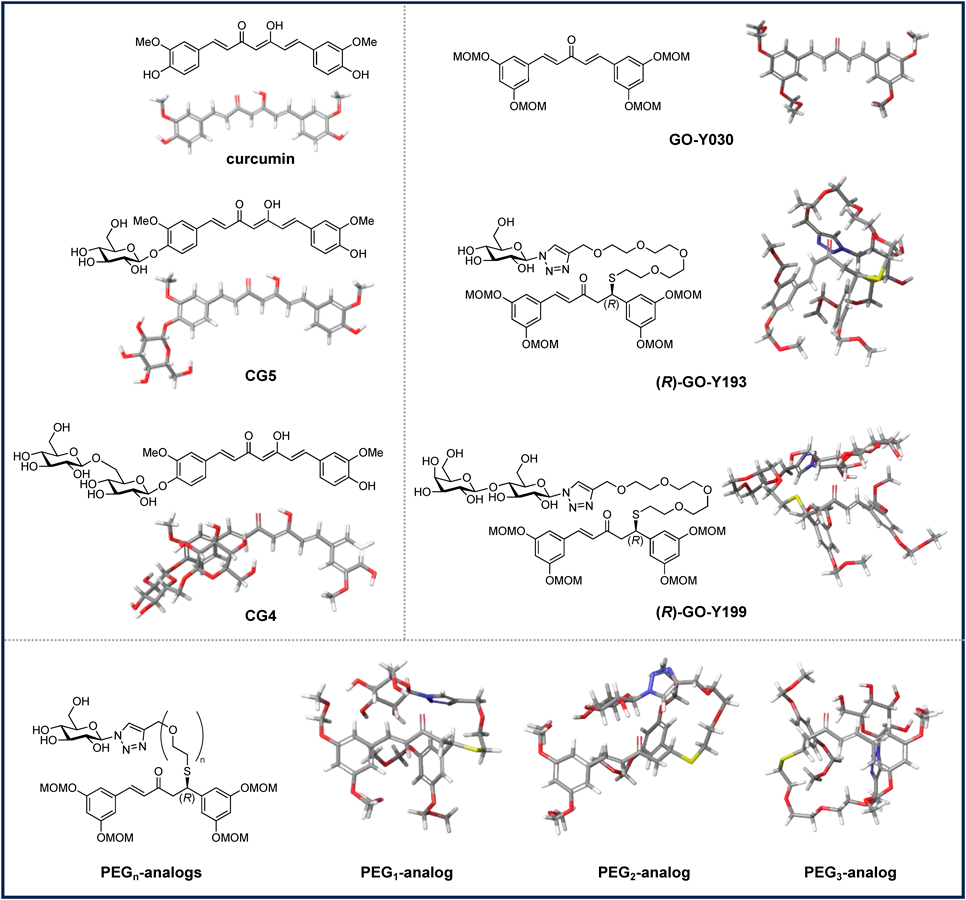
For the rapid acquisition of compounds exhibiting the desired hydrophilicity, we considered the impact of the length of polyethylene glycol (PEG) linkers in designing a water-soluble prodrug. We first performed a computational study to predict the lipophilicity (QPlogPo/w) and water solubility (QPlogS) of monosaccharide-linked GO-Y030–SPEG4–sugars (PEG1–3 analogs and GO-Y193) and disaccharide-linked GO-Y199 (Fig. 2, Table 1). The known monosaccharide CG5 and disaccharide CG4 were selected for comparison because their water solubility has already been reported.22) As expected, both curcumin and GO-Y030 exhibited a decrease in lipophilicity with an increase in the number of linked sugars. The aqueous solubility of curcumin increased with the increasing number of sugars, strongly correlating with the reported water solubility (CG5: 7.0 × 10−3 mM, CG4: 73 mM). Interestingly, the glycoconjugates of GO-Y030 were predicted to have a higher water solubility (0.787–2.000) than that of curcumin derivatives having a similar number of sugar moieties (−4.615 and −1.908). Considering the molecular modeling shown in Fig. 2, we believe that the PEG linkers would allow the adoption of a conformation in which sugars wrap around the aromatic ring to minimize the exposure of the hydrophobic aromatic moieties. Water solubility increased with the increasing PEG chain length, reaching the maximum computational value (QPlogS: 2.000) for the PEG4-monosaccharide. Therefore, we used a PEG4 linker in our prodrug design.
