2024 Volume 72 Issue 4 Pages 393-398
2024 Volume 72 Issue 4 Pages 393-398
Preparation of drug metabolites at the milligram scale is essential for determining the structure and toxicity of drug metabolites. However, their preparation using recombinant proteins and human liver microsomes (HLM) is often difficult because of technical and ethical issues. Reproducing human drug metabolism in food-derived microorganisms may be useful for overcoming these challenges. In this study, we identified an unknown metabolite of the anaesthetic drug lidocaine, which is metabolised by HLM. By screening for lidocaine metabolic activity in five types of foods (blue cheese, shiitake mushroom, natto, yoghurt, and dry yeast), we found that bacteria isolated from natto reproduced the lidocaine metabolic reaction that occurs in HLM. A fraction containing the unknown lidocaine metabolite was prepared through mass cultivation of a Bacillus subtilis standard strain, ethyl acetate extraction, open column chromatography, and HPLC purification. We identified the unknown metabolite as 3-(2,6-dimethylphenyl)-1-ethyl-2-methyl-4-imidazolidinone using NMR. Our results showed that food-derived microorganisms can produce large amounts of human drug metabolites via large-scale cultivation. Additionally, food microorganisms that can reproduce drug metabolism in humans can be used to examine drug metabolites at a low cost and without ethical issues.
In the field of pharmaceutical research and development, compounds are often initially considered as promising with high therapeutic efficacy but encounter developmental discontinuation during clinical trials or therapeutic applications because of unexpected adverse reactions. Instances of development discontinuation during clinical trials attributed to drug metabolism and dynamics have decreased over time, with rates of 40, 9, and 1% in 1991, 2000, and 2011, respectively. However, cases stemming from adverse reactions and toxicity remained high at 13, 20, and 19%, respectively, during the same years.1–3) Numerous cases of discontinuation resulted from the onset of drug-induced liver injury,4–6) which is thought to be associated with metabolites generated by drug-metabolising enzymes. Consequently, there is growing demand for testing systems capable of accurately assessing the metabolism and toxicity of candidate compounds in the early stages of development. Human liver microsomes (HLM) is often used in early-stage metabolic studies of drug development. However, there are ethical concerns regarding the large amounts of HLM used to prepare milligram quantities of metabolites for structural analysis and toxicity evaluation. Mass-cultivatable microorganisms capable of replicating HLM metabolism and producing metabolites on a large scale for assessment purposes would enable highly precise toxicity studies to be conducted during the early stages of drug development.
The exact mode of action of lidocaine, discovered in 1948, as a local anaesthetic and antiarrhythmic agent is not completely understood. This study was conducted to determine the mechanism of action of lidocaine by investigating its metabolites. For lidocaine, major metabolites generated in rat and sheep include 3-hydroxylidocaine (A), 4-hydroxylidocaine (B), monoethylglycinexylidine (MEGX) (C), 2-(diethylamino)-N-[2-(hydroxymethyl)-6-methylphenyl]acetamide (D), 3-hydroxy MEGX (E), 4-hydroxy MEGX (F), glycinexylidine (G), 2,6-dimethylaniline (2,6-xylidine, DMA) (H), N-hydroxylysine and hydroxy DMA7–9) (Fig. 1).
In this study, we searched for lidocaine metabolites produced by HLM using LC/MS and identified a new metabolite not previously been reported to be produced by HLM. We also screened for lidocaine-metabolising microorganisms present in food. The screened microorganisms were cultivated in large quantities to produce lidocaine metabolites, which were purified and structurally characterised. Replacing HLM with microorganisms that can be cultivated in large quantities and produce large amounts of metabolites may curtail unexpected dropouts, enhance success rates, and reduce the costs associated with drug discovery research and development.
Unspecified reagents were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Lidocaine was of biochemical grade. Pure water, methanol, and acetonitrile were used in LC/MS analysis.
