2024 Volume 72 Issue 11 Pages 1014-1023
2024 Volume 72 Issue 11 Pages 1014-1023
In nuclear medicine, molecular imaging of the tumor microenvironment using radiopharmaceuticals (RPs) targeting cancer-associated fibroblasts is gaining significant interest. Among these RPs, [68Ga]Ga-FAPI-46 for positron emission tomography (PET) imaging is frequently used in clinical research protocols. To ensure that the production of this RP complies with good manufacturing practices, process automation is widely adopted. In this context, an automated method for preparing [68Ga]Ga-FAPI-46 was designed using a GAIA® synthesizer. Additionally, a HPLC method was developed and validated to determine the radiochemical purity (RCP) of [68Ga]Ga-FAPI-46 and ensure product quality. The validated HPLC method showed excellent repeatability, with coefficients of variation (%CV) for RCP and retention time (tR) below 0.03 and 0.16%, respectively, across 10 measurements. The radiochemical identification of [68Ga]Ga-FAPI-46 showed comparable tr values to [natGa]Ga-FAPI-46 (6.65 and 6.59 min, respectively). The limits of detection (LOD) and quantification (LOQ) were 79 and 42 kBq/mL, respectively, with a linear detector response between 62.9 and 0.08 MBq/mL (R2 = 0.9999). The method proved robust, tolerating minor variations in mobile phase flow rate and composition. This validated radio-HPLC method can be used routinely for the quality control of [68Ga]Ga-FAPI-46. Finally, three RP validation batches were produced using the automated method described and subjected to multiple quality controls. All three synthesis products met the expected specifications, notably regarding appearance, chemical and isotope identification, pH, sterility, stability, and radionuclidic and radiochemical purity.
The tumor microenvironment (TME) is a complex interplay among cancer cells, stromal cells, immune cells, and the extracellular matrix.1) It exerts significant influence on tumor progression, metastasis, immune evasion and therapy resistance.2–4) With the variety of cell types composing the TME and the multitude of potential molecular targets offered, there has been a growing interest in targeting specific TME components for both diagnostic and therapeutic purposes.5) Specifically, fibroblast activation protein (FAP) has emerged as a target of choice due to its highly specific overexpression on the surface of cancer-associated fibroblasts.6) As a member of the dipeptidyl peptidase protein family, FAP exhibits an endopeptidase enzymatic activity for substrates containing glycine–proline patterns.7,8) Based on this structural criterion, small molecule FAP inhibitors (FAPI) were developed, centered on a quinoline scaffold bearing a glycyl-2-cyano-4,4-difluoropyrrolidine motif.9,10) In particular, derivatives functionalized with a linker containing a 1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetic acid (DOTA) chelating moiety have shown promise as molecular targeting agents, allowing convenient labeling with radiometals such as gallium-68 (t1/2 = 67.7 min, β+ = 89%, electron capture = 11%) for positron emission tomography (PET) imaging.11–13) As a result, [68Ga]Ga-FAPI-46 (Fig. 1) became one of the most widely developed FAP-targeting nuclear imaging probes in clinical applications to date.14) This radiopharmaceutical (RP) and its closely related derivatives have been studied in many types of cancer, with particular interest in diseases with low to moderate [18F]fluorodeoxyglucose (FDG) uptake such as liver and biliary tract cancers,15–22) gastrointestinal carcinomas23–30) and peritoneal carcinomatosis.31–34)

Over the past two decades, there has been considerable interest in 68Ga PET imaging, mainly due to the inherent chemical and physical characteristics of this radioisotope. Gallium-68, obtained from a 68Ge/68Ga generator, enables convenient in-house production processes, particularly useful for research and development purposes and speeding up the translation of experimental PET imaging agents into clinical applications.35) To ensure robust, repeatable processes, with low radiation exposure for operators and in full compliance with good manufacturing practices (GMP) specifications, 68Ga radiolabeling protocols of investigational medicinal products for clinical applications benefit from automation via synthesis modules.36) Strict pH control of the 68Ga radiolabeling reaction medium is necessary, which involves using a buffer solution tailored in nature and concentration to the vector molecule being labeled.37) Moreover, the adjunction of an antioxidant compound during radiolabeling, such as gentisic acid or ascorbic acid, typically enhances the overall reaction process.38) Similarly, the presence of such compounds in the final RP formulation allows, through their anti-radiolysis properties, to maintain a stable radiochemical purity (RCP) over time.39) At the end of synthesis, it is essential to verify the RCP of 68Ga RPs, typically using techniques such as radio-TLC and radio-HPLC. This is especially important for experimental RP preparations like [68Ga]Ga-FAPI-46, which require the creation of an investigational medicinal product dossier (IMPD). Such documents are then submitted to regulatory authorities to demonstrate the full capability of user centers in producing and controlling the experimental RP drug, thereby ensuring its quality and safe clinical use.
In this context, we present herein the development and validation of an automated radiolabeling method for the preparation of [68Ga]Ga-FAPI-46 on a specific synthesizer, in accordance with GMP criteria. Additionally, we report in detail the implementation and validation of associated quality control procedures, particularly radio-HPLC for determining the RCP of the final preparation. The results of quality controls performed on 3 test preparations will be discussed in detail. This report is the first description of a turnkey automated protocol for the preparation of [68Ga]Ga-FAPI-46 involving a GALLIAD® generator and a GAIA® module.
