2024 Volume 72 Issue 8 Pages 751-761
2024 Volume 72 Issue 8 Pages 751-761
Gout is the second largest metabolic disease worldwide after diabetes, with acute gouty arthritis as most common symptom. Xanthine oxidase (XOD) and the NOD like receptor-3 (NLRP3) inflammasome are the key targets for acute gout treatment. Chlorogenic acid has been reported with a good anti-inflammatory activity, and Apigenin showed an excellent potential in XOD inhibition. Therefore, a series of chlorogenic acid–apigenin (CA) conjugates with varying linkers were designed and synthesized as dual XOD/NLRP3 inhibitors, and their activities both in XOD and NLRP3 inhibition were evaluated. An in vitro study of XOD inhibitory activity revealed that the majority of CA conjugates exhibited favorable XOD inhibitory activity. Particularly, the effects of compounds 10c and 10d, with an alkyl linker on the apigenin moiety, were stronger than that of allopurinol. The selected CA conjugates also demonstrated a favorable anti-inflammatory activity in RAW264.7 cells. Furthermore, compound 10d, which showed the optimal activity both in XOD inhibition and anti-inflammatory, was chosen and its inhibitory ability on NLRP3 and related proinflammatory cytokines was further tested. Compound 10d effectively reduced NLRP3 expression and the secretion of interluekin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) with an activity stronger than the positive control isoliquiritigenin (ISL). Based on these findings, compound 10d exhibits dual XOD/NLRP3 inhibitory activity and, therefore, the therapeutic effects on acute gout is worthy of further study.
Gout is a multifaceted disease that originates from hyperuricemia, which can be attributed to reduced uric acid (UA) excretion or purine metabolism disorders. The deposition of monosodium urate (MSU) crystals in tissues and subsequent clinical manifestations, such as acute gouty arthritis, gouty stones, joint deformities, and dysfunction, occur when serum uric acid levels exceed the normal concentration (≥7 and ≥6 mg/dL for males and females, respectively).1) Currently, gout is the second largest metabolic disease following diabetes, and there is a substantial market demand for anti-gout drugs.2)
Presently, the clinical agents for treating gout including3,4): (1) xanthine oxidase inhibitors (XODIs), which inhibit the conversion of xanthine to uric acid, including allopurinol, febuxostat, and topiroxostat5–7) and (2) anti-inflammatory agents for managing acute gout, such as colchicine, nonsteroidal anti-inflammatory drugs (NSAIDs), and glucocorticoids.8,9) The XODIs is primarily effective in intermittent or chronic gout, rather than acute gout. The NOD-like receptor-3 (NLRP3) inhibitors was reported as a potent anti-inflammatory agent in treating acute gout, nevertheless, the NLRP3 inhibitors are ineffective in reducing uric acid levels, results in monotherapy of NLRP3 inhibitors being insufficient in gout therapy.10,11) Then, the combination therapy of XOD and NLRP3 inhibitors may be more effective for gout treatment. However, there was still no bifunctional XOD/NLRP3 inhibitor for treating gout, therefore, based on traditional XOD inhibitor, and importance of NLPR3 in acute gout attacks, it is of great significance to design dual XOD-NLPR3 inhibitors with uric acid lowering and anti-inflammatory activities for treating gout.12)
Previous studies suggested that flavonoids possess multiple functions, such as antioxidant, antibacterial, and antiviral properties. The XOD inhibitory activity of flavonoids has also been extensively investigated.13–15) Notably, apigenin, quercetin, and morin showed strong binding affinity to XOD (Fig. 1). Compared to the clinical XODIs such as febuxostat, flavonoid-based XODIs exhibit superior activity with minimal toxicity and adverse effects.16,17) However, the physicochemical properties and pharmacokinetics of flavonoids limit their application in treating gout.18)