Metabolism by HLMEach reaction mixture consisted of HLM (Life Technologies, Carlsbad, CA, U.S.A.; HMNCPL, 0.5 mg protein/mL), substrate (50 mg/mL lidocaine), and reduced nicotinamide adenine dinucleotide phosphate (NADPH) regeneration system solution A and solution B (Promega, Madison, WI, U.S.A.) in 0.1 mol/L potassium phosphate buffer pH 7.4 in a final volume of 400 µL. The reactions were initiated by addition of NADPH regeneration system solution A and B after incubation for 5 min at 37 °C. The reaction mixture (100 µL) was collected at 0, 30, 60, and 90 min and mixed with 100 µL of methanol, followed by centrifugation at 15000 ×g for 3 min. The supernatant was analysed using LC/MS.
Metabolism by Microorganisms Present in FoodFood materials were purchased from a supermarket near Osaka University. For natto, shiitake mushroom, and blue cheese, 1 g of each edible material was suspended in 10 mL of phosphate-buffered saline, and the supernatant was applied to Luria–Bertani (LB) (Nacalai Tesque, Kyoto, Japan) agar plates for natto and to yeast peptone dextrose supplemented with adenine hemisulfate (YPDA) (TaKaRa Bio, Shiga, Japan) agar plates for shiitake mushroom and blue cheese. Three colonies were isolated from each sample. All isolates from the same material showed similar results in subsequent experiments. The natto isolates were pre-cultured overnight on LB liquid medium, and the shiitake mushroom and blue cheese isolates on YPDA liquid medium were used for lidocaine metabolism experiments. For metabolism experiments, precultures were added to 3 mL of liquid medium (LB or YPDA) containing 1 mg/mL lidocaine. Natto isolates were incubated in LB liquid medium at 37 °C for 7 d, and shiitake mushroom and blue cheese were incubated in YPDA medium at 25 °C for 7 d.
Dry yeast and yoghurt were used for lidocaine metabolism experiments as a material suspension. Dry yeast was suspended in 400 mL pure water containing 0.5 g/L lidocaine. Yoghurt was suspended in 360 mL of De Man, Rogosa, and Sharpe liquid medium containing 1 mg/mL lidocaine. The suspension was incubated at 37 °C for 2 h.
All reactions were terminated by adding an equal volume of methanol and then centrifuged at 15000 ×g for 3 min. The supernatant was analysed using LC/MS.
For metabolite purification, Bacillus subtilis ssp. Spizizenii (NBRC3134), a standard strain of B. subtilis purchased from the Biological Resource Center, National Institute of Technology and Evaluation (NBRC, Tokyo, Japan), was pre-cultured overnight in LB liquid medium and cultured in LB liquid containing 0.5 g/L lidocaine.
Identification of Natto IsolateThe natto isolate was identified using 16S ribosomal DNA (rDNA) sequencing. This analysis was conducted by the Fasmac Corporation (Kanagawa, Japan).
LC/MSLC/MS was performed on an ACQUITY Ultra-Performance Liquid Chromatography (UPLC) system (Waters, Milford, MA, U.S.A.) and a Quattro-Premier XE mass spectrometer (Waters). Sample solutions were separated on an ACQUITY UPLC BEH C18 column (50 × 2.1 mm i.d., 1.7 µm, Waters) using a binary gradient system (solvent A–0.1% formic acid in water; solvent B–acetonitrile) at a flow rate of 0.5 mL/min. The column temperature was maintained at 40 °C, and the sample injection volume was 5 µL. The gradient program for solvent B was as follows: 15% (0–0.5 min), 100% (2.5–3.5 min), and 15% (3.6–4.5 min). The mass spectrometer was operated in positive electrospray ionisation mode. Operating parameters for MS were as follows: cone voltage of 35 V, capillary voltage of 4.5 kV, and desolvation temperature of 350 °C. The MS scan range was set from m/z 50 to 500. The scan time was set to 0.95 s.
High-resolution MS and MS/MS were performed by using ACQUITY UPLC H-Class and Vion Q TOF MS (Waters). Separation conditions were the same as described above. Lock spray for acquisition of accurate mass was conducted. Leucine-enkephalin was used for lock spray reference. Reference mass was set at m/z 556.2766 according to manufacturer’s manual. Collision energy ramp for MS/MS was set at 10 to 40 eV. Capillary voltage and scan time were set to 3.0 kV and 0.1 s, respectively. The other parameters for MS were the same as described above.