GMP-grade FAPI-46 and [natGa]Ga-FAPI-46 reference standard for HPLC were provided by SOFIE Bioscience and produced by ABX Pharmaceuticals (Advanced Biochemical Compounds, Germany) as lyophilized products, available in vials containing 50 µg and 1.24 mg, respectively. Additionally, sterile, single-use fluidic labeling kits for 68Ga labeling (reference RT-01-H) and corresponding reagent kits (reference RT-101) were procured from ABX Pharmaceuticals. The reagents used from the kit included C18 cartridge (Sep-Pak® Plus 360 mg sorbent), isotonic saline 0.9% vial (approximately 10 mL), ethanol 60% vial (1.5 mL), absolute ethanol vial (5 mL), water for injection Ecoflac® (WFI, 100 mL), and a 0.22 µm filter (Minisart® 0.22 µm, 13 mm). Sodium acetate 0.8 M and ascorbic acid 70 mM solutions were prepared extemporaneously in 15 mL sterile type 1 glass vials from fresh chemicals of the highest available purity grade and meeting Ph. Eur. requirements. Gallium-68 was obtained by eluting a pharmaceutical-grade 68Ge/68Ga generator (GALLIAD® 1850 MBq, IRE Elit, Belgium) with approximately 1.1 mL of a 0.1 M HCl solution. The automated radiosynthesis of [68Ga]Ga-FAPI-46 was conducted using a GAIA® synthesis module (Elysia-Raytest, Germany) operated by the appropriate software (GAIA control, Elysia-Raytest, Germany). Production took place within a RP preparation unit (GMP grade C clean room) in a shielded, GMP grade A cell with laminar airflow (MEDI 9000 Research 4R, LemerPax, France), where both the automated synthesis module and the 68Ge/68Ga generator were housed. Radioactivity in the product vial and in patient doses was measured using a calibrated ionization chamber (CRC®-25R, Capintec, U.S.A.).
Production Process of [68Ga]Ga-FAPI-46Prior to synthesis start, the C18 cartridge underwent appropriate manual preconditioning with 5 mL of absolute ethanol and 5 mL of WFI. Subsequently, the single-use kit was installed on the automated system and the necessary reagents were prepared and connected to the kit. Specifically, the FAPI-46 vector (50 µg) was solubilized in 0.25 mL of 0.8 M sodium acetate buffer inside a 1 mL syringe of low dead volume (Injekt®-F 1 mL, B. Braun, Germany). Then, 1.5 mL of 0.07 M ascorbic acid solution was prepared in a 3 mL syringe for future adjunction in the reaction medium. Ethanol 60% for C18 cartridge elution was also conditioned in a 3 mL syringe to precisely adjust the volume at 1.5 mL. In addition, a final formulation solution was prepared by adding 700 µL of 0.07 M ascorbic acid to 7.9 mL of NaCl 0.9% in a 10 mL syringe. Figure 2 describes the installation and layout of the kit.

The ramps are identified A, B and C and the valves of each ramp are numbered from 1 to 5. The low dead volume syringe with FAPI-46 in 0.8 M sodium acetate buffer is connected at B3; the formulation solution is connected at B4; the ethanol 60% solution for C18 elution is connected at B5; the 1.5 mL ascorbic acid 0.07 M solution is connected at C1.
After initiation of the custom synthesis process, the system performed a kit integrity test to ensure that no leakage would occur during production. Next, FAPI-46 solution in 250 µL sodium acetate buffer was transferred to the reaction vessel, followed by the 1.5 mL of ascorbic acid 0.07 M. Then, the C18 cartridge was conditioned with WFI, before the tubing lines were purged with filtered air. The 68Ge/68Ga generator was subsequently eluted with 1.1 mL of 0.1 N HCl, the vacuum suction transferring the eluate directly to the reaction vial. After peristaltic pumping to finish the elution process, the radiolabeling reaction proceeded for approximately 8 min at 98 °C. A temperature ramp up to 120 °C for 30 s at the start of heating followed by a drop to 98 °C ensured that the target temperature was effectively reached. The crude product was then transferred to the C18 cartridge, with subsequent rinsing of the reaction vial and tubing with around 10 mL WFI. Free 68Ga3+, which was not retained by the C18 cartridge, was directed to the waste vial through a washing step with WFI, while [68Ga]Ga-FAPI-46 was trapped on the cartridge. Subsequently, the active substance was eluted from the C18 cartridge into the product vial (type 1 glass TC-ELU-5®, Curium, France) using alternating fractions of 60% ethanol (totaling 1.5 mL) and 1 mg/mL ascorbic acid in NaCl 0.9% (totaling 8.6 mL). The majority of 68Ga colloids potentially formed during radiolabeling was retained on the cartridge. Sterilizing filtration was ensured using a 0.22 μ end filter, and the filter’s integrity was confirmed by the automaton at the end of the preparation through a bubble point integrity test (with a minimum pressure value set at 2.5 bar). At the end of the synthesis, the reaction yield was calculated to estimate the proportion of radioactivity actually recovered in the terminal vial at the end of preparation. This was calculated by comparing the activity collected in the product vial with the sum of the post-synthesis activities in the reaction vial, on the C18 cartridge, in the waste vial and in the product vial. The complete [68Ga]Ga-FAPI-46 radiosynthesis process on the GAIA® module is outlined in Fig. 3, and details of the automated sequence are provided as Supplementary materials.

