2024 年 72 巻 3 号 p. 298-302
2024 年 72 巻 3 号 p. 298-302
The current study aimed to explore the impact of buffer species on the dissolution behavior of orally disintegrating tablets (ODT) containing a basic polymer and its influence on bioequivalence (BE) prediction. Fexofenadine hydrochloride ODT formulations were used as the model formulations, Allegra® as the reference formulation, and generic formulations A and B as the test formulations. Allegra®, generic A, and generic B are ODT formulations that contain aminoalkyl methacrylate copolymers E (Eudragit® E, EUD-E), a basic polymer commonly used to mask the bitter taste of drugs. Both generic A and generic B have been known to be bioequivalent to Allegra®. The dissolution tests were conducted using a compendial paddle, with either bicarbonate (10 mM, pH 6.8) or phosphate buffer (25 mM, pH 6.8) as the dissolution media. A floating lid was employed to cover the surface of the bicarbonate buffer to prevent volatilization. Results indicated that in phosphate buffer, the dissolution profiles of Allegra and generic B significantly varied from that of generic A, whereas in the bicarbonate buffer, the dissolution profiles of Allegra, generic A, and generic B were comparable. These findings suggest that the use of bicarbonate buffer may offer a more precise prediction of human bioequivalence compared to phosphate buffer.
In generic drug development, it is essential to establish the bioequivalence (BE) of the test formulation compared to the reference formulation in humans.1–3) Dissolution testing is widely used to predict BE in humans. Phosphate buffer (PB) has widely been used as a dissolution medium.4–6) However, dissolution testing using PB often fails to predict BE in humans. To increase the success rate of human BE studies, it is critical to develop a dissolution test with appropriate buffer conditions to reflect in vivo dissolution. Since human intestinal pH is regulated by bicarbonate buffer (BCB), it is reasonable to use BCB for dissolution testing.7–10)
Bicarbonate shows the following chemical equilibrium in aqueous media, leading to its unique properties for drug dissolution.11–13)
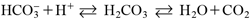 | (1) |
The usefulness of BCB for dissolution testing has been successfully demonstrated with enteric coated and amorphous solid dispersion formulations using acidic polymers.9,14–16) However, the applicability of BCB to basic polymers has yet to be extensively explored. Basic polymers are primarily used for orally disintegrating tablets (ODT).17–19) Because ODT formulations disintegrate in the oral cavity usually within a few tens of seconds, the taste of drugs is more pronounced.20–23) Therefore, taste masking is required for ODT development. Particle coating with basic polymers has been used to delay the dissolution of a drug in the oral cavity.18,24) Furthermore, basic polymers have been used as carrier polymers for solid dispersion and controlled release formulations.25,26) They stabilizes the supersaturated state and thus has the potential to enhance the bioavailability of drugs.27–29) Thus, usefulness of basic polymers has been recognized with their versatility. However, due to its pH-dependent solubility, basic polymers may influence the dissolution profiles in the gastrointestinal tract. Therefore, it is important to investigate the effect of buffer species on the dissolution profile of ODT with basic polymer.
The purpose of the present study was to investigate the effect of buffer species on the dissolution profile of ODT with basic polymer and how it influences BE prediction. Fexofenadine hydrochloride ODT formulations were used as the model formulations (Allegra® as a reference formulation, and generic A and B as test formulations). Allegra®, generic A, and generic B are ODT formulations containing aminoalkyl methacrylate copolymers E (Eudragit® E, EUD-E), a basic polymer widely used for masking the bitterness of drugs. The dissolution profile of generic A has been reported to be different from those of Allegra and generic B in PB, while both generic A and B have been demonstrated to be bioequivalent to Allegra®.30)
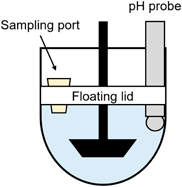
In this study, the floating lid method was employed to use BCB for dissolution testing.31) In a conventional dissolution test setting, when BCB is exposed to the atmosphere, the pH value rapidly rises as CO2 volatilizes from the solution. To overcome the pH increase, CO2 bubbling has previously been used for dissolution testing (the CO2 bubbling method).32–34) However, the CO2 bubbling method presents certain challenges as it requires special equipment and intricate operational procedures. In addition, CO2 bubbling causes foaming when used with a surfactant, which makes the CO2 bubbling method impractical for biorelevant dissolution testing. Furthermore, the CO2 bubbles may enhance drug precipitation. Recently, we have proposed the floating lid method for BCB. A floating lid is placed in the paddle dissolution vessel to prevent CO2 volatilization, thereby maintaining the pH value more easily than the CO2 bubbling method (Fig. 1).