PurificationThe culture medium of the B. subtilis standard strain was extracted with ethyl acetate and then purified using an octadecyl silica (ODS) open column packed with YMC-GEL ODS-A 120-230/70 (YMC Co., Ltd., Kyoto, Japan); distilled water and acetonitrile were used as eluents. The target metabolite was purified using reversed-phase HPLC. An ACQUITY UPLC BEH C18 (2.1 mm i.d. × 50 mm, 1.7 µm, Waters) and 15% acetonitrile in distilled water were used as the column and eluent, respectively. UV absorbance was monitored at 210 and 254 nm.
NMR1H-NMR (600 MHz) and 13C-NMR (150 MHz) spectra were obtained using a Varian-INOVA600 instrument (Palo Alto, CA, U.S.A.). Chemical shifts are reported in parts per million, with deuteriochloroform used as the reference standard.
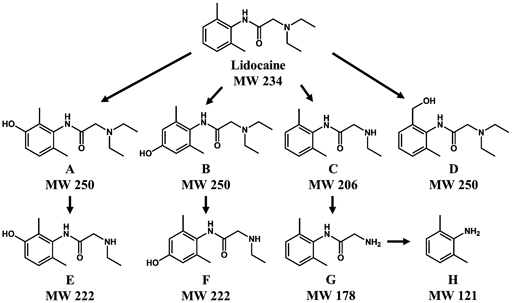
Lidocaine was metabolised by HLM and subjected to LC/MS. The results are shown in Fig. 2. The peak detected at 1.89 min in the mass chromatogram of m/z 235 was lidocaine (peak iv in Fig. 2). The peaks in the mass chromatograms with m/z 122 (tR 1.84 min, peak i in Fig. 2), 207 (tR 1.89 min, peak iii in Fig. 2) and 251 (tR 1.75 and 1.95 min, peak v and vi in Fig. 2) appeared after incubation in HLM, which were presumed to be the known metabolites DMA (H), MEGX (C), and 3- and 4-hydroxylidocaine (A and B). These detected ions would be protonated molecules ([M + H]+) because we selected positive electrospray ionisation in LC/MS. Additionally, a peak at 2.00 min in the mass chromatograms with m/z 148 (peak ii in Fig. 2) appeared after incubation; however, we did not find corresponding lidocaine metabolites in the literature.
Peaks i–vi correspond to DMA (H), an unknown metabolite, MEGX (C), lidocaine, and 3- and 4-hydroxylidocaine (A, B), respectively. The incubation time was 90 min. For other reaction conditions, see the Experimental.
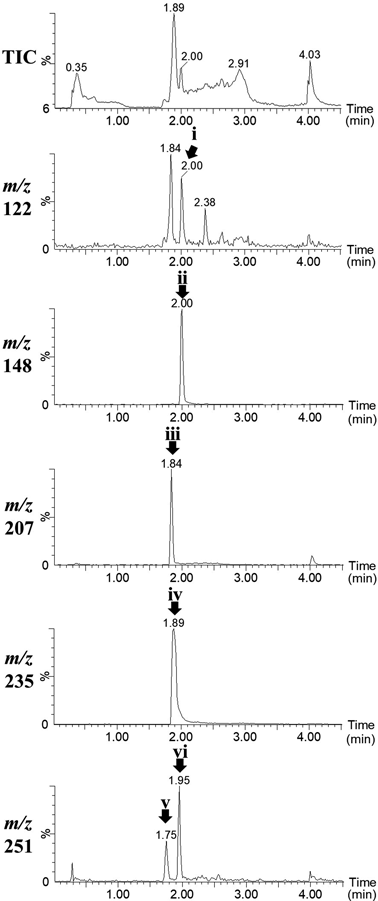
We searched for food-derived microorganisms that metabolise lidocaine and produce the metabolite at m/z 148. The foods used in this study included blue cheese, shiitake mushroom, dry yeast, yoghurt, and natto. The culture solution containing lidocaine did not appear to inhibit the growth of the isolated bacteria. The LC/MS data were shown in Supplementary Fig. 1. Any peaks corresponding to lidocaine metabolites produced in the abovementioned experiment using HLM were not observed in the culture of microorganisms in any foods other than natto. In lidocaine-containing culture of the natto isolate, the peaks at m/z 207, which was thought to be MEGX (C), was detected (Supplementary Fig. 2). Moreover, the peak at m/z 148, an unknown metabolite detected in HLM, was also detected in the lidocaine-containing culture of the natto isolate (Fig. 3(a), Supplementary Fig. 2).