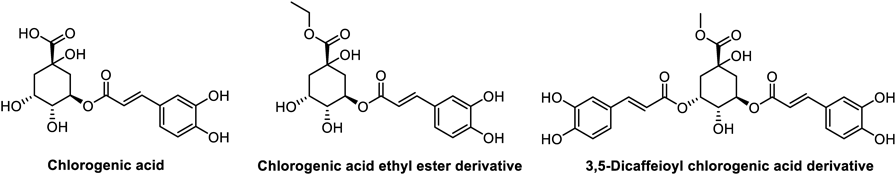
Meanwhile, the chlorogenic acid has been reported with anti-inflammatory activity and demonstrated promising therapeutic effects for inflammatory-related diseases both in vitro and in vivo.19) Notably, chlorogenic acid effectively suppresses the activation of NLRP3 inflammasomes and maybe potential for treating acute gout.20) Moreover, the modification of chlorogenic acid can enhance its anti-inflammatory activity. Esterification of the 1-position of quinic acid moiety on chlorogenic acid has been found to greatly enhance its anti-inflammatory activity. Additionally, the anti-inflammatory activity of 3,5-disubstituted caffeic acid derivatives has been observed to be stronger than that of chlorogenic acid21–24) (Fig. 2).
Due to the notable XOD inhibitory properties of flavonoids and the NLRP3 inhibitory properties of chlorogenic acid, as well as their potential therapeutic applications in the management of acute gout, we developed and synthesized a series of dual XOD–NLRP3 natural product conjugates linked by various linkers, based on the principle of drug hybridization.25,26) The linkers have been extensively studied in the development of protein hydrolysis targeting chimeras (PROTAC) and antibody coupled drugs (ADC), in which, through employing linking chains not only afforded bifunctional molecules, but also exert a significant influence on the physicochemical and pharmacological properties of the conjugates. Through variation and optimization of the linkers, the good bifunctional inhibitory activity along with good pharmaceutical and physicochemical properties might obtained.27–30) Therefore, in this study, a series of chlorogenic acid–apigenin conjugates connected by various linking chains were designed and synthesized (Chart 1). The present study aimed to acquire inhibitory activity against XOD and NLRP3 through conjugation, which had the potential to emerge lead compounds to treat acute gout for further exploration.

The novel Chlorogenic acid–apigenin (CA) conjugates were listed in Charts 2 and 3. The Apigenin moiety was synthesized as in Chart 2, Apigenin was methoxymethyl (MOM) protected, and followed by linking alkyl ester chains with varying lengths to the 4ʹ-position given compound 2. Compound 2 was then deprotected of MOM and subsequently hydrolyzed the ester groups to afford intermediates 4a–4d31,32) (Chart 2). The chlorogenic acid was totally acetylazed with acetic anhydride given compound 5. Compound 5 was amidated with different mono tert-butoxycarbonyl (Boc)-protected diamine, and subsequently deprotection of the Boc group afforded intermediates 7a–7d or 9a–9d33) (Chart 2). CA conjugates were synthesized through the condensation of compounds 4a–4d with intermediates 7a–7d or 9a–9d in the presence of condensation agents (Chart 3).


XOD is a crucial and rate-limiting enzyme in the production of uric acid, therefore, the in vitro XOD inhibitory activity of the synthesized CA conjugates was firstly evaluated. As shown in Fig. 3 and Table 1, the majority of CA conjugates showed XOD inhibitory activity. The inhibitory effects of compounds 11a–11c and 12a–12d are weaker than that of equimoler allopurinol, especially compounds 11a, 12a, 12b, and 12c, with little inhibition effect. Compound 11d showed a similar effect as allopurinol. The 10a–10d series, which featured an alkyl chain in the apigenin moiety, exhibited a more potent inhibitory effect than the 11a–11d and 12a–12d series. The inhibitory activity of conjugates 10c and 10d (IC50: 30.74, 22.42 µM, respectively), which contain longer alkyl chains, is stronger than positive control allopurinol (IC50: 40.55 µM).