| Number of sugars | Number of (PEG)n | QPlogPo/w | QPlogS | Water solubility22) (mM) | |
|---|---|---|---|---|---|
| Curcumin | 0 | — | 3.734 | −5.093 | 3.0 × 10−5 |
| CG5 | 1 | — | 1.530 | −4.615 | 7.0 × 10−3 |
| CG4 | 2 | — | −0.730 | −1.908 | 73 |
| GO-Y030 | 0 | — | 3.279 | −2.908 | — |
| PEG1-analog | 1 | 1 | 1.094 | 0.787 | — |
| PEG2-analog | 1 | 2 | 0.991 | 1.205 | — |
| PEG3-analog | 1 | 3 | 0.960 | 1.644 | — |
| (R)-GO-Y193 | 1 | 4 | 0.633 | 2.000 | — |
| (R)-GO-Y199 | 2 | 4 | −1.228 | 2.000 | — |
With the aforementioned points in mind, the sugar moiety was planned to be introduced via the copper-catalyzed azide–alkyne cycloadditions (CuAAC reaction) in the final step of the synthesis, affording GO-Y030–SPEG–alkyne with a 1-azido sugar and a terminal acetylene moiety as coupling partners. The intended final conjugation step should enable the divergent and protecting group-free synthesis of GO-Y030–sugar conjugates.
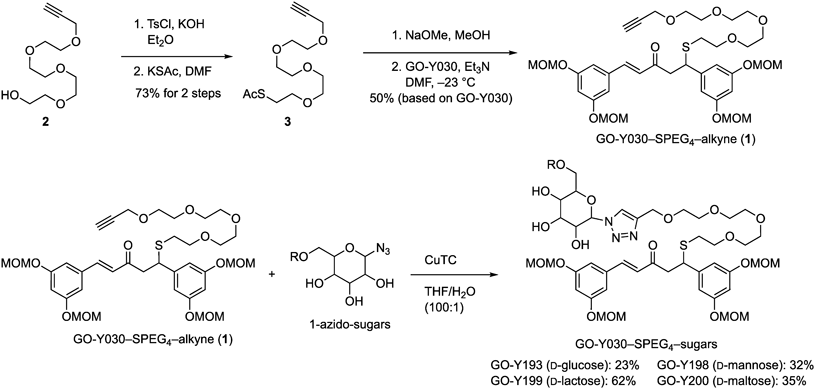
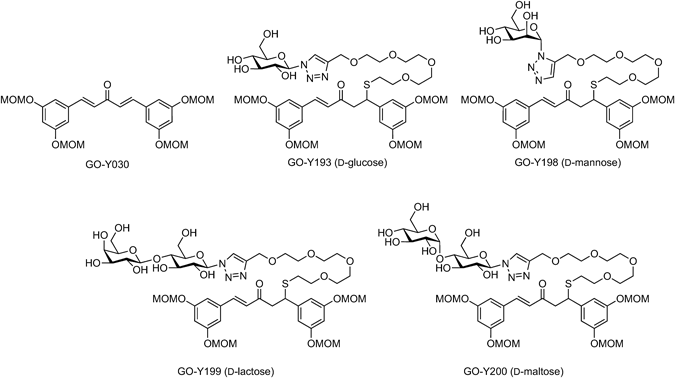
We synthesized GO-Y030–SPEG4–sugars from known alcohol 1 by utilizing the aforementioned design (Chart 2). Tosylation, followed by substitution with potassium thioacetate, yielded protected thiol 2 in 73% yield in two steps. After the methanolysis of thioacetate with NaOMe, the resulting thiol was subjected to a thia-Michael reaction in the presence of triethylamine to produce GO-Y030–SPEG4–alkyne (3). This was followed by the final derivatization, in which the click reaction of 3 with 1-azidosugars in aqueous THF afforded five GO-Y030–SPEG4–sugars in 23–62% yield. Aqueous conditions were necessary to dissolve azido sugars. Gratifyingly, the retro-thia-Michael reaction had minimal involvement within a few hours. The GO-Y030–SPEG4–sugars synthesized by utilizing D-glucose, D-mannose, D-lactose, and D-maltose were named GO-Y193, GO-Y198, GO-Y199, and GO-Y200, respectively. These glycoconjugates are a mixture of epimers with sulfur-substituted chiral centers. Their calculated lipophilicity (QPlogPo/w) and water solubility (QPlogS) are listed in the Supplementary Materials (Supplementary Fig. S1, Supplementary Table S1).