Allegra 60 mg ODT was purchased from Sanofi Co., Ltd. (Tokyo, Japan). Generic products of Fexofenadine 60 mg ODT (Generic A and Generic B) were purchased in the Japanese market. The composition of the formulation is shown in Table 1. Sodium bicarbonate (NaHCO3), NaCl, and 6 N HCl were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Japanese pharmacopeia 2nd dissolution fluid (×10) (phosphate buffer) was purchased from Kanto Chemical (Tokyo, Japan). The floating lid was purchased from Nihon Validation Technologies Corporation.
| Excipients | Allegra | Generic A | Generic B |
|---|---|---|---|
| Amino alkyl methacrylate copolymers E | + | + | + |
| Crystalline cellulose | + | — | + |
| Crystalline cellulose (granular) | + | — | — |
| D-Mannitol | — | + | + |
| Hydroxypropyl cellulose | — | + | — |
| Magnesium stearate | + | + | — |
| Sodium stearyl fumarate | — | — | + |
| Povidone | + | + | + |
| Crospovidone | + | + | + |
The compendial paddle dissolution apparatus was used for the dissolution test. A NaHCO3 solution (10.2 mM, 490 mL, 0.14 M NaCl) was added to a vessel. The solution surface was covered by a floating lid. The floating lid (acrylate, thickness: 5 mm) was designed to cover the surface of a buffer solution almost completely (more than 95% area, but not a tight sealing).31) An HCl solution (0.113 N, 10 mL) was added to adjust the pH value to pH 6.8 (final concentration: bicarbonate = 10 mM, NaCl = 140 mM, buffer capacity: 4.4 mM/pH). The paddle was rotated at 50 rpm and the temperature was set at 37 °C. One tablet was placed in the vessel and the dissolution test was started (NTR-6200A, Toyama Sangyo Co., Ltd., Osaka, Japan). A sample solution (1 mL) was collected at each sampling time and immediately filtered (0.22 µm polyvinylidene difluoride (PVDF), Millipore Corporation, Billerica, MA, U.S.A.). The first few drops of the filtrate were discarded to avoid filter adsorption. The filtrate was diluted with 0.1 N HCl. The concentration of drugs was measured at 225 nm by UV absorbance (SH1000lab, Hitachi High-Technologies Corporation, Tokyo, Japan). Each dissolution test was performed in triplicate.
The compendial paddle dissolution test using PB was performed as follows. 500 mL of PB (25 mM sodium phosphate buffer, 50 mM NaCl, pH 6.8) was added to the vessel. The paddle was rotated at 50 rpm and the temperature was set at 37 °C. One tablet was placed in the vessel and the dissolution test was started (NTR-6200A, Toyama Sangyo Co., Ltd., Osaka, Japan). The sampling method is described above.
F2 CalculationThe f2 function (Eq. 2) was used to quantify the similarity between the dissolution profiles of two formulations. The samples are judged to be similar in dissolution when f2 > 50. All sampling points (6 time points) were used for comparison.
 | (2) |
where n is the number of sampling time points, and Rt and Tt are the dissolved % values at time t for reference and test formulations, respectively.3)
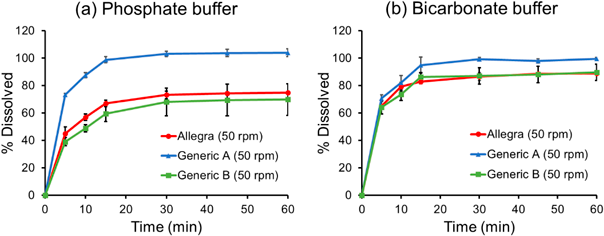
Figure 2(a) shows the dissolution profiles of Allegra, generic A, and generic B in PB. Coning formation was observed at the bottom of the vessels in all cases. The dissolution profile of generic A was judged to be dissimilar to that of Allegra and generic B based on the f2 value (Table 2). These results are in good agreement with the results in the literature. Figure 2(b) shows the dissolution profiles of Allegra, generic A, and generic B in BCB. The pH changes before and after the dissolution test were less than 0.1 in all cases. The dissolution profiles of Allegra, generic A, and generic B were judged to be similar based on the f2 value (Table 3). Allegra and generic B dissolved faster in BCB than in PB. On the other hand, the dissolution rate of generic A did not change between these buffers.
| Formulation | Phosphate buffer (25, 50 mM NaCl, pH 6.8) | |||
|---|---|---|---|---|
| Dissolution % at 15 min (mean ± S.D.) | f2 value | |||
| 50 rpm | 100 rpm | 50 rpm | 100 rpm | |
| Allegra | 67 ± 2.3 | 71 ± 5.5 | — | — |
| Generic A | 98 ± 2.3 | 95 ± 2.2 | Dissimilar (32) | Dissimilar (37) |
| Generic B | 58 ± 5.6 | 53 ± 5.8 | Similar (64) | Dissimilar (48) |
S.D., standard deviation.
| Formulation | Bicarbonate buffer (10, 140 mM NaCl, pH 6.8) | |||
|---|---|---|---|---|
| Dissolution % at 15 min (mean ± S.D.) | f2 value | |||
| 50 rpm | 100 rpm | 50 rpm | 100 rpm | |
| Allegra | 83 ± 1.5 | 88 ± 5.6 | — | — |
| Generic A | 95 ± 5.9 | 96 ± 3.3 | Similar (55) | Similar (59) |
| Generic B | 86 ± 3.0 | 90 ± 5.9 | Similar (83) | Similar (81) |
The paddle rotation speed was increased from 50 to 100 rpm to eliminate the coning formation. In both PB and BCB, the dissolution% at 15 min was little changed (< ± 5%) when comparing 50 and 100 rpm (Fig. 3).