Data were expressed as mean ± standard error of the mean (S.E.M.) (n = 3). * p < 0.05, ** p < 0.01, *** p < 0.001, compared with allopurinol group.
| Compound | IC50 (µM) | Compound | IC50 (µM) |
|---|---|---|---|
| Allopurinol | 40.55 | 11c | 73.83 |
| 10a | 73.97 | 11d | 44.45 |
| 10b | 75.50 | 12a | >100 |
| 10c | 30.74 | 12b | >100 |
| 10d | 22.42 | 12c | >100 |
| 11a | >100 | 12d | 84.4 |
| 11b | 97.25 |
The preliminary structure–activity relationship analysis revealed that: (1) Compounds linked with alkyl chains is significantly superior to that with polyethylene glycol (PEG) chains. (2) The longer the linked chain, the better inhibitory activity, and (3) The alkyl chain on the apigenin moiety show better inhibitory activity than that on the chlorogenic acid moiety.
Preliminary Study of the Anti-inflammatory Activities in RAW264.7 CellsEffects of Selected CA Conjugates (10a–10d and 12a–12d) on the Viability of Lipopolysaccharide (LPS) Treated RAW264.7 CellsFirstly, the anti-inflammatory activity of CA conjugates (compounds 10a–10d and 12a–12d) on RAW264.7 macrophages was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The results showed in Fig. 4a, LPS (1 µg/mL) treatment induced a significant cell death. However, at a concentration of 10 µg/mL, compounds 10a, 10d, and 12a significantly rescued RAW264.7 cells from LPS-induced cell death. Compounds 10b, 10c, 12b, and 12d showed protective effects without statistical significance. Conversely, compound 12c has no protective activity against LPS-induced cytotoxicity (Fig. 4a).

Data were expressed as mean ± S.E.M. (n = 3). #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001, compared with control group; * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, compared with LPS treated vehicle group.
NO is a crucial inflammatory marker in LPS-induced RAW264.7 cells,34) therefore, Griess assay was performed to evaluate the inhibitory effects of selected CA conjugates on LPS induced NO production. As shown in Fig. 4b, LPS (1 µg/mL) treatment for 24 h induced significantly NO overproduction compared with control group. At concentration of 10 µg/mL, conjugates 10a, 10b, 10c, 10d, and 12b, could suppress LPS-induced NO production in RAW264.7 cells, while compounds 12a, 12c, and 12d exhibited no discernible inhibitory effect on NO production. These findings indicated that 10a, 10b, 10c, and 10d play a significant role in inhibiting the inflammatory response of LPS-induced RAW264.7 macrophages (Fig. 4b).
Compounds 10a, 10b, 10c, and 10d showed cytoprotective effects as well as inhibitory activity on NO production in LPS-treated RAW264.7 cells. In consideration of the inhibitory activity on XOD of all the CA conjugates, 10d was chosen for further evaluation of its anti-inflammatory activity.
Determination of NLRP3 Inflammasome Inhibitory Effects of 10d in RAW264.7 CellsThe effects of compound 10d on the production of NLRP3 inflammasomes and cytokines (caspase-1, interluekin-1β (IL-1β), and tumor necrosis factor-α (TNF-α)) in LPS + ATP treated RAW264.7 cells were further evaluated,35,36) and the Isoliquiritigenin (ISL), which has been regarded as a typical inhibitor of NLRP3, was selected as positive control.37) A notable increase in the levels of NLRP3 and inflammatory factors in PCR was observed following LPS stimulation. However, pretreatment with 10d at a concentration of 100 µM significantly reduced the elevation of NLRP3, IL-1β, and TNF-α induced by LPS, comparable with the positive control ISL (Fig. 5).