The water solubility of GO-Y030–SPEG4–sugars was evaluated based on the turbidity of the compounds in water (Table 2, Supplementary Fig. S2). The turbidity data are expressed as NTUs. When the aqueous solutions of GO-Y030 with 1% dimethyl sulfoxide (DMSO) were prepared, cloudy mixtures were formed. The turbidity of these mixtures increased with increasing concentration of GO-Y030, and the NTU value was 333.8 at 100 µM. In contrast, all the glycoconjugates almost completely dissolved in aq. 1% DMSO. Their NTU values (0.6–1.2) were comparable to that of the control (0.4). Monosaccharide adducts GO-Y193 and GO-Y198 produced slightly cloudy solutions with NTU values of 19.3 and 23.5, respectively. To our delight, disaccharide adducts GO-Y199 and GO-Y200 dissolved in water to produce a clear solution without DMSO. Taken together, all GO-Y030–SPEG4–sugars dissolve well in water, with disaccharides showing particularly excellent solubility. These results highlight the effectiveness of our designed linkers.
 | |||
|---|---|---|---|
| Compound | Concentration (µM) | NTU in solvent | |
| H2O with 1% DMSO | H2O | ||
| GO-Y030 | 10 | 29.4 | — |
| GO-Y030 | 30 | 83.0 | — |
| GO-Y030 | 100 | 333.8 | — |
| GO-Y193 | 100 | 1.2 | 19.3 |
| GO-Y198 | 100 | 0.6 | 23.5 |
| GO-Y199 | 100 | 0.6 | 2.2 |
| GO-Y200 | 100 | 0.6 | 2.2 |
| Control | 0 | 0.4 | — |
NTU: nephelometric turbidity unit.
Next, we evaluated the cytotoxicity of GO-Y030–SPEG4–sugars in colon cancer HCT-116 cells (Table 2). GO-Y030 exhibited a more potent inhibition of the growth of cancer cells than that demonstrated by curcumin, with a sub-micromolar IC50 value (0.71 µM compared to 16 µM for curcumin). The IC50 values for glycoconjugates GO-Y193, GO-Y198, GO-Y199, and GO-Y198–200 are 0.55, 0.44, 0.43, and 0.42, respectively (Table 3), all of which exhibited comparable or slightly higher toxicity than that of GO-Y030. These results, along with the previous observation that the stable thiol adducts of GO-Y030 (derived from hydrophobic thiols such as GO-Y143) exhibit weaker cytotoxicity,19) suggest that GO-Y030 is rapidly generated from the glycoconjugates in situ via a retro-thia-Michael reaction.
| Compound | Curcumin | GO-Y030 | GO-Y193 | GO-Y198 | GO-Y199 | GO-Y200 |
|---|---|---|---|---|---|---|
| IC50 (µM) | 16 | 0.71 | 0.55 | 0.44 | 0.43 | 0.42 |
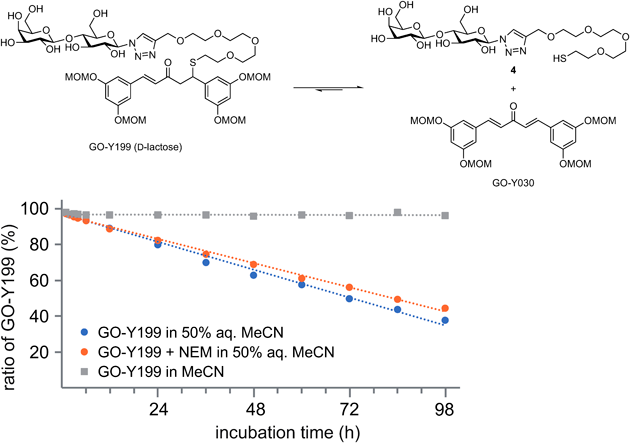
To verify the production of GO-Y030 through the retro-thia-Michael reaction, the degradation rate of GO-Y199 in solution was analyzed using HPLC (Fig. 3). A solution of GO-Y199 in a 1 : 1 mixture of acetonitrile and water was prepared. The molar ratio of GO-Y199 to GO-Y030 was plotted as a function of time. The degradation of GO-Y199 proceeds gradually, producing only 20% GO-Y030 after 24 h. Thereafter, the proportion of GO-Y030 increases linearly, eventually reaching 62% after 96 h. The contribution of the reverse reaction (thia-Michael reaction between GO-Y030 and thiol 4) was investigated using N-ethylmaleimide (NEM), a thiol scavenger. Under the same solvent conditions, NEM did not affect the degradation rate of GO-Y199, indicating that GO-Y199 was degraded almost irreversibly. Despite its inherent degradability, GO-Y199 is stable enough to be stored for long periods, except when exposed to water. GO-Y199 can be purified using silica gel chromatography. Furthermore, the HPLC analysis of GO-Y199 dissolved solely in acetonitrile showed only a trace formation of GO-Y030 even after 96 h. Note that the retro-thia-Michael reaction of GO-Y199 proceeded in water in the same way as it did in water/MeCN (1 : 1). However, the precipitation of GO-Y030, which occurred gradually, made it challenging to calculate the molar ratio of GO-Y199 to GO-Y030. Therefore, we have not presented the results in this paper. Prodrugs require both storable stability and release properties in response to specific stimuli or conditions. GO-Y030–SPEG4–sugars are promising prodrugs that can be stored and that gradually release GO-Y030 upon exposure to water.