Radio-HPLC analyses were conducted using a Nexera X3 system (Shimadzu, Japan) with HPLC-grade solvents. The radio-HPLC setup included a solvent degasser (DGU-405), a solvent pump (LC40D), an autosampler (SIL-40, 20 µL injection volume), a column oven (CTO-40S, thermostated at 30 °C), a UV detector (SPD-40, wavelength set at 254 and 280 nm), and a radioactivity detector (GABI Nova with mid-energy probe and 2 × 5 µL flow cell) connected in series. The stationary phase consisted of a C18 ACE® Equivalence™ column with dimensions of 3.0 × 150 mm, 110 Å pore size, and 3 µm particle size. The flow rate was maintained at 0.6 mL/min and a segmented gradient program was applied from 0.1% TFA in water (A) to 0.1% TFA in acetonitrile (B) as follows: 0–2.5 min 98/2 A/B; 2.5–3.5 min linear gradient from 98/2 A/B to 70/30 A/B; ; 3.5–12 min linear gradient from 70/30 A/B to 35/65 A/B; 12–13 min linear gradient from 35/65 A/B to 98/2 A/B; 13–15 min 98/2 A/B. Radio-HPLC analyses were processed using the suitable acquisition and analysis software (Gina Star 10, Elysia-Raytest, Germany). Validation of the radio-HPLC analytical method for determining RCP of [68Ga]Ga-FAPI-46 was performed by aligning with the principles outlined in the ICH Q2 (R1) guidelines adapted for the analysis of radioactive compounds.40)
LinearityTo assess the linearity of radio detection, given the short physical half-life of 68Ga, consecutive analyses were conducted on samples from a single [68Ga]Ga-FAPI-46 final product batch. The peak area of interest was plotted against the volume activity at the time of analysis, and the coefficient of determination (R2) was calculated via linear regression. A coefficient R2 ≥ 0.99 was anticipated. Specifically, 47 HPLC measurements were carried out between 5 min and 710 min post-synthesis on a [68Ga]Ga-FAPI-46 sample with an initial volume activity of 62.94 MBq/mL. The activity volume range utilized for linearity assessment encompassed values encountered in clinical settings.
SpecificitySpecificity was assessed by analyzing triplicates of three distinct batches of [68Ga]Ga-FAPI-46, each containing all components that could potentially be present during the HPLC quality control of a preparation for clinical use. Potential impurities considered for detection included free 68Ga3+ (identified by 2 radio-HPLC peaks) and other impurities containing 68Ga. The specificity of a method is usually considered sufficient for a resolution >1.5 between the peak of interest and adjacent peaks.
Repeatability (Part of Precision)Repeatability of the method was evaluated by performing 10 consecutive measurements of a single sample of [68Ga]Ga-FAPI-46 preparation under consistent operating conditions and within a short time frame (2.5 h). Each analysis involved determining RCP of the sample, retention time, and area of the [68Ga]Ga-FAPI-46 peak (adjusted for decay). Coefficient of variation (%CV) was calculated for each parameter using the formula: %CV = (s/m) × 100, where ‘s’ is the standard deviation of the 10 values and ‘m’ is the average of the 10 values. CV values ≤5.0% were expected for each parameter.
AccuracyIn order to assess accuracy of the radio-HPLC method and prevent the underestimation of impurities due to irreversible retention on the chromatography column, the fraction of injected activity effectively recovered at the column outlet was measured in triplicate during the RCP analyses of a single [68Ga]Ga-FAPI-46 preparation. Samples of both the final product and the post-column recovery were counted in a gamma-counter, corrected for decay, and their volume activities were compared to determine the recovery percentage.
To confirm the consistency between the RCP values obtained from HPLC and TLC, the results of analyses performed in triplicate using both methods on three independent [68Ga]Ga-FAPI-46 syntheses were compared. For each triplicate, the % deviation between mean RCP values obtained by HPLC and TLC should be ≤5.0%.
RobustnessRobustness of the radio-HPLC method was assessed by analyzing a single sample of [68Ga]Ga-FAPI-46 with minor variations in chromatographic experimental conditions. This evaluation included gradient variations of ±2.0% and flow rate variations of ±0.1 mL/min, with each condition tested in triplicate. RCP value, area and retention time of the [68Ga]Ga-FAPI-46 peak obtained under each condition were compared to reference values obtained under normal analytical conditions. For each parameter, the % deviation between the mean of the parameter studied and the reference mean should be ≤5.0%.
Limit of Quantification and Limit of DetectionThe limit of quantification (LOQ) for the method was determined through successive analyses of samples from the same [68Ga]Ga-FAPI-46 preparation, until the lowest volume activity that yielded a signal-to-noise ratio >10 was reached. The determination of LOQ was conducted for both [68Ga]Ga-FAPI-46 and the smallest 68Ga3+ impurity peak. Similarly, the limit of detection (LOD) for the method was determined for [68Ga]Ga-FAPI-46 and 68Ga3+ using the same approach, until the lowest volume activity yielding a signal-to-noise ratio >3 was achieved.
High-Resolution TestTo evaluate the capability of the method in effectively separating closely eluting chromatographic peaks and identify potential compound co-elution, a high-resolution test was performed on three batches of [68Ga]Ga-FAPI-46. During this assessment, the gradient time in the HPLC method was doubled to enhance compound separation, with all other parameters maintained unchanged.