Data were expressed as mean ± S.E.M. (n = 3). ##p < 0.01, compared with control group; ** p < 0.01, compared with LPS treated vehicle group.
The inhibitory effect of compound 10d on IL-1β secretion in RAW264.7 cells was further determined by enzyme-linked immunosorbent assay (ELISA). The results showed LPS stimulation induced a dramatically increase in IL-1β production, while 25 µM of ISL significantly revised the elevation (Fig. 6). Compound 10d also inhibited the IL-1β production in a concentration-dependent manner and the inhibitory effect at high concentration (100 µM) was equivalent to that of ISL (Fig. 6).

Through the Western blotting assay, we further confirmed the suppressive effect of compound 10d on the proinflammatory cytokine IL-1β and the NLRP3/caspase-1 inflammasome in RAW264.7 cells. Compound 10d exhibited a dose-dependent reduction on the expression levels of NLRP3 and IL-1β, while no notable inhibitory effect on caspase-1 expression was observed (Fig. 7).

The findings revealed that compound 10d effectively inhibited the production and release of the NLRP3 inflammasome, IL-1β, and TNF-α, thus highlighting the potential of compound 10d as a promising therapeutic agent for regulating the inflammatory response during acute gout attacks.
Effects of 10d on the Expression of Inflammatory Proteins in LPS-Induced RAW264.7 CellsThe iNOS and COX2 enzymes play a critical role in the synthesis process of NO and prostaglandin E2 (PGE2) in LPS-induced RAW264.7 macrophages, respectively. To further investigate the anti-inflammatory effects of compound 10d, the suppressive effect on protein expression levels of inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX2) were assessed using Western blotting. As depicted in Fig. 8, the induction of iNOS protein was observed in RAW264.7 macrophages upon LPS stimulation, while treatment with 10d at a low concentration resulted in a slight attenuation of its expression (Fig. 8a). Similarly, the expression level of COX2 protein was upregulated by LPS and mildly suppressed by 10d in a similar manner in RAW264.7 cells (Fig. 8b).

The binding model of compound 10d with XOD and NLRP3 was determined using AutoDock Vina software. The results indicated that the Apigenin moiety of 10d exhibited a preference for binding with XOD protein, while the chlorogenic acid moiety tends to bind with the ATP binding pocket of NLRP3. Additionally, although the middle alkyl chain acts as only a linker and does not participate in binding process, the suitable linker maintained good rigidity of CA conjugate, could fully expose both hits of CA conjugate, allowing for accurate binding with XOD or NLRP3, as depicted in Fig. 9. Moreover, Fig. 10 illustrates that the 5′,7′-dihydroxyl groups on the Apigenin moiety of 10d form two hydrogen bonds with amino acids GLY588 and ASP595 at distances of 3.2 and 2.6 Å, respectively. Additionally, the planar ring of the Apigenin moiety exhibits van der Waals interactions with Tyr592 and Ser587 on the XOD protein. Notably, the docking score of the Apigenin moiety with XOD is −7.67 kcal/mol, which significantly exceeds that between allopurinol and XOD (−6.280 kcal/mol), indicating a more potent inhibitory activity.

Hydrogen bonds are shown as arrows, and cation–π interaction is shown by red lines.

Figure 11 illustrates well binding of the chlorogenic acid fragment on 10d with the NLRP3 protein. The binding is facilitated by the cation–π interaction from the amino acid Arg259 and the benzene ring on chlorogenic acid moiety at a distance of 3.0 Å. Additionally, two hydrogen bonds was also formed between Arg259 and the carbonyl groups on 4′-acetyl and 1-amide bond at distances of 2.9 and 3.1 Å, respectively. Furthermore, Arg149 and Arg234 on the NLRP3 protein formed two additional hydrogen bonds with the carbonyl groups of 1,4-diacetyls at distances of 2.9 and 2.8 Å, respectively. Other amino acids in close proximity engage in van der Waals interactions with the chlorogenic acid fragment of 10d. The calculated binding energy of compound 10d with NLRP3 (−10.870 kcal/mol) surpasses that of allopurinol and NLRP3 (−8.479 kcal/mol), thus indicating a higher level of inhibitory activity for 10d.

