2024 Volume 72 Issue 4 Pages 360-364
2024 Volume 72 Issue 4 Pages 360-364
Batrachotoxin (1) is a potent cardio- and neurotoxic steroid isolated from certain species of frogs, birds, and beetles. We previously disclosed two synthetic routes to 1. During our synthetic studies toward 1, we explored an alternative strategy for efficiently assembling its 6/6/6/5-membered steroidal skeleton (ABCD-ring). Here we report the application of intermolecular Weix and intramolecular pinacol coupling reactions. While Pd/Ni-promoted Weix coupling linked the AB-ring and D-ring fragments, SmI2-mediated pinacol coupling did not cyclize the C-ring. Instead, we discovered that SmI2 promoted a 1,4-addition of the α-alkoxy radical intermediate to produce the unusual 11(9→7)-abeo-steroid skeleton. Thus, this study demonstrates the convergent assembly of the skeleton of the natural product matsutakone in 11 steps from 2-allyl-3-hydroxycyclopent-2-en-1-one.
Steroids possess a 6/6/6/5-membered ABCD-ring system, C10,C13-angular methyl groups, and a C17-carbon chain.1) A wide range of biologically important steroids are created in nature through structural modifications of a shared carboskeleton, typically including oxidation, unsaturation, esterification, and glycosylation. Migration and cleavage of the C–C bonds of the tetracycle further lead to the formation of abeo-steroids and secosteroids, respectively.2) The diverse bioactivities of steroidal natural products originate from the functional groups attached to the tetracycle and the overall three-dimensional structures conferred by the ring fusion patterns. Because of their profound biological activities, many naturally occurring steroids as well as synthetic and semi-synthetic steroidal compounds are utilized as biological probes and pharmaceuticals.
Batrachotoxin (1, Chart 1) is a unique steroidal natural product first isolated from poison-dart frogs of the genus Phyllobates and later isolated from certain species of birds3,4) and beetles.5) It is utilized as an arrow poison in the Columbian rain forest,6) and its potent toxicity derives from its ability to activate voltage-gated sodium channels.7) The tetracyclic carboskeleton of 1 contains two double bonds (C7 and C16), and has one nitrogen (C18) and five oxygen substituents (C3, C9, C11, C14, and C20).8–10) These multiple functional groups together with a six-membered C3,C9O-hemiacetal across the cis-fused AB-ring and a seven-membered oxazepane on the cis-fused CD-ring make the chemical construction of 1 a formidable synthetic challenge.11–13) The Wehrli group achieved a semisynthesis of batrachotoxinin A, a C20-alcohol derivative of 1, from 11α-acetoxyprogesterone,14,15) and the Kishi,16) Du Bois,17) and Luo18) groups subsequently accomplished the total synthesis of batrachotoxinin A or 1 by applying distinct and creative synthetic strategies.

a) MOM = methoxymethyl, TBS = tert-butyldimethylsilyl, Tf = trifluoromethanesulfonyl, pin = pinacolate.
In 2023, we disclosed two convergent synthetic routes19,20) to 1 using AB- and D-ring fragments. While the first approach employed intermolecular Pd/Ag-promoted Suzuki–Miyaura coupling and intramolecular Dieckmann condensation reactions,21) the second approach used intermolecular radical coupling and intramolecular Pd/Ni-promoted Weix coupling reactions.22) As a result, 1 was assembled in 22 and 28 steps, respectively, from (+)-Wieland–Miescher ketone. During the synthetic studies toward 1, we explored an alternative third approach adopting the intramolecular SmI2-mediated pinacol coupling reaction. Although this effort did not result in the total synthesis of 1, we found that the reaction efficiently formed an unusual 11(9→7)-abeo-steroid skeleton, corresponding to the bioactive rearranged steroid matsutakone (a.k.a. pleurocin A).23,24)
Our previous convergent total synthesis of 1 based on the first approach is summarized in Chart 1. AB-ring and D-ring fragments were prepared. A five-step sequence converted (+)-Wieland–Miescher ketone into C8-vinyl bromide 6a, on which the C3-methyl acetal and C9-tetrasubstituted carbon were constructed in four steps to yield C8-vinyl iodide 4. Triflate 5a and pinacol boronate 5b were derivatized from 2-allyl-3-hydroxycyclopent-2-en-1-one in seven and eight steps, respectively. When AB-ring iodide 4 and D-ring triflate 5a were subjected to the Pd/Ni-promoted reductive coupling conditions developed by the Weix group,25,26) adduct 3 was obtained in only 18% yield, presumably reflecting the steric hindrance induced by the C9-methoxy carbonyl group. In contrast, 3 was obtained in 70% yield by powerful Pd/Ag-promoted Suzuki–Miyaura coupling27) between iodide 4 and pinacol boronate 5b. Ensuing Dieckmann condensation and decarboxylation then forged the C-ring, leading to tetracyclic intermediate 2 in four steps from 3. The following eight steps from 2 completed the total synthesis of batrachotoxin (1).