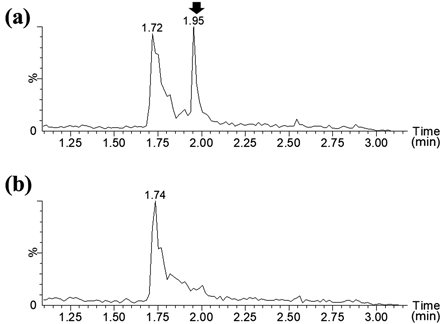
The natto isolate that produced this metabolite was subjected to 16S rDNA sequencing analysis and showed the highest homology to B. subtilis (ATCC6051) (99.94%). We obtained B. subtilis ssp. Spizizenii NBRC 3134 from NBRC and cultivated this strain in LB medium containing 0.5 g/L lidocaine. A peak at m/z 148 was observed. Figure 4 shows the mass chromatograms at m/z 148 of the natto isolate culture (Fig. 4(a)), B. subtilis standard strain culture (Fig. 4(b)), and HLM (Fig. 4(c)). A peak at m/z 148 with a retention time of 1.95 min was detected in all samples.

(a)–(c): LC/MS mass chromatograms at m/z 233 of (a) culture of natto isolate, (b) culture of B. subtilis ssp. Spizizenii No. 3134, (c) human liver microsome.
Bacillus subtilis standard strain culture (4 L) supplemented 0.5 g/L lidocaine was prepared. The culture medium was extracted with ethyl acetate and purified on an ODS open column. The purified product was further purified using reversed-phase HPLC. The LC/MS results of the culture medium, ethyl acetate extract, combined fractions after ODS open-column chromatography, and HPLC purification are shown in Fig. 5. We obtained 0.8 mg of residue after reverse-phase HPLC. HPLC-photodiode array (PDA) analysis showed that the purity of purified fraction was 92% (Supplementary Fig. 3). Based on the amount of lidocaine added to the culture solution, the recovery rate was calculated to be 0.037%.
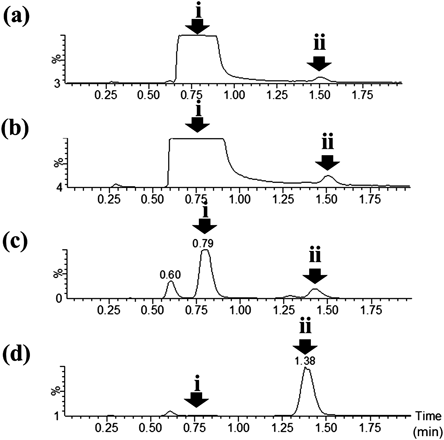
(a) Culture medium of Bacillus subtilis ssp. Spizizennii No. 3134 supplemented with lidocaine, (b) ethyl acetate extract, (c) combined fractions after ODS open column, and (d) after HPLC purification. Peak i: lidocaine, ii: target metabolite showing m/z 148 and 233.
The HPLC fractions were subjected to high-resolution MS. Similar to the results of LC/MS analysis, a peak at m/z 148 was detected. The accurate mass was m/z 148.1133 (Fig. 6(a)). The estimated molecular formula is C10H14N (calculated mass: 148.1126, difference: 4.7 ppm). Moreover, a peak at m/z 233.1660 was detected for this fraction (Fig. 6(a)). The estimated molecular formula was C14H21N2O (calculated mass: 233.1654, difference: 2.6 ppm). MS/MS analysis of m/z 233.1660 revealed a product ion at m/z 148.1133 (Fig. 6(b)), indicating that the peak at m/z 148 was a fragment of the ion at m/z 233.1660.