Chemical Identity Determined by HPLCA cold reference solution was prepared by dissolving 1.24 mg of [natGa]Ga-FAPI-46 reference complex in 1.24 mL of a mixture containing 0.9% NaCl and 60% ethanol (85/15 ratio). The reference solution was measured in triplicate under the same HPLC conditions used for RCP determination of [68Ga]Ga-FAPI-46. Additionally, three different [68Ga]Ga-FAPI-46 preparation batch were measured in triplicate and the relative retention times (RRT) between [natGa]Ga-FAPI-46 and [68Ga]Ga-FAPI-46 were calculated using the following formula:
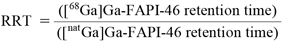 |
RRT between the radiocomplex and its cold reference should be between 0.95 and 1.05.
Other Quality Controls Performed on [68Ga]Ga-FAPI-46 Final ProductAppearance and pHAfter synthesis completion, visual inspection of [68Ga]Ga-FAPI-46 preparations ensured the absence of particulates and confirmed that the product is a clear, colorless solution. pH assessment of the final compounded [68Ga]Ga-FAPI-46 preparations used 2-zone Rota pH 1–11 indicator paper (VWR, PA, U.S.A.) to confirm that the pH fell within the expected range (4 to 8) and was suitable for intravenous administration.
Radionuclide Identity and Radionuclide PurityGamma-spectrometry analysis was performed on a low activity sample (around 100 kBq in 1 mL) from each validation batch of [68Ga]Ga-FAPI-46 using a Hidex AMG® gamma counter (LabLogic, U.K.). Emitted energies were measured, particularly focusing on the 511 and 1077 keV peaks from annihilation photons, in accordance with European Pharmacopoeia specifications for 68Ga RPs.41,42) The half-life was determined by conducting repeated measurements on each validation batch sample over approximately 4 h (6 to 8 measurements per batch). Results were expected within the 61–75 min range and centered on the theoretical value of 67.71 min.43,44) Linearity of the gamma counter response was previously validated for 1 mL samples between 430 kBq/mL and 13 Bq/mL.45)
To assess radionuclide purity, the validation batch samples previously used for radionuclide identity assays underwent measurement in the gamma spectrometer for 120 min, after a 48-h decay period and maintaining consistent geometric conditions. This procedure allowed the detection of any residual 68Ga activity formed in situ from 68Ge breakthrough and other radionuclide impurities with half-lives exceeding 5 h. The total radioactivity measured after the 48-hour decay period should not exceed 0.001% of the initial radioactivity measured in each sample.43)
Radiochemical Purity Determined by TLCRadio-TLC analyses used iTLC-SG strips as the stationary phase and a mixture of aqueous 1 M NH4OAc in methanol (1 : 1) as the mobile phase. The assessment of % areas of radioactivity at the origin and at the solvent front was performed using a radio-TLC scanner (miniGITA® Star, Elysia-Raytest, Germany). The corresponding acquisition software (TLC Control v.2.30, Raytest, Germany) and analysis software (GINA Star TLC™ v.6.0, Elysia-Raytest, Germany) were used for data analysis. Under these experimental conditions, Rf values of 0.0–0.2 for 68Ga impurities and 0.8–1.0 for [68Ga]Ga-FAPI-46 were expected. LOQ for the 68Ga impurities signal was previously found to be 5.65 kBq (Supplementary Fig. S21).45)
Radiochemical StabilityThe RCP of the three validation batches was assessed by radio-TLC and radio-HPLC, right after synthesis and subsequently at hourly intervals (radio-TLC) or every 15 min (radio-HPLC) for up to 4 h post-preparation. Throughout this duration, the final product was stored at room temperature, maintained at 22 ± 2°C.
Bacterial EndotoxinsThe endotoxin level was assessed using a calibrated Endosafe® nexgen-PTS™ unit (Charles River, MA, U.S.A.) in accordance with Ph. Eur. 2.6.14 standards. A recommended limit of 175 endotoxin units (EU) per maximal injected dose was applied. For [68Ga]Ga-FAPI-46 preparations with a final volume of 10.1 mL, the theoretical maximum limit would therefore be 17.3 EU/mL. To determine the maximum significant dilution (MSD) of the [68Ga]Ga-FAPI-46 preparation for testing, the formula MSD = L/S was used, with L representing the detection limit for endotoxins in the sample, set at <5.0 EU/mL, and S representing the intrinsic sensitivity of the technique, which is 0.05 EU/mL. This dilution strategy ensures that the sample does not interfere with the spectrophotometric detection of the assay, maintaining the % recovery between 50 and 200 and achieving a low %CV between measurements.
Sterility TestingSterility testing was conducted on the three validation batches using the direct inoculation method, in accordance with Ph. Eur. 2.6.1 and 0125. A GMP-certified laboratory performed the testing on three 1 mL aliquots of the drug products, following Ph. Eur. guidelines. The expectation was that the culture media containing the preparation samples would remain free from microbial growth.
Residual Ascorbic Acid ContentAccording to the defined preparation protocol, the final ascorbic acid content in the [68Ga]Ga-FAPI-46 preparation should be between 0.5 and 1 mg/mL. To check this concentration, 5 µL of [68Ga]Ga-FAPI-46 were spotted onto a MQuant® colorimetric strip for ascorbic acid test (Sigma-Aldrich, MO, U.S.A.). After 5 s, the color of the strip was compared with the color scale on the ascorbic acid strip container. Prior to use, suitability of the strips was checked with 3 ascorbic acid standard solutions prepared extemporaneously (0.5, 0.7, and 1 mg/mL).