In the new approach, we envisioned shortening the synthetic route by simultaneously installing the C9-tetrasubsituted and C11-trisubsituted carbons of 8 using the intramolecular pinacol coupling reaction. We expected that the strategy would allow us to employ structurally simpler vinyl bromide 6a and vinyl triflate 5a as the AB- and D-ring fragments, respectively, and would enable their efficient Weix coupling to produce the adduct 7 because of the absence of the sterically imposing C9-methoxycarbonyl group. After the derivatization of 7, the pinacol coupling reaction would cyclize the C-ring of 8, which would be readily transformed into 2 by changing the protective groups.
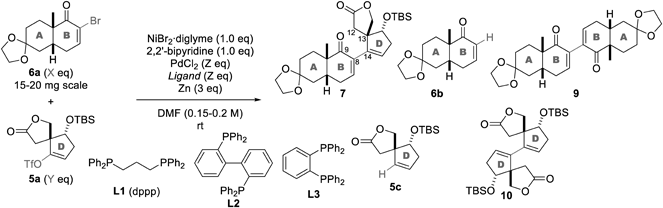
According to the plan of the third approach, the previously synthesized AB-ring 6a and D-ring 5a were subjected to Pd/Ni-promoted Weix coupling conditions (Table 1). To optimize the reaction, we screened Pd ligands and changed the equivalents of 6a (X), 5a (Y), and PdCl2/ligand (Z) while maintaining the Ni ligand to 2,2′-bipyridine and the amount of NiBr2·diglyme and Zn to one and three equivalents (equiv.), respectively. In entry 1, 6a (1 equiv.) and 5a (1 equiv.) were subjected to NiBr2·diglyme, 2,2′-bipyridine, PdCl2 (0.5 equiv.), 1,3-bis(diphenylphosphino)propane (dppp, L1, 0.5 equiv.), and Zn in N,N-dimethylformamide (DMF) at room temperature. The desired adduct 7 was obtained in 39% yield along with the recovered AB-ring 6a (20%), hydrogenated AB-ring 6b (15%), AB-ring dimer 9 (10%), hydrogenated D-ring 5c (4%), and D-ring dimer 10 (2%). Exchange of L1 with 2,2′-bis(diphenylphosphino)biphenyl (L2, entry 2) and 1,2-bis(diphenylphosphino)benzene (L3, entry 3) decreased the yield of 7 to 31 and 16%, respectively, and increased the yields of byproducts 6b (49 and 44%), 5c (31 and 28%), and 10 (15 and 14%).
 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Entry | X | Y | Z | Ligand | Yield [%]b) | ||||||
| 7 | 6a | 6b | 9 | 5a | 5c | 10 | |||||
| 1a) | 1.0 | 1.0 | 0.5 | L1 | 39 | 20 | 15 | 10 | 0 | 4 | 2 |
| 2a) | 1.0 | 1.0 | 0.5 | L2 | 31 | 5 | 49 | 9 | 0 | 31 | 15 |
| 3a) | 1.0 | 1.0 | 0.5 | L3 | 16 | 0 | 44 | 0 | 32 | 28 | 14 |
| 4a) | 1.0 | 1.5 | 0.5 | L1 | 34 | 5 | 48 | 6 | 41 | 26 | 12 |
| 5a) | 1.3 | 1.0 | 0.5 | L1 | 49c) | 0 | 18 | 5c) | 0 | 1 | 12c) |
| 6a) | 1.3 | 1.0 | 0.25 | L1 | 51 | 0 | 34 | 6 | 0 | 3 | 14 |
| 7d) | 1.3 | 1.0 | 0.25 | L1 | 54c) | 0 | —e) | —e) | 0 | —e) | —e) |
a) 15–20 mg scale. b) Yield calculated from 1H-NMR using CH2Br2 as an internal standard. c) Isolated yield. d) 2.6 g scale. e) Not isolated. dppp = 1,3-bis(diphenylphosphino)propane.