We developed water-soluble prodrugs of GO-Y030 based on the retro-thia-Michael reaction in an attempt to improve its water solubility. We conducted a computational prediction of the water solubility of these prodrugs for the rational design of water-soluble prodrugs. Disaccharide-linked prodrugs GO-Y199 and GO-Y200 were soluble in water without the aid of DMSO. GO-Y199 undergoes spontaneous degradation in water, resulting in the gradual generation of the active compound GO-Y030. Cancer cells can be targeted using this retro-thia-Michael reaction-based prodrug system with appropriate sugar structures or cancer-targeting functions. These studies are ongoing in our laboratory, and we will report the results in due time.
All calculations were performed using the Schrödinger Suite (Schrödinger Release 2023-1).
Ligand PreparationAll the calculated compounds were prepared using the LigPrep module of the Schrödinger Suite with the OPLS4 force field. The ionization state was set for pH = 7.0 ± 2.0 by using Epik. Geometry optimization and conformational searches of these compounds were performed using the MacroModel program. An OPLS4 force field was used. The calculations were performed without a solvent. A conformational search method was used for torsional sampling (Monte-Carlo Multiple Minimum, or MCMM).
Prediction of Lipophilicity and Water SolubilityThe lipophilicity and water solubility of the energetically most stable conformer were predicted using the QikProp module of the Schrödinger Suite.
Turbidity AssayNephelometric turbidity units (NTUs) were measured using a digital turbidimeter TBD700 (AS ONE, Osaka, Japan) equipped with an infrared radiation diode (wavelength: 850 nm). DMSO solutions containing each compound were diluted 1 : 100 in phosphate-buffered saline (PBS) (pH 7.4). After mixing, the measurements were conducted four times and the average NTU values were calculated.
Cell Growth AssayGrowth-suppressive effects of curcumin analogs against human colon cancer HCT116 cells were measured for 72 h. The percentage cell growth of the control, which was treated with 1% DMSO alone, was calculated and plotted, and the IC50 value was then determined. Cell viability was assayed as previously described.18,23) The data were obtained from three independent experiments.
General Information for Chemical Synthesis1H-NMR spectra were recorded with tetramethylsilane (δH 0.00), CHCl3 (δH 7.26), or CH3OH (δH 3.31) as an internal standard. Coupling constants (J) are reported in hertz (Hz). Abbreviations of multiplicity are as follows: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; br, broad. Data are presented as follows: chemical shift, multiplicity, coupling constants, and integration. 13C-NMR spectra were recorded with CDCl3 (δC 77.0) and CD3OD (δC 49.0) as an internal standard. IR spectra were recorded on an FT-IR spectrophotometer and absorbance bands are reported in wavenumber (cm−1).
Column chromatography was carried out on silica gel 60 N (63–210 or 40–50 µm). Analytical TLC was carried out with 0.25 mm silica gel plates. Visualization was accomplished with ultraviolet light and anisaldehyde or phosphomolybdic acid stain, followed by heating. Reagents and solvents were purified by standard means or used as received unless otherwise noted. Dehydrated dichloromethane (CH2Cl2) and tetrahydrofuran (THF, stabilizer free) were purchased. All reactions were conducted under an argon atmosphere unless otherwise noted.