Residual Ethanol ContentIn order to ensure that the ethanol concentration in the final product remained below 10% (v/v), the volume of NaCl 0.9% in the final formulation was adjusted to 8.6 mL. This adjustment ensured that the addition of 1.5 mL of 60% ethanol resulted in a concentration inevitably lower than 9% (v/v). Furthermore, gas chromatography (GC) analyses were conducted on the three standardized validation batches using a GC-2010 AF instrument (Shimadzu, Japan) to quantify the final ethanol concentration.
A 56-step automated synthesis method (Supplementary Fig. S1) has been successfully set up on the GAIA® module for 68Ga radiolabeling of FAPI-46 in 25.1 min (18.25 min from generator elution to final RP formulation). This GMP-compliant process is suitable for manufacturing RPs for medical applications. As in most previously reported automated [68Ga]Ga-FAPI-46 radiolabeling protocols,46–49) significant amounts of vector (50 µg) were used to ensure a large excess compared to 68Ga3+, thus achieving high complexation yields. Since specific activity is an essential parameter in PET imaging,50) using smaller quantities of FAPI-46 could be considered to maintain sufficient activity per vector mass,38,51) especially when using an end-of-life generator that yields low 68Ga activities.
Although N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES) buffer usually displays excellent properties for 68Ga radiolabeling,52) including with FAPI-46,48,49,53) sodium acetate buffer was preferred for regulatory reasons, as the Ph. Eur. limits HEPES content to 500 µg per injected dose in RPs for parenteral administration.41,42) Sodium acetate has been proved reliable for the preparation of [68Ga]Ga-FAPI-46 at both low48,51) and high concentrations,54) particularly when combined with ascorbic acid. When added to the reaction medium, the antioxidant properties of ascorbic acid limit the degradation of the tracer molecule caused by the formation of free radical species during radiolabeling.55) These benefits have been specifically described with [68Ga]Ga-FAPI-46, resulting in a cleaner radio-HPLC profile and, therefore, better RCP.48) Furthermore, sodium ascorbate is sometimes used on its own, also serving as a buffer.46,47) Overall, there are only few reports on the preparation of [68Ga]Ga-FAPI-46 without the use of ascorbic acid or other anti-radiolysis compounds.49,56) When added to the final formulation of the RP product, sodium ascorbate may prevent radiolysis over time. Interestingly, the [68Ga]Ga-FAPI-46 synthesis is one of the few 68Ga radiolabeling processes to use this approach,51,53) whereas the addition of ascorbate to improve radiochemical stability of the final formulation is more common in 177Lu radiolabeling.57–60) For more convenience, since ascorbate is not retained by the C18 cartridge,61) the automated method described herein features the addition of the antioxidant agent at the formulation step. This step involves diluting the radiolabeling product freshly eluted from the C18 cartridge with a total of 8.6 mL of 1 mg/mL ascorbic acid solution in saline. This eliminates the need for manual addition to the product vial, either before or after synthesis, and takes advantage of terminal sterilizing filtration provided by the 0.22 µm filter mounted on the product vial.
Contrary to other groups that report a prepurification step of the 68Ga eluate using a strong cation exchange (SCX) cartridge,46,49,51) a process relying on the direct use of the generator eluate was preferred here. In particular, this allowed the total synthesis time to be shortened, as the pre-purification process configured on the GAIA® module typically lasts around 160 s.45) If a generator eluate prepurification step were to be added to this method, a SCX cartridge could be connected between the A2 and B1 horizontal positions. In this way, the 68Ga3+ ions in the eluate would bind to the cartridge, and the 0.1 M HCl would be discarded to the waste vial. Then, an SCX cartridge elution step using 1 mL of 5 M NaCl in 0.1 M HCl would be necessary to recover the 68Ga3+ ions and direct them to the reaction vial. The SCX cartridge eluent syringe should be connected at C1, which would require pooling the 250 µL of buffer solution containing FAPI-46 and the 1.5 mL of 0.07 M ascorbic acid solution in a single syringe at position B2 to free up position C1. Of note, such configuration would enable the use of multiple generators eluted simultaneously for the same radiolabeling.49,62) Similarly, the use of cyclotron-derived 68Ga could be considered.53,54)
For the solid-phase extraction step, a Sep-Pak® C18 cartridge containing 360 mg of sorbent allowed good liquid flow, particularly during the formulation step. Contrary to free 68Ga3+, [68Ga]Ga-FAPI-46 was selectively retained and readily eluted into the product vial by alternating fractions of 60% ethanol (1.5 mL total) and 1 mg/mL ascorbate in saline (approx. 1.5 mL before the formulation step). Although various cartridge types have been reported for FAPI-46 purification after automated synthesis, such as CM (hydrophilic weak cation-exchanger to retain free 68Ga3+),46,54) OASIS HLB (balanced copolymer with hydrophilic N-vinylpyrrolidone and lipophilic divinylbenzene)47,48,51) or Sep-Pak® C18 (conventional hydrophobic phase),48,49,53,56) only a minor influence of this parameter on the RCP of the final product has been noted.38)
Radio-HPLC Method ValidationSince standard quality control tests for RCP determination in RPs are needed for the release of a pharmaceutical preparation, a radio-HPLC method for [68Ga]Ga-FAPI-46 analysis was validated. Under the above described chromatographic conditions, the substance of interest appeared as a single, well-resolved peak, with an average retention time of 6.654 ± 0.008 min (n = 10). Interestingly, no significant shoulder peaks next to the main signal were identifiable, indicating the effectiveness of ascorbic acid in preventing the formation of radiolysis products during the reaction.48) The [natGa]Ga-FAPI-46 cold reference, solubilized in the same matrix as the RP preparation and analyzed in UV HPLC under the same conditions, showed an average retention time of 6.59 ± 0.17 min (n = 6) (Supplementary Fig. S14). Thus, with a mean RRT of 1.01, the chemical identity of the radiocomplex was confirmed (Supplementary Table S6). A delay of 3 to 5 s between UV and radio detection is due to the connection in series of the two detectors. The system suitability test performed on the [natGa]Ga-FAPI-46 reference standard, diluted to 0.6 µg/mL to better reflect the concentration observed in current practice, confirmed that the chosen analytical conditions were relevant for the quality control of the corresponding RP (Table 1). The linearity of UV detection at 280 nm was confirmed for [natGa]Ga-FAPI-46 over the concentration range 0.0135–10 µg/mL (R2 = 0.9998) (Supplementary Fig. S15). The LOD and LOQ of [natGa]Ga-FAPI-46 were calculated at 0.18 and 0.545 µg/mL, respectively. Of note, although all spectra were strictly comparable, one tailing factor value was higher than 1.8, due to its automatic calculation by the integration software. A manual calculation of the same parameter yielded a value within the specified range.