The proposed mechanism of the Weix coupling is shown in Chart 2. Zn0 reduces NiII and PdII to Ni0 and Pd0, respectively. The Ni0 and Pd0 reagents oxidatively insert into the C–Br of 6a and the C–OTf bond of 5a, respectively, to afford 11 and 13. ZnII species 12, which is formed from NiII species 11, then reacts with PdII species 13 to produce adduct 7 via the reductive elimination of Pd0 from 14. Considering this mechanism, hydrogenated 6b and 5c would originate from the protonation of vinyl metal species such as 11/12 and 13 upon the aqueous work-up. Alternatively, dimers 9 and 10 would be produced through the coupling of 12 and 11, and the generation of the vinyl zincate from 13 and the subsequent coupling of the vinyl zincate and 13. Hence, all these byproducts, 6b, 5c, 9, and 10, are likely produced as a result of the slow transmetallation from 12 and 13 to the key intermediate 14. The lower yields of 7 upon using the bulkier phosphine ligands L2 (entry 2) and L3 (entry 3) rather than L1 (entry 1) supports the importance of the steric effect for the effective formation of 14.
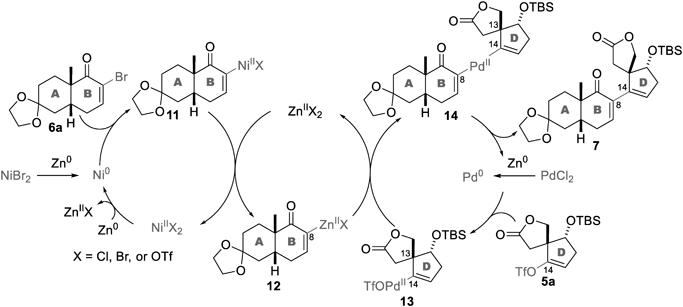
To improve the efficiency, the X, Y, and Z valuables were altered using L1 as the Pd ligand (Table 1, entries 4–6). When the equivalent of D-ring 5a was increased from 1 to 1.5, the yield of 7 decreased from 39 to 34% (entry 4). The high yields of the obtained hydrogenated AB-ring 6b (48%) and the recovered D-ring 5a (41%) indicated the facile consumption of AB-ring 6a during the reaction. Therefore, 1.3 equiv. of AB-ring 6a and 1 equiv. of D-ring 5a were used for the coupling reaction (entry 5), improving the yield of 7 (49% yield). Even when the amount of PdCl2 and dppp was halved (0.25 equiv.), the yield of 7 remained consistent (51%, entry 6). Application of the optimized conditions to 2.6 g of 5a furnished 7 in 54% yield (entry 7), thereby establishing a scalable procedure of Weix coupling between 6a and 5a under mild conditions. Noteworthily, a comparison of the coupling yields of 3 (18%, Chart 1) and 7 (54%, Table 1) corroborated the negative effect of the bulky C9-methoxycarbonyl group on the coupling.
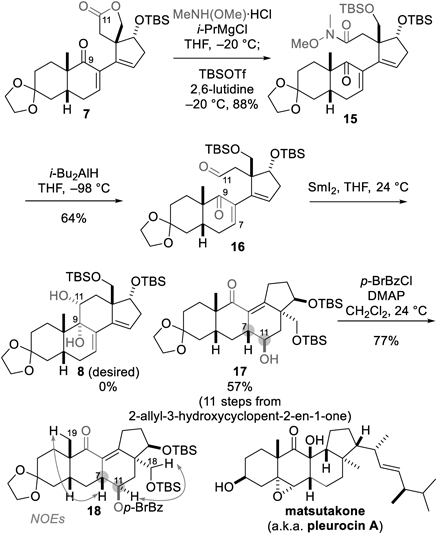
Having realized the requisite intermolecular reaction between the AB- and D-ring fragments, we attempted to apply the SmI2-promoted intramolecular reaction to form the C-ring of batrachotoxin (1) (Chart 3). Accordingly, dicarbonyl compound 16 was prepared from 7 in two steps. One-pot treatment of 7 with MeNH(OMe)·HCl and i-PrMgCl28) and then with TBSOTf and 2,6-lutidine opened the lactone and capped the resultant primary hydroxy group, leading to bis-TBS-protected Weinreb amide 15. The amide moiety of 15 was in turn reduced chemoselectively over the C9-carbonyl group using i-Bu2AlH at −98 °C in tetrahydrofuran (THF), giving rise to aldehyde 16. Subsequently, 16 was submitted to SmI2 in THF at room temperature, aiming to construct tetracycle 8.29,30) The desired bond formation at the C9 and C11 positions, however, did not occur under these conditions. Because of the α,β-unsaturated ketone structure of 16, the alternative cyclization mode was operative: the reductive 1,4-addition stereoselectively linked C7 and C11 to