(a) Full scan mass spectrum, (b) Product ion scan of m/z 233.1660.
The HPLC fractions were subjected to 1H- and 13C-NMR. Although some components were mixed, clear peaks were observed. 1H-NMR revealed the following peaks: δ = 7.15 (m, 2H), 7.09 (m, 1H), 4.46 (q, J = 4.5 Hz, 1H), 3.79 (dd, J = 12.5, 1.5 Hz, 1H), 3.19 (dd, J = 12.3, 1.5 Hz, 1H), 2.94 (m, 1H), 2.46 (m, 1H), 2.27 (s, 3H), 2.21 (s, 3H), 1.19 (t, J = 6.0 Hz, 3H), 1.14 (d, J = 5.0 Hz, 3H) ppm (Fig. 7(a)). 13C-NMR revealed the following peaks: δ = 170.2, 137.9, 135.7, 133.0, 128.8, 128.7, 128.4, 55.2, 47.5, 18.8, 18.7, 18.3, 13.4 ppm (Fig. 7(b)). Because these data and the molecular formula estimated from the exact mass of m/z 233.1660 were mostly consistent with those reported by Ren et al.,10) the unknown metabolite was 3-(2,6-dimethylphenyl)-1-ethyl-2-methyl-4-imidazolidinone. Although Ren et al.10) detected a peak at δ = 77.36 ppm in 13C-NMR, this peak was completely overlaid by the peaks of deuteriochloroform.
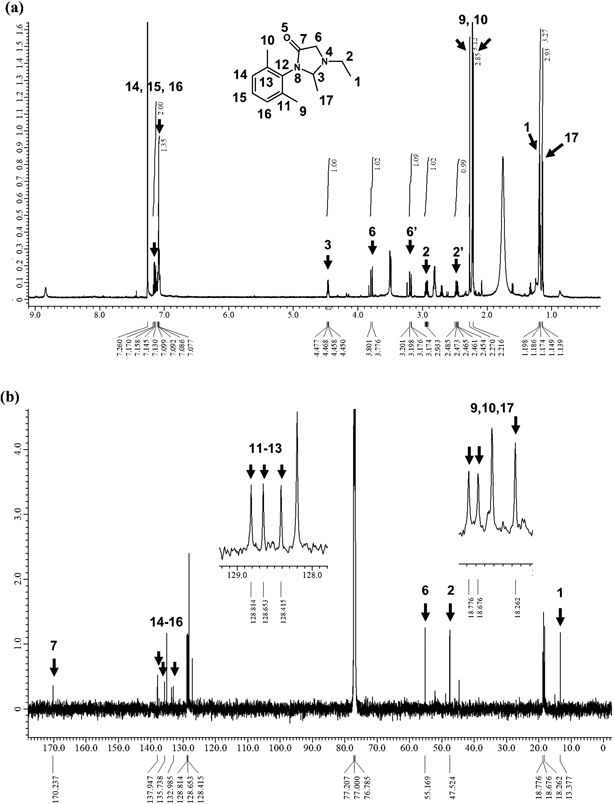
Lidocaine is metabolised by HLM. Many studies have reported several lidocaine metabolites such as 4-hydroxylidocaine, DMA, and MEGX.7–9) Our results on the metabolism of lidocaine by HLM suggest the presence of an unknown lidocaine metabolite corresponding to the peak at m/z 148 (Fig. 2).