| Parameter | Specification | Results (n = 6) |
|---|---|---|
| Retention time | CV ≤ 1.0% | 0.17% |
| Peak area | CV ≤ 1.0% | 0.42% |
| Symmetry factor (tailing factor) | 0.8 ≤ As ≤ 1.8 | 1.71 ± 0.17 |
| Number of theoretical plates | N > 2000 | 45506 ± 7337 |
| Capacity factor | 1 ≤ k’ ≤ 5 | 4.07 ± 0.01 |
The radio detection of [68Ga]Ga-FAPI-46 was linear over the volume activity range 62.94–0.12 MBq/mL, covering the values usually expected for clinical applications. The regression equation was found to be y = 0.0008x + 0.0125 with a correlation coefficient R2 = 0.9999 (Fig. 4), in accordance with the acceptance criteria.

Free gallium-68 impurities, resulting in two distinct peaks (tr = 1.25 and 1.68 min), were barely or not retained by the column (Fig. 5). The apparent resolution values for the [68Ga]Ga-FAPI-46 peak compared to 68Ga-impurity 1 and 68Ga-impurity 2 were calculated to be fairly low (0.73 ± 0.03 and 1.30 ± 0.05, respectively). Nevertheless, this can be explained as the two impurity integrations do not form peak shapes and are negligible compared to the massive [68Ga]Ga-FAPI-46 peak. In addition, the large base width of the [68Ga]Ga-FAPI-46 signal (0.55 min vs. 0.22 and 0.25 for 68Ga-impurityies, respectively), resulting from the excellent reaction yield and the almost exclusive presence of radiolabeling product, artificially reduces the resolution values. Overall, no significant interference signals were identified near the peak of interest, supporting the specificity of the method (Supplementary Table S3). Likewise, the high-resolution test did not reveal any extra peak when the solvent gradient time was extended, validating the good HPLC discrimination of the radioactive species contained in the final [68Ga]Ga-FAPI-46 preparation (Supplementary Fig. S13).

Results of repeatability tests are summarized in Table 2 and detailed in Supplementary Table S4. Consecutive analyses of a [68Ga]Ga-FAPI-46 preparation displayed little variation in the parameters studied for the product of interest (%CV ≤0.52% for tr, signal area and RCP). Essentially due to their extremely low surface area, higher variability was found for the free gallium-68 impurities peaks (%CV of 5.41 and 10.28 for decay-corrected area; %CV of 11.07 and 35.00 for proportion, respectively).
| Parameter | Mean ± S.D. (n = 6) | %CV | |
|---|---|---|---|
| [68Ga]Ga-FAPI-46 | Retention time | 6.66 ± 0.01 | 0.16 |
| Decay-corrected area | 160924.55 ± 836.68 | 0.52 | |
| Proportion (RCP) (%) | 99.26 ± 0.03 | 0.03 | |
| Free 68Ga signal 1 | Retention time | 1.25 ± 0.004 | 0.31 |
| Decay-corrected area | 81.61 ± 4.41 | 5.41 | |
| Proportion (%) | 0.047 ± 0.005 | 11.07 | |
| Free 68Ga signal 2 | Retention time | 1.68 ± 0.005 | 0.31 |
| Decay-corrected area | 19.25 ± 1.98 | 10.28 | |
| Proportion (%) | 0.012 ± 0.004 | 35.00 |
S.D., standard deviation.
The selected analytical conditions did not seem to cause irreversible resorption on the column or excessive retention of the radioactive species present in the tested samples, as the recovery rates of the activity injected at the column outlet proved to be acceptable for a radioisotope with a half-life as short as 68Ga (mean = 88.65 ± 2.94%, n = 3) (Supplementary Table S5). Accuracy of the method was also supported by the consistency between RCP values obtained by HPLC and TLC on identical samples (mean deviation = 0.51 ± 0.22%, n = 3).
