2024 年 72 巻 2 号 p. 186-189
2024 年 72 巻 2 号 p. 186-189
As a part of our continuing exploration to discover new potential promising fungicide candidates, eighteen sulfonate derivatives (3a–3r) containing a kakuol moiety were designed and synthesized. Synthetic sulfonate derivatives were tested comprehensively for antifungal activities against four plant pathogenic fungi (Botrytis (B.) cinerea, Valsa (V.) mali, Fusarium (F.) graminearum, Sclerotinia (S.) sclerotiorum), and their structure activity relationships were summarized. Especially, derivatives 3i and 3j exhibited remarkable activity against V. mali, with the inhibition rates of 99.8 and 100%, which were slightly superior to that of carbendazim (98.9%), a reference fungicide. Moreover, derivatives 3a, 3k and 3q possess the broader antifungal spectrum against three tested plant pathogenic fungi with inhibition rates over 60%. Structure–activity relationship (SAR) analysis indicated that the introduction of 2-F or 3-F into the benzene ring would give rise to a remarkable increase of the antifungal activity against V. mali.
In a variety of agricultural production worldwide, infections of crops with phytopathogenic fungi have caused very serious problem especially due to the development of fungal resistance, not only affecting the quality of crops but also resulting in a large-scale crop yield reduction.1,2) More seriously, many plant pathogenic fungi could produce mycotoxins and the secondary metabolic products that may threaten the health of animals and human beings.3) In the past few years, various chemical synthetic fungicides have been widely used in agricultural production to effectively control infection of crops with phytopathogenic fungi. However, continuous and extensive use of chemical fungicides will induce to develop plant pathogens resistant, further leading to the gradual failure of chemical commercia fungicides.4) As a result, the developments of new substitutes with novel molecular frameworks or new action mechanisms to replace the traditional chemical synthetic pesticides are necessary at present.
Chemical crop protectants are still considered as the efficient measures for preventing and controlling fungal diseases in modern agricultural production.5,6) Natural products derived from plants usually provide an abundant source for developing potential lead compounds in the agrochemical field because of the significant advantages, such as low toxicity to animals and pollution-free to the environment.7–9) In recent years, kakuol (I, Fig. 1) and its derivative (IA, Fig. 1) have aroused the great interest of many agricultural chemists because of their remarkable antifungal activity.10,11) Kakuol firstly isolated from the rhizomes of Asarum sieboldii (Miq) exhibited prominent and extensive biological activities.10) Studies on the structure–activity relationships (SAR) of kakuol derivatives in the previous report demonstrated that the conjugated terminal C=C bond (IA, Fig. 1) is extremely important for improvement of the antifungal activity.11) As mentioned above, previous research strongly indicated that the plant sourced kakuol has been developed as a potential fungicide candidate.
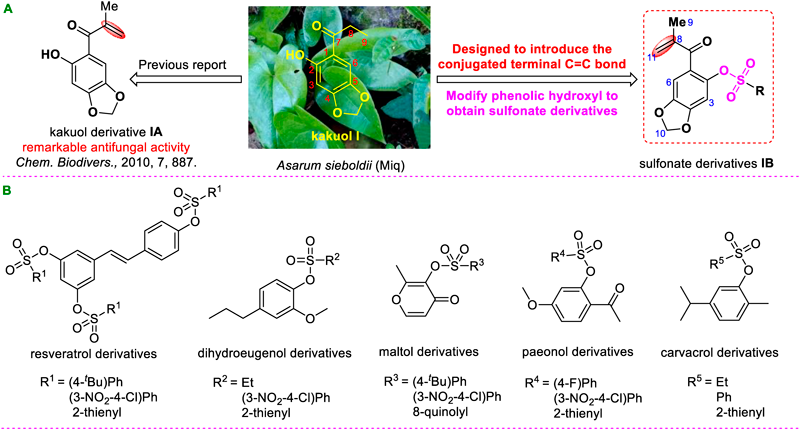
Meanwhile, it is found that the introduction of sulfonate structure into molecules of some potential natural products can also significantly improve their antifungal and anti-oomycete activities, such as natural products resveratrol, dihydroeugenol, maltol, paeonol and carvacrol12–16) (Fig. 1). Above studies indicated that the sulfonate fragment may well be a significant pharmacophore to improve antifungal activity.
Considering the active antifungal effects of the two types of pharmacophores described above, in current work, we plan to combine the active structure of kakuol derivative, the terminal C=C bond conjugated to the C=O group, with sulfonate fragment to design and synthesize a series of novel sulfonate derivatives (IB, Fig. 1), aiming for obtaining potential natural-product-based fungicidal candidates, especially against many resistant fungi, also establishing the preliminary SAR for synthetic target compounds. Based on this design goal, antifungal activity of sulfonate derivatives was measured and evaluated systematically.