Next, we attempted to estimate the structure of this unknown metabolite; however, none of the compounds were found on mass spectrum database11) and literature. Further structural determination is required. Structure determination requires unknown metabolites in the order of milligrams, which is impractical to produce in HLM. Therefore, we focused on microorganisms commonly used in food products to prepare adequate amounts of this metabolite. Food products pose no ethical problems, have a small environmental impact, and have relatively little effect on the human body. According to Kato, antibacterial and bactericidal activities of lidocaine to bacteria including Escherichia coli, Staphylococcus aureus and so on were expressed at concentrations of >3.0 and >8.0 g/L, respectively.12) In this study, the concentration of lidocaine was 0.5 g/L, which is one-sixth of the concentration showing antibacterial activity, therefore we set the concentration at 0.5 g/L for screening. Screening to identify microorganisms that metabolise lidocaine suggested that the bacteria isolated from natto can metabolise lidocaine. When the bacteria were cultured on lidocaine-containing and lidocaine-free media and the peaks from LC/MS analysis were compared, a difference in peaks was observed only in bacteria isolated from natto (Fig. 3, Supplementary Figs. 1, 2). Furthermore, the m/z and retention time of the lidocaine metabolite produced by bacteria isolated from natto were consistent with those of an unknown metabolite produced by HLM. Therefore, focusing on a bacterium isolated from natto may be useful for obtaining adequate amounts of unknown metabolites and determining their structure and properties. It should be noted that these results do not indicate that lidocaine can not be metabolized in foods other than natto. It is possible that lidocaine can be metabolized in other foods by changing the incubation conditions, such as the medium, temperature, and reaction time.
Because B. subtilis was the most homologous bacterium to that isolated from natto, the same experiment was conducted using the standard B. subtilis ssp. Spizizenii No. 3134 strain. The results obtained using the standard B. subtilis strain revealed the same peak observed for the natto isolate (Fig. 4). Thus, B. subtilis has lidocaine-metabolising activity and produces an unknown lidocaine metabolite.
Purification yielded 0.8 mg product. HPLC-PDA analysis showed that the purity of purified fraction was 92% (Supplementary Fig. 3). Based on the amount of lidocaine added to the culture solution, the recovery rate was calculated to be 0.037%. Although the recovery rate is not very high, it is important to be able to obtain a sufficient amount by large scale culture. High-resolution MS revealed peaks at m/z 148.1133 and 233.1660. Importantly, MS/MS analysis of m/z 233.1660 detected a product ion at m/z 148.1133 (Fig. 6(b)), indicating that the protonated molecular and fragment ion of the unknown metabolite were m/z 233.1660 and 148.1133, respectively. The estimated molecular formula for m/z 233.1660 is C14H21N2O. Because these data and 1H- and 13C-NMR of purified fraction containing metabolite at m/z 233.1660 were mostly consistent with those reported by Ren et al.,10) the unknown metabolite was 3-(2,6-dimethylphenyl)-1-ethyl-2-methyl-4-imidazolidinone. This compound was reported by Ren et al. to be produced as a metabolite by Bacillus megaterium P450BM3 (CYP102A1) during lidocaine metabolism10) and was reported as a lidocaine metabolite in urine of man.13) However, these properties have not been reported previously in HLM.
In this study, we were only able to obtain the amount of the metabolite required to determine its structure, and were unable to evaluate cytotoxicity and physiological functions. However, it is easy to increase the culture scale of safe food-derived microorganism, if we continue this approach, it seems possible to obtain the amounts necessary for these studies. Moreover, the detection and structure determination of a novel metabolite in HLM is an important result, thus we think it is worth reporting. Evaluation of cytotoxicity and physiological function will be the subject of future study.
The mechanism of action of lidocaine is not completely understood. Therefore, the discovery of this compound as a lidocaine metabolite in HLM may contribute to the understanding of the mechanism of action of lidocaine. Additionally, we demonstrated the food microorganisms that can reproduce drug metabolism in humans can be used to examine drug metabolites at low cost and without ethical issues. Furthermore, similar metabolites were produced by both HLM and B. subtilis, suggesting that there may be common CYPs or different CYPs that perform similar metabolic roles between HLM and B. subtilis. Our results provide insight for future studies on lidocaine, as well as for identifying drug metabolites using microorganisms and in studies on CYPs in HLM.
This research was financially supported by the Undergraduate Research Project of Osaka University. This research was partially supported by Research Support Project for Life Science and Drug Discovery (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) form AMED under Grant Number JP22ama121054 (Support Number: 5799). We thank Professor Mitsuhiro Arisawa, Professor Tsuyoshi Inoue, and Dr. Misa Muraoka for their support. We also thank Professor Masayoshi Arai and Dr. Haruyasu Asahara for helpful discussions.
The authors declare no conflict of interest.
This article contains supplementary materials.