produce tetracycle 17 as the sole stereoisomer in 57% yield. The newly introduced C7- and C11-stereochemistries of 17 were unambiguously determined by a nuclear Overhauser effect spectroscopy (NOESY) experiment after its conversion to C11-p-bromobenzoate 18. Although the single-step cyclization of the C-ring of 1 was unsuccessful, the synthetic sequence efficiently provided the structure of the functionalized 11(9→7)-abeo-steroid 17 in 11 steps from 2-allyl-3-hydroxycyclopent-2-en-1-one. Matsutakone (a.k.a. pleurocin A) is an example of 11(9→7)-abeo-steroids,23,24,31,32) in which the B- and C-rings are fused at C7–C11 instead of C9–C11. This natural product was isolated from the edible mushroom Tricholoma matsutake or Pleurotus eryngii and displays inhibitory activity against acetylcholinesterase and nitric oxide production.
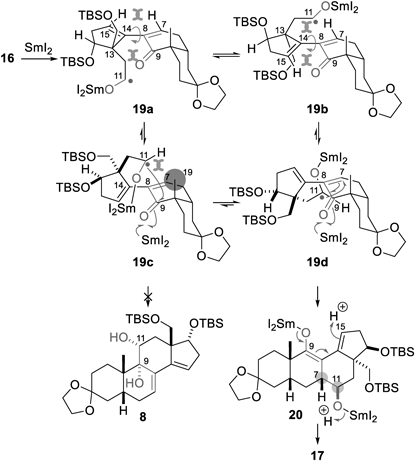
A plausible mechanism for the formation of 11(9→7)-abeo-steroid skeleton 17 is illustrated in Chart 4. First, SmI2 reacts with C11-aldehyde 1633,34) to afford C11-α-alkoxy radical 19, which potentially adopts four representative conformations 19a–19d regarding the orientation of the D-ring. While the C11-α-alkoxy radical is located such that it avoids the AB-ring system in all conformers 19a–19d, the C7=C8 and C14=C15 planes of 19a/19b and 19c/19d are parallel and perpendicular, respectively. Thus, 19a/19b would be energetically higher than 19c/19d because the 1,3-allylic strain of the two double bonds of 19a and 19b induces large steric repulsions among the C7,C9,C13,C15-substituents. The SmI2-attached C11-α-alkoxy radical is proximal to the C9-ketone in 19c and the C7-olefin in 19d. As the wanted C9–C11 bond formation from 19c to 8 is impeded by the sterically cumbersome C19-methyl group on the top face, the unwanted C7–C11 bond formation proceeds at the bottom face of 19d without experiencing the unfavorable steric interaction, leading to 11(9→7)-abeo-steroid skeleton 20 in a C7- and C11-stereoselective manner. Then, protonation of the C9-enolate at the less sterically shielded C15 position proceeds to afford 17.
In summary, we devised a new convergent route to the functionalized 11(9→7)-abeo-steroid skeleton in 11 steps from 2-allyl-3-hydroxycyclopent-2-en-1-one during our synthetic studies toward batrachotoxin (1). Scalable Pd/Ni-promoted Weix coupling conditions were established by screening various factors to realize the coupling between AB-ring bromide 6a and D-ring triflate 5a. The two-step process from adduct 7 generated aldehyde 16, and ensuing treatment with SmI2 delivered the 11(9→7)-abeo-steroid 17 corresponding to matsutakone/pleurocin A. Because of the overall efficiency, the strategy developed here will be applicable for efficient total syntheses of diverse rearranged steroids with important biological activities.
This research was financially supported by Grants-in-Aid for Scientific Research (S) (JP22H04970) to M.I. and for Early Career Scientists (JP21K14622) and Scientific Research (C) (JP23K04749) to K.H. from JSPS. Fellowships from JSPS (JP17J09521 and JP23KJ0578) to H.M. and Y.W. are gratefully acknowledged.
The authors declare no conflict of interest.
This article contains supplementary materials.