Slight changes in experimental chromatography conditions did not significantly impair the results of [68Ga]Ga-FAPI-46 analyses. The robustness of the method was demonstrated by showing that variations in gradient and flow had minimal impact on signal area, retention time, and RCP of the preparation (Table 3). Notably, variations in the solvent gradient had a slight influence on the retention time of [68Ga]Ga-FAPI-46, contrasting with variations in flow rate.
| Modified parameter | Decay-corrected peak area | Retention time (min) | RCP (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± S.D. | %CV | %Dev | Mean ± S.D. | %CV | %Dev | Mean ± S.D. | %CV | %Dev | |
| Gradient +2% | 176059.3 ± 712.8 | 0.4 | 101.2 | 6.59 ± 0.01 | 0.18 | 98.65 | 98.88 ± 0.16 | 0.16 | 99.90 |
| Gradient −2% | 172125.1 ± 4257.0 | 2.47 | 98.93 | 6.74 ± 0.01 | 0.17 | 100.80 | 97.88 ± 0.73 | 0.74 | 98.89 |
| Flow 0.7 mL/min | 176733.0 ± 357.6 | 0.20 | 101.58 | 6.66 ± 0.01 | 0.17 | 99.70 | 98.97 ± 0.15 | 0.15 | 99.99 |
| Flow 0.5 mL/min | 176786.0 ± 821.8 | 0.46 | 101.61 | 6.66 ± 0.01 | 0.17 | 99.7 | 99.10 ± 0.09 | 0.09 | 100.12 |
| Reference conditions | 173988.4 ± 7740.6 | 4.45 | - | 6.68 ± 0.04 | 0.62 | - | 98.98 ± 0.27 | 0.27 | - |
%Dev is the percentage deviation between the mean of the parameter studied and the reference mean.
The LOQ and LOD for [68Ga]Ga-FAPI-46 were reached for samples of 0.079 MBq/mL (Supplementary Fig. S9) and 0.042 MBq/mL (Supplementary Fig. S11), respectively, which is less than the minimum volume activity that would allow clinical use of the preparation (set at 14 MBq/mL). Similarly, the LOQ and LOD for free 68Ga impurities were previously determined at 0.228 MBq/mL (Supplementary Fig. S10) and 0.097 MBq/mL (Supplementary Fig. S12), respectively.45) These higher values can be explained by the width of the corresponding signals (see Supplementary materials). As a summary, the main results from the validation of [68Ga]Ga-FAPI-46 radio-HPLC quality control method are compiled in Table 4.
| Parameter | Criteria | Specifications | Results |
|---|---|---|---|
| Chemical identity | RRT | 0.95–1.05 | 1.01 |
| Linearity | R2 value | R2 ≥0.99 | R2 ≥0.9999 |
| Specificity | R value | R >1.5 | R >1.5 (Absence of significant adjacent peak) |
| Repeatability (%) | %CV | %CV ≤2 | RCP: CV <0.03 |
| tr: CV <0.16 | |||
| Area: CV <0.52 | |||
| Accuracy (%) | Recovery | — | Recov = 88.7 ± 2.9 |
| %Dev HPLC vs. TLC | %Dev ≤5 | %Dev <0.75 | |
| Robustness | %Dev | %Dev ≤5 | RCP: %Dev <1.11 |
| tr: %Dev <1.35 | |||
| Area: %Dev <1.61 | |||
| LOQ (MBq/mL) | Radio detection | Signal/noise ratio >10 | [68Ga]Ga-FAPI-46 : 0.079 |
| 68Ga3+ : 0.228 | |||
| LOD (MBq/mL) | Radio detection | Signal/noise ratio >3 | [68Ga]Ga-FAPI-46 : 0.042 |
| 68Ga3+ : 0.097 | |||
| HR test | Radio detection | No extra signal | No extra signal |
Three GMP-quality batches of [68Ga]Ga-FAPI-46 were produced using the automated synthesis protocol described above, and the resulting preparations underwent an extensive series of quality controls. Product specifications and acceptance criteria were defined on the basis of the Ph. Eur. monograph for gallium (68Ga) edotreotide injection.42)
The 3 test preparations were clear, colorless, particle-free solutions with a pH of 6, as expected for a filtered mixture of saline, WFI and ethanol. Very good synthesis yields have been reached (92.5 ± 1.6%) with minimal loss of activity in the reaction vial (0.5 ± 0.2%), on the C18 cartridge (0.7 ± 0.1%) and in the waste vial (6.2 ± 1.7%). Mean final activities of the 3 preparations were 684 ± 18 MBq using a 5 months-old 68Ge/68Ga generator, giving an average specific activity of 13.68 ± 0.37 MBq/µg of FAPI-46.
Gamma-spectrometry analysis on the three validation batches found the 511 keV and 1077 keV energy peaks expected for 68Ga, confirming the identity of the radionuclide (Supplementary Figs. S2–S4). An average half-life of 69.03 ± 0.76 min (gamma counter linearity: R2 = 0.99) was calculated from successive activity measurements on samples from each batch and was consistent with the physical properties of 68Ga (Supplementary Figs. S5–S7). The 3 solutions showed excellent radionuclide purity, with 68Ge and gamma-emitting impurities in a mean proportion of 2.52 × 10−5 ± 2.07 × 10−5%, as expected from a RP-grade 68Ga eluate and after purification by solid phase extraction (Supplementary Table S2).
The radiolabeling products showed excellent RCP at the end of the synthesis, both in radio-TLC (99.74 ± 0.20%) and radio-HPLC (99.23 ± 0.06%), as only traces of free 68Ga3+ and other radioimpurities could be detected. The typical profile of the corresponding spectra is presented in Fig. 5.