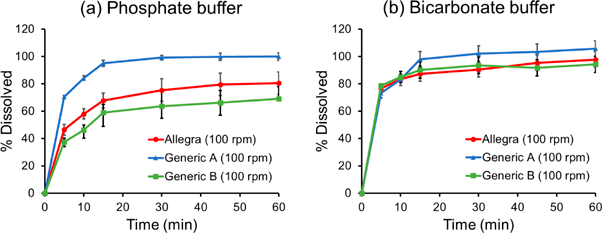
Figure 4 and Table 4 show the results of dissolution tests of Allegra, generic A, and generic B in PB of different concentrations (2.5, 12.5, and 25 mM, paddle rotation speed: 50 rpm). In both PB and BCB, the dissolution% at 15 min was little affected by PB concentration.

| PB conc. (mM) | Buffer capacity (mM/pH) | Dissolution % at 15 min (mean ± S.D.) | ||
|---|---|---|---|---|
| Allegra | Generic A | Generic B | ||
| 2.5 | 0.92 | 63 ± 4.9 | 96 ± 2.5 | 54 ± 2.9 |
| 12.5 | 4.62 | 62 ± 8.4 | 94 ± 4.0 | 55 ± 3.8 |
| 25 | 9.24 | 67 ± 2.3 | 98 ± 2.3 | 58 ± 5.6 |
In PB, the dissolution profiles of Allegra and generic B were significantly different from generic A, whereas, in BCB, the dissolution profiles of Allegra, generic A, and generic B were similar. These results suggest that the use of BCB may provide a more accurate prediction of human BE. Allegra and generic B displayed faster dissolution profiles in BCB compared with PB. In contrast, generic A exhibited little change in the dissolution profile between PB and BCB. Possible explanations for these differences may relate to factors such as coning formation, buffer capacity (β), and buffer species itself. Therefore, we next examined these factors to elucidate the underlying reasons for the difference among the formulations.
Coning formation was observed in the dissolution test using PB but not BCB. Therefore, it was speculated that the presence of coning formation might have hindered the dissolution profile. However, the paddle speed did not affect the dissolution profiles in PB. This observation suggests that coning formation was not the cause of delayed dissolution.
To investigate the impact of buffer capacity, the dissolution testing was conducted at 50 rpm using various concentrations of PB (2.5 mM (β = 0.92 mM/pH), 12.5 mM (β = 4.62 mM/pH), and 25 mM (β = 9.24 mM/pH)). Buffer capacity did not affect the dissolution profiles. These findings suggested that the differences in the dissolution profiles between PB and BCB observed in Allegra and generic B were not attributed to the differences in buffer capacity, but rather to the difference in buffer species. It has been well-documented in the literature that, in the case of enteric-coated and solid dispersion formulations using acidic polymers, the buffer capacity exerts a remarkable effect on the dissolution profiles in PB.9,14–16) In contrast, the result of the present study suggested that the buffer capacity of PB would not affect the dissolution profiles of formulations with basic polymers.
While the mechanism of the dissolution profiles of Allegra and generic B in BCB being faster than those in PB remains unclear, it is postulated that ionic interactions might have occurred between the amino groups of EUD-E and the carboxyl and piperidine groups of fexofenadine in BCB. This interaction leads to an important role in the formation of supersaturated solutions, thereby increasing drug dissolution in BCB.28,35)
The absence of difference in the dissolution profiles of generic A between PB and BCB can be attributed to the unique manufacturing process. Generic A is manufactured with the Rapid and Comfortable Tablets (RACTAB®) technology, which is characterized by a faster disintegration time compared to that of conventional ODT formulations.22) The RACTAB® Technology employs the suspension spray coating method where rapidly disintegrating particles less than 100 µm are produced by coating with a water-dispersible wicking agent. The particles are mixed with drugs or drug particles possessing various functionalities (e.g. taste masking, controlled release, enteric coating) and then dry-pressurized to make the RACTAB®. Consequently, the rapid disintegration of generic A may explain the consistent dissolution profiles observed in both PB and BCB.
Since we used only one example of ODT formulations, more extensive evaluation should be needed to elucidate the dissolution process of ODT formulations incorporating basic polymers in BCB.
In conclusion, the dissolution profiles of fexofenadine hydrochloride ODT formulations containing EUD-E in BCB were consistent with the results of human BE studies, suggesting that BCB is a promising medium for accurately predicting BE in humans for formulations with basic polymers. Although each company’s decision differs, there are many cases in which companies take on the risk of proceeding to BE studies even when the dissolution profiles of the reference and the test formulation are not similar.4,36) The results of this study have the potential to make a significant contribution to enhancing the accuracy of BE predictions by in vitro dissolution tests.
The authors declare no conflict of interest.