Chemical Synthesis and Compound DataAcetyl 3,6,9,12-tetraoxapentadec-14-yn-1-yl sulfide (3): KOH (2.85 g, 50.1 mmol) was added to an ice-cooled (0 °C) solution of 3,6,9,12-tetraoxapentadec-14-yn-1-ol (2, 3.87 g, 16.7 mmol) in Et2O (25 mL) After 10 min of stirring at 0 °C, TsCl (3.82 g, 20.0 mmol) was added, and the resulting mixture was stirred at room temperature for 50 min. The reaction was quenched with half-saturated aqueous NH4Cl (50 mL), and the mixture was extracted with AcOEt (160 and 80 mL). The combined organic extracts were washed with brine (80 mL), and dried over anhydrous Na2SO4. Filtration and evaporation in vacuo furnished the crude product (6.67 g), which was used without further purification.
KSAc (2.30 g, 20.0 mmol) was added to an ice-cooled (0 °C) solution of the crude tosylate in N,N-dimethylformamide (DMF) (20 mL). After 2.5 h of stirring at room temperature, the reaction was quenched with saturated aqueous NaHCO3 (50 mL). The resulting mixture was filtered through cotton, and the filtrate was extracted with Et2O (4 × 100 mL). The combined organic extracts were washed with brine (100 mL) and dried over anhydrous Na2SO4. Filtration and evaporation in vacuo furnished the crude product (10.7 g), which was purified by flash column chromatography (silica gel 50 g, n-hexane/AcOEt 3 : 1→AcOEt) to obtain sulfide 3 (3.54 g, 73% for two steps) as an orange oil. Rf 0.63 (AcOEt); IR (neat) 3259, 2869, 2113, 1692, 1456, 1353, 1292, 1249, 1105, 1034, 954 cm−1; 1H-NMR (400 MHz, CDCl3) δ: 2.34 (s, 3H), 2.43 (t, J = 2.3 Hz, 1H), 3.09 (t, J = 6.5 Hz, 2H), 3.60 (t, J = 6.5 Hz, 2H), 3.63–3.72 (m, 12H), 4.21 (d, J = 2.3 Hz, 2H); 13C-NMR (100 MHz, CDCl3) δ: 28.8, 30.5, 58.4, 69.1, 69.7, 70.3, 70.4, 70.5, 70.6, 74.4, 74.5, 79.6, 195.5; HRMS (FAB) m/z [M + H]+ Calcd for C13H23O5S 291.1261. Found 291.1238.
GO-Y030-SPEG4-alkyne (1): NaOMe (347 mg, 6.42 mmol) was added to an ice-cooled (0 °C) solution of sulfide 3 (620 mg, 2.13 mmol) in MeOH (10 mL). After 1.5 h of stirring, the reaction mixture was neutralized with the Dowex 50 W × 8 acidic resin. In vacuo filtration and evaporation furnished the crude product (734 mg), which was used without further purification.
A solution of crude thiol in DMF (12 plus 2 mL rinse) was added to a cooled (−23 °C) solution of GO-Y030 (1.02 g, 2.14 mmol) and Et3N (0.35 mL, 2.52 mmol) in DMF (6.0 mL). After 2 h of stirring at −23 °C, H2O (50 mL) was added and the resulting mixture was extracted with Et2O (3 × 100 mL). The combined organic extracts were washed with brine (150 mL) and dried over anhydrous Na2SO4. In vacuo filtration and evaporation furnished the crude product (1.89 g), which was purified by flash column chromatography (silica gel 50 g, n-hexane/AcOEt 1 : 1) to give sulfide GO-Y030-SPEG4-alkyne (1, 776 mg, 50%) as a pale-yellow oil. Rf 0.15 (n-Hexane/AcOEt 1 : 1); IR (neat) 3279, 2901, 2827, 2114, 1688, 1663, 1592, 1454, 1401, 1332, 1281, 1248, 1215, 1146, 1185, 1033, 965 cm−1; 1H-NMR (400 MHz, acetone-d6) δ: 2.56 (t, J = 6.5 Hz, 2H), 2.92 (t, J = 2.5 Hz, 1H), 3.22 (dd, J = 7.3, 16.4 Hz, 1H), 3.30 (dd, J = 7.3, 16.4 Hz, 1H), 3.41 (s, 6H), 3.43 (s, 6H), 3.50–3.61 (m, 14H), 4.54 (d, J = 2.5 Hz, 2H), 4.53 (t, J = 7.3 Hz, 1H), 5.16 (s, 4H), 5.22 (s, 4H), 6.59 (t, J = 2.2 Hz, 1H), 6.76 (t, J = 2.3 Hz, 1H), 6.78 (d, J = 2.3 Hz, 2H), 6.82 (d, J = 15.9 Hz, 1H), 7.00 (d, J = 2.3 Hz, 2H), 7.57 (d, J = 15.9 Hz, 1H); 13C-NMR (150 MHz, acetone-d6) δ: 31.3, 45.6, 47.5, 56.1, 56.2, 58.5, 69.8, 70.9, 71.0, 71.2, 71.6, 75.7, 81.0, 95.1, 95.2, 104.1, 107.7, 110.20, 110.23, 127.8, 137.7, 143.1, 145.7, 159.3, 159.6, 197.0; HRMS (FAB) m/z [M + H]+ Calcd for C36H51O13S 723.3045. Found 723.3062.