The RCP of each batch was tested up to 4 h post-synthesis on final products stored at room temperature (Supplementary Table S7). Whether measured by radio-TLC or radio-HPLC, the RCP values were consistently above the acceptable limit of 95% over time (Fig. 6). As previously stated, adding an antioxidant compound such as ascorbic acid to the final RP prevents a significant decrease in RCP, which might otherwise drop below 90% within 2 h post-synthesis.38) Of note, the ethanol contained in the final formulation also acts as an anti-radiolysis agent owing to its reductive properties.63,64)

For microbiological evaluation of the test batches, limulus amebocyte lysate test for bacterial endotoxin required a 1/100e dilution (20 µL preparation in 1980 µL water for bacterial endotoxin testing) and resulted in <5 EU/mL in the three [68Ga]Ga-FAPI-46 productions. Similarly, sterility testing on fluid thioglycollate medium and tryptic soy broth treated with [68Ga]Ga-FAPI-46 samples and incubated at 30–35 °C and 20–25 °C, respectively, validated the sterility of the parenteral drugs.
As a limit of 10% (v/v) ethanol in 68Ga RP preparations is recommended,65) the volumes used in the synthesis process described above were calculated to ensure this limit would not be exceeded. Thus, 1.5 mL of 60% (v/v) ethanol was sufficient to properly elute the C18 cartridge, and the addition of 8.6 mL of 1 mg/mL ascorbic acid in saline fixed the theoretical final ethanol content at approximately 8.9%. This was proved experimentally by gas chromatography, which confirmed average residual ethanol amounts of 9.50 ± 0.28% (Supplementary Figs. S22–S24). Similarly, the ascorbic acid concentration in the final vial (theoretically approx. 850 µg/mL) was confirmed to be within the pre-determined range of 500 to 1000 µg/mL, attesting to the proper execution of the formulation. Table 5 summarizes the main data from quality controls of the three validation batches.
| Test | Specifications | Batch 1 | Batch 2 | Batch 3 |
|---|---|---|---|---|
| Appearance | Clear, colorless solution | Clear, colorless solution | Clear, colorless solution | Clear, colorless solution |
| Identification | ||||
| Energy of gamma photons (MeV) | 0.511 and 1.077 | 0.511 and 1.077 | 0.511 and 1.077 | 0.511 and 1.077 |
| Half-life (min) | 64–72 | 68.87 ± 0.49 | 68.64 ± 0.87 | 69.56 ± 0.56 |
| Chemical identity | RRT between 0.95 and 1.05 | 1.012 | 1.012 | 1.012 |
| pH | 4–7 | 5 | 6 | 6 |
| Sterility | Sterile | Sterile | Sterile | Sterile |
| Bacterial endotoxins | <17.5 EU/mL | <5 EU/mL | <5 EU/mL | <5 EU/mL |
| Radionuclidic purity | ||||
| (68Ga) Gallium | ≥99.9% | 99.99995092% | 99.99998735% | 99.99998624% |
| (68Ge) Germanium and γ-emitting impurities | ≤0.001% | 4.9078 × 10−5% | 1.26467 × 10−5% | 1.37563 × 10−5% |
| Radiochemical purity | ||||
| [68Ga]Ga-FAPI-46 (HPLC) | ≥95% | 99.22% | 99.29% | 99.18% |
| [68Ga]gallium impurities (HPLC) | <5% | 0.05% | 0.05% | 0.03% |
| [68Ga]Ga-FAPI-46 (TLC) | ≥95% | 99.53% | 99.76% | 99.92% |
| [68Ga]gallium impurities (TLC) | <3% | 0.47% | 0.24% | 0.08% |
| Ascorbic acid concentration | ||||
| Determined by colorimetric strips | 0.5–1 mg/mL | 0.7 mg/mL | 0.7 mg/mL | 0.7 mg/mL |
| Filter integrity test | Bubble point measurement ≥50 psi (3450 mbar) | 4007 mbar | 4015 mbar | 4296 mbar |
| Volume activity at end of synthesis | >14 MBq/mL | 66.24 MBq/mL | 69.80 MBq/mL | 67.13 MBq/mL |
| Specific activity at end of synthesis | — | 13.38 MBq/µg | 14.10 MBq/µg | 13.56 MBq/µg |
| Radiochemical yield (based on RCP determined by TLC) | — | 93.54% | 92.19% | 90.89% |
| Stability over 4 h | ≥95% (HPLC) | ≥99.07% (HPLC) | ≥99.20% (HPLC) | ≥99.02% (HPLC) |
| ≥95% (TLC) | ≥99.48% (TLC) | ≥99.41% (TLC) | ≥99.52% (TLC) | |
| Ethanol content | <10% | 9.46% | 9.80% | 9.24% |
The method described herein for the automated production of [68Ga]Ga-FAPI-46 using a GAIA® synthesis module was successfully implemented through the production of 3 test batches that fulfilled all acceptance criteria for injectable RP products according to Ph. Eur. This GMP-compliant process, coupled with a rigorously validated radio-HPLC protocol for RCP determination and a comprehensive set of quality controls, allows reliable routine production of [68Ga]Ga-FAPI-46 for clinical applications.
This research was partially funded by the Société Française de Pharmacie Oncologique (SFPO) and the Académie Nationale de Pharmacie. The authors are very grateful to Jean-Christophe Boyer of Nîmes University Hospital for kindly carrying out the gas chromatography analyses presented in this manuscript.
The authors declare no conflict of interest.
This article contains supplementary materials.