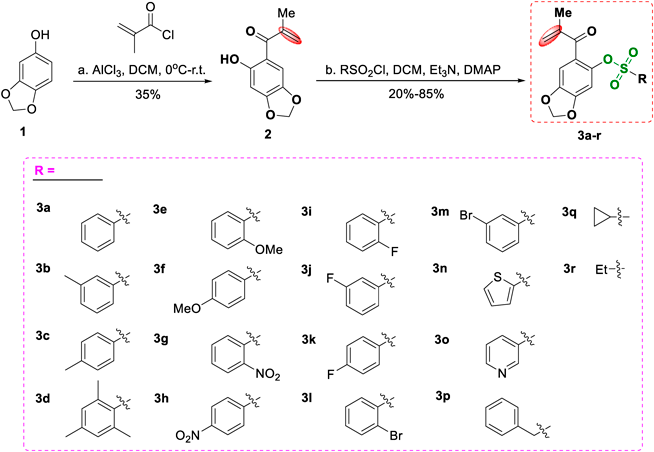
Detailed synthetic process to sulfonate derivatives (3a–r) was displayed in Chart 1, first step, commercially available sesamol (1) was reacted with methacryloyl chloride via aluminum chloride as catalyst to give acylation product (2) in 35% yield. Then, in the presence of triethylamine and N,N-dimethyl-4-aminopyridine (DMAP), the prepared product 2 was further reacted with various sulfonyl chlorides to obtain the corresponding sulfonate derivatives (3a–r) in 20–85% yield. The chemical structures of all the synthesized sulfonate derivatives were characterized by 1H-NMR, 13C-NMR and electrospray ionization (ESI)-MS, and the details of the synthesis procedures and structural characterization can be found in Supporting Information.
Meanwhile, almost all sulfonate derivatives exhibited generally consistent spectrogram characteristics because of the similarity of chemical structure. In particular, the chemical shifts of the hydrogen atoms at C9-position, C10-position, and C11-position basically appear in the same position for different derivatives 3b, 3n and 3p (Fig. 2).

In vitro antifungal activity of sulfonate derivatives 3a–r against four plant pathogenic fungi (Botrytis cinerea, Valsa mali, Fusarium graminearum, Sclerotinia sclerotiorum) was performed according to the reported method previously.17,18) Carbendazim (≥99%), a commercial fungicide, was used as the positive control. The screening results of preliminary antifungal activity of all sulfonate derivatives were listed in Table 1, and the screening results indicated all sulfonate derivatives displayed the antifungal activity. Especially, derivatives 3i and 3j exhibited excellent activity against V. mali, with the inhibition rates of 99.8 and 100%, which was slightly higher than that of carbendazim (98.9%), a commercial fungicide. Moreover, derivative 3k also displayed preferable antifungal activity against V. mali with average inhibition rate of 81.4%. As for S. sclerotiorum, derivatives 3a, 3i–l, 3o–r all displayed good antifungal activity with average inhibition rate of 80.7–88.8%. Disappointingly, almost all sulfonate derivatives exhibited poorer antifungal activity against B. cinerea and F. graminearum, and none of the derivatives approached or exceeded the positive control. Surprisingly, derivatives 3a, 3k and 3q exhibited obvious inhibitory activities against three tested plant pathogenic fungi with average inhibition rates over 60%, which indicated that derivatives mentioned above possess the broader antifungal spectrum from the perspective of horizontal comparison.
 |
Standard deviation (S.D.)
By comparison of the antifungal activity data in Table 1, several important SARs can be discovered and deduced in Fig. 3. First, the results in Fig. 3 revealed that the type and position of the substituents on benzene ring can produce huge influences on the antifungal activity. Compared with the other derivatives, the presence of 2-F or 3-F would lead to a remarkable increase of the antifungal activity against V. mali. For half or most plant pathogenic fungi, the introduction of methoxy, nitro or bromine atom into ortho position (or introducing the substituents like methyl or bromine atom into the meta-position; or the introduction of methyl, methoxy or nitro into the para-position) all can remarkably reduce the antifungal activity. Second, there is almost no significant change in antifungal activity for most pathogenic fungi when aliphatic structure is used instead of aromatic part.

In conclusion, eighteen novel sulfonate derivatives were designed and synthesized. Systematic structural optimization and SAR studies contribute to the discovery of three sulfonate derivatives (3i, 3j, 3k) with strong inhibition activity (inhibition rates >80%) in vitro against two strains of phytopathogenic fungi. Among them, derivatives 3i, 3j exhibited the wonderful antifungal activity against V. mali with the average inhibition rate of 99.8 and 100%, which was slightly higher than that of carbendazim (98.9%). SAR analysis preliminarily indicated that the type and position of substituents on the benzene ring can produce significant effects on the antifungal activity. Especially, the introduction of 2-F or 3-F into the benzene ring would give rise to a remarkable increase of the antifungal activity against V. mali. The present results strongly suggest that derivatives 3i and 3j are potential for the development of new potential fungicidal candidates in crop protection for the effective control of plant fungal pathogen V. mali.
The authors are grateful for the financial support for the present work from the doctoral research start-up fund of Weifang University. In addition, we also want to express special gratitude to postgraduate Lili Shu for biological activity assay in the whole bioassay experiment.
Dr. Guoqing Sui is mainly responsible for experimental design, data collection, manuscript writing and submitting manuscript. Master student Lili Shu is mainly responsible for biological assay. Dr. Ailing Zhang and prof. Dan Li are mainly responsible for supervision. And prof. Shuhua Cao is mainly responsible for validation.
The authors declare no conflict of interest.
This article contains supplementary materials.