Typical Procedure for CuAAC ReactionGO-Y193: CuTC (4.1 mg, 22 µmol) was added to a mixture of GO-Y030-SPEG4-alkyne (1, 214 mg, 296 µmol) and β-D-glucopyranosyl azide24) (358 mg, 1.75 mmol) in aqueous THF (8.1 mL, THF : H2O = 100 : 1). After 2 h of stirring, the reaction mixture was concentrated in vacuo, and the residue was purified by flash column chromatography (silica gel 2.4 g, CHCl3/MeOH 19 : 1→9 : 1) to give GO-Y193 (62.0 mg, 23%) as a colorless amorphous. Rf 0.67 (CHCl3/MeOH 4 : 1); IR (neat) 3398, 1688, 1660, 1593, 1455, 1401, 1281, 1215, 1146, 1085, 1033, 924 cm−1; 1H-NMR (600 MHz, CD3OD) δ: 2.55 (t, J = 6.5 Hz, 2H), 3.18 (dd, J = 7.0, 16.1 Hz, 1H), 3.24 (dd, J = 7.9, 16.1 Hz, 1H), 3.41 (s, 6H), 3.44 (s, 6H), 3.50–3.64 (m, 17H), 3.72 (dd, J = 5.5, 12.2 Hz, 1H), 3.88 (dd, J = 2.0, 12.2 Hz, 1H), 3.90 (t, J = 9.0 Hz, 1H), 4.47 (dd, J = 7.0, 7.9 Hz, 1H), 4.62 (s, 2H), 5.12 (d, J = 2.2 Hz, 2H), 5.13 (d, J = 2.2 Hz, 2H), 5.18 (s, 4H), 5.60 (d, J = 9.1 Hz, 1H), 6.59 (t, J = 2.2 Hz, 1H), 6.75 (d, J = 16.1 Hz, 1H), 6.76 (t, J = 2.2 Hz, 1H), 6.76 (d, J = 2.2 Hz, 2H), 6.92 (d, J = 2.2 Hz, 2H), 7.48 (d, J = 16.1 Hz, 1H), 8.15 (s, 1H); 13C-NMR (150 MHz, CD3OD) δ: 31.6 (CH2), 46.4 (CH), 48.0 (CH2), 56.3 (CH3), 56.4 (CH3), 62.4 (CH2), 64.9 (CH2), 70.8 (CH2), 70.9 (CH), 71.3 (CH2), 71.51 (CH2), 71.53 (CH2), 71.55 (CH2), 72.0 (CH2), 74.0 (CH), 78.5 (CH), 81.1 (CH), 89.5 (CH), 95.5 (CH2), 95.6 (CH2), 104.7 (CH), 108.2 (CH), 110.6 (CH), 110.7 (CH), 124.3 (CH), 127.8 (CH), 137.9 (C), 144.6 (CH), 145.8 (C), 146.1 (C), 159.7 (C), 160.0 (C), 199.5 (C); HRMS (FAB) m/z [M + H]+ Calcd for C42H62N3O18S 928.37442. Found 928.3762.
GO-Y198: The reaction was performed according to the typical procedure (7.0 mL THF, 70 µL H2O, r.t., 2.5 h) employing α-D-mannopyranosyl azide25) (127 mg, 340 µmol), alkyne 1 (247 mg, 342 µmol), and CuTC (5.2 mg, 27.3 µmol). GO-Y198 (102 mg, 32%) was obtained as a colorless amorphous solid from the crude product after flash column chromatography (silica gel 2.3 g, CHCl3/MeOH 19 : 1→9 : 1). Rf 0.13 (AcOEt/MeOH 9 : 1); IR (neat) 3399, 2903, 1685, 1654, 1593, 1456, 1400, 1332, 1281, 1215, 1146, 1084, 1033, 924 cm−1; 1H-NMR (600 MHz, CD3OD) δ: 2.53 (t, J = 6.5 Hz, 2H), 3.16 (dd, J = 7.0, 15.8 Hz, 1H), 3.20 (dd, J = 7.9, 15.8 Hz, 1H), 3.39 (s, 6H), 3.43 (s, 6H), 3.49–3.52 (m, 4H), 3.55–3.63 (m, 11H), 3.72–3.82 (m, 3H), 4.07 (dd, J = 3.3, 8.7 Hz, 1H), 4.45 (dd, J = 6.9, 7.9 Hz, 1H), 4.68 (t, J = 3.3 Hz, 1H), 4.80 (s, 2H), 5.11 (d, J = 6.8 Hz, 2H), 5.14 (d, J = 6.8 Hz, 2H), 5.16 (s, 4H), 6.02 (d, J = 2.6 Hz, 1H), 6.57 (t, J = 2.1 Hz, 1H), 6.72 (d, J = 16.1 Hz, 1H), 6.74 (t, J = 2.1 Hz, 1H), 6.74 (d, J = 2.0 Hz, 2H), 6.90 (d, J = 2.0 Hz, 2H), 7.46 (d, J = 16.1 Hz, 1H), 8.12 (s, 1H); 13C-NMR (150 MHz, CD3OD) δ: 31.6 (CH2), 46.3 (CH), 48.0 (CH2), 56.37 (CH3), 56.42 (CH3), 62.5 (CH2), 64.9 (CH2), 68.5 (CH), 70.1 (CH), 70.8 (CH2), 71.2 (CH2), 71.45 (CH2), 71.48 (CH2), 71.51 (CH2), 71.9 (CH2), 72.5 (CH), 78.5 (CH), 88.3 (CH), 95.5 (CH2), 95.6 (CH2), 104.7 (CH), 108.2 (CH), 110.6 (CH), 110.7 (CH), 125.0 (CH), 127.8 (CH), 137.9 (C), 144.5 (CH), 145.8 (C), 146.3 (C), 159.7 (C), 159.9 (C), 199.3 (C); HRMS (FAB) m/z [M + H]+ Calcd for C42H62N3O18S 928.3744. Found 928.3752.
GO-Y199: The reaction was performed according to the typical procedure (6.0 mL THF, 120 µL H2O, r.t., 2 h) employing β-D-lactosyl azide24) (109 mg, 296 µmol), alkyne 1 (214 mg, 296 µmol), and CuTC (6.2 mg, 32.5 µmol). GO-Y199 (199 mg, 62%) was obtained as a colorless amorphous solid from the crude product after flash column chromatography (twice, silica gel 3 g, CHCl3/MeOH 9 : 1→4 : 1, silica gel 2.3 g, CHCl3/MeOH 9 : 1→17 : 3). Rf 0.20 (CHCl3/MeOH 4 : 1); IR (neat) 3389, 1660, 1593, 1455, 1440, 1401, 1333, 1281, 1241, 1215, 1146, 1084, 1034, 924 cm−1; 1H-NMR (600 MHz, CD3OD) δ: 2.55 (t, J = 6.6 Hz, 2H), 3.18 (dd, J = 7.1, 15.9 Hz, 1H), 3.23 (dd, J = 7.9, 15.9 Hz, 1H), 3.41 (s, 6H), 3.45 (s, 6H), 3.47–3.64 (m, 17H), 3.74–3.83 (m, 6H), 3.89 (m, 2H), 3.97 (t, J = 9.1 Hz, 1H), 4.41 (d, J = 7.8 Hz, 1H), 4.47 (dd J = 6.6, 7.9 Hz, 1H), 4.62 (s, 2H), 5.12 (d, J = 6.9 Hz, 2H), 5.14 (d, J = 6.9 Hz, 2H), 5.18 (s, 4H), 5.63 (d, J = 9.1 Hz, 1H), 6.58 (t, J = 2.2 Hz, 1H), 6.74 (d, J = 16.2 Hz, 1H), 6.76 (t, J = 2.1 Hz, 1H), 6.76 (d, J = 2.2 Hz, 2H), 6.92 (d, J = 2.1 Hz, 2H), 7.48 (d, J = 16.2 Hz, 1H), 8.16 (s, 1H); 13C-NMR (150 MHz, CD3OD) δ: 31.6 (CH2), 46.4 (CH), 48.1 (CH2), 56.3 (CH3), 56.4 (CH3), 61.6 (CH2), 62.5 (CH2), 65.0 (CH2), 70.3 (CH), 70.8 (CH2), 71.3 (CH2), 71.51 (CH2), 71.56 (CH2), 71.57 (CH2), 71.59 (CH2), 72.0 (CH2), 72.5 (CH), 73.7 (CH), 74.9 (CH), 76.9 (CH), 77.1 (CH), 79.6 (CH), 79.8 (CH), 89.3 (CH), 95.56 (CH2), 95.62 (CH2), 104.8 (CH), 105.1 (CH), 108.2 (CH), 110.65 (CH), 110.72 (CH), 124.3 (CH), 127.8 (CH), 137.9 (C), 144.6 (CH), 145.8 (C), 146.1 (C), 159.8 (C), 160.0 (C), 199.6 (C); HRMS (FAB) m/z [M + H]+ Calcd for C48H72N3O23S 1090.4272. Found 1090.4265.
GO-Y200: The reaction was performed according to the typical procedure (10 mL THF, 100 µL H2O, r.t., 2 h) employing β-D-maltosyl azide24) (160 mg, 436 µmol), alkyne 1 (226 mg, 313 µmol), and CuTC (3.3 mg, 17.3 µmol). GO-Y200 (133 mg, 35%) was obtained as a colorless amorphous solid from the crude product after flash column chromatography (silica gel 5.5 g, CHCl3/MeOH 9 : 1→17 : 3). Rf 0.08 (CHCl3/MeOH 8 : 1); IR (neat) 3386, 2904, 1684, 1661, 1594, 1541, 1506, 1456, 1400, 1334, 1281, 1216, 1146, 1084, 1033, 968 cm−1; 1H-NMR (600 MHz, CD3OD) δ: 2.56 (t, J = 6.5 Hz, 2H), 3.18 (dd, J = 6.9, 16.0 Hz, 1H), 3.24 (dd, J = 7.9, 16.0 Hz, 1H), 3.42 (s, 6H), 3.46 (s, 6H), 3.48 (m, 1H), 3.53–3.56 (m, 4H), 3.59–3.70 (m, 15H), 3.76 (t, J = 9.2 Hz, 1H), 3.82–3.90 (m, 4H), 3.96 (t, J = 9.2 Hz, 1H), 4.48 (dd J = 6.9, 7.9 Hz, 1H), 4.63 (s, 2H), 5.13 (d, J = 6.8 Hz, 2H), 5.15 (d, J = 6.8 Hz, 2H), 5.19 (s, 4H), 5.24 (d, J = 3.9 Hz, 1H), 5.63 (d, J = 9.2 Hz, 1H), 6.59 (t, J = 2.2 Hz, 1H), 6.75 (d, J = 16.3 Hz, 1H), 6.77 (t, J = 2.2 Hz, 1H), 6.77 (d, J = 2.2 Hz, 2H), 6.94 (d, J = 2.2 Hz, 2H), 7.49 (d, J = 16.3 Hz, 1H), 8.17 (s, 1H); 13C-NMR (150 MHz, CD3OD) δ: 31.6 (CH2), 46.4 (CH), 48.1 (CH2), 56.3 (CH3), 56.4 (CH3), 61.9 (CH2), 62.8 (CH2), 65.0 (CH2), 70.8 (CH), 71.3 (CH2), 71.53 (CH), 71.57 (CH2), 71.58 (CH2), 71.60 (CH2), 72.1 (CH2), 73.6 (CH), 74.2 (CH), 74.9 (CH), 75.1 (CH), 78.2 (CH), 79.7 (CH), 80.4 (CH), 89.4 (CH), 95.57 (CH2), 95.63 (CH2), 103.0 (CH), 104.8 (CH), 108.2 (CH), 110.66 (CH), 110.72 (CH), 124.3 (CH), 127.8 (CH), 138.0 (C), 144.6 (CH), 145.8 (C), 146.1 (C), 159.8 (C), 160.0 (C), 199.6 (C); HRMS (FAB) m/z [M + H]+ Calcd for C48H72N3O23S 1090.4272. Found 1090.4303.
This research was supported in part by a Grant-in-Aid for Scientific Research (C) (JSPS KAKENHI Grant Number: 22K06495 to H.Y.), Drug Discovery and Life Science Research [Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)] (Grant Number: JP22ama121040j0001 to Y.I.) from AMED, and Kowa Life Science Foundation (to H.Y.).
The authors declare no conflict of interest.
This article contains supplementary materials.