All chemicals (reagent grade) used in this study were purchased from commercial sources. 1H-NMR and 13C-NMR spectra were measured using a Bruker Ascend 400 MHz spectrometer at 25 °C and referenced to TMS. High-resolution mass spectra (HR-MS) were recorded on Fourier transform (FT)-ICR MS (Ionspec, 7.0 T). Analytical TLC was performed on glass-backed silica gel sheets (silica gel 60 Å GF254). All compounds were detected using UV light (254 or 365 nm). All solvents and reagents were analytically pure, and no further purification was required. All starting materials were commercially available. The detailed synthesis procedure of compounds 4a–4d, and the compounds 7a–7e and 9a–9d were presented in supplementary materials.
Synthesis of 10a–10d, 11a–11d, and 12a–12d: Compound 4 (0.2 mmol, 1.0 equivalent (equiv.)), N-hydroxybenzotriazole (HOBT) (27 mg, 0.2 mmol, 1.0 equiv.), and 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride (EDCI) (76.4 mg, 0.4 mmol, 1.0 equiv.) were added into a 25-mL flask, followed by the addition of anhydrous N,N-dimethylformamide (DMF) (5 mL). The mixture was stirred for 10 min at room temperature, and then compound 7 or 9 (0.24 mmol, 1.2 equiv.) was added and stirred at room temperature for 2–3 h. With completion of the reaction monitored by TLC, then water (5 mL) was added, followed by extraction with ethyl acetate (15 mL ×5). Next, the organic layers were combined, sequentially washed with water (15 mL) and brine (10 mL), and then dried over anhydrous sodium sulfate. Compound 10–12 were obtained after evaporation to dryness and purification via silica gel column chromatography (DCM/MeOH = 100/1–20/1).
(1R,2R,4S,6R)-6-(((E)-3-(3,4-Diacetoxyphenyl)acryloyl)oxy)-4-((2-(2-(4-(5,7-dihydroxy-4-oxo-4H-chromen-2-yl)phenoxy)acetamido)ethyl)carbamoyl)cyclohexane-1,2,4-triyltriacetate (10a)Pale yellow foam solid (112.9 mg, yield 56%). 1H-NMR (400 MHz, CDCl3) δ: 12.75 (s, 1H), 9.34 (s, 1H), 7.60–7.57 (m, 3H), 7.39–7.36 (m, 4H), 7.20 (d, J = 6.8 Hz, 1H), 6.80 (d, J = 5.8 Hz, 2H), 6.31 (t, J = 11.6 Hz, 4H), 5.67 (s, 1H), 5.57 (s, 1H), 5.17 (d, J = 9.2 Hz, 1H), 4.39 (s, 2H), 3.59–3.45 (m, 4H), 2.86 (d, J = 13.8 Hz, 1H), 2.75–2.48 (m, 3H), 2.30 (s, 6H), 2.22 (s, 3H), 2.10 (s, 3H), 1.99 (s, 3H); 13C-NMR (101 MHz, CDCl3) δ: 182.0, 171.3, 170.4, 170.3, 169.4, 168.2, 168.0, 167.0, 165.9, 163.7, 163.0, 161.7, 159.7, 157.6, 144.1, 143.8, 142.5, 132.8, 127.9, 126.6, 124.2, 124.0, 122.9, 118.2, 114.9, 104.7, 99.4, 94.5, 81.1, 71.8, 68.2, 67.3, 66.9, 40.7, 38.8, 38.2, 30.7, 21.8, 21.1, 20.7, 20.6, 20.58; HR-MS: C45H44N2O19 + H+, Calcd 917.2617. Found, 917.2607; C45H44N2O19 + Na+, Calcd 939.2436. Found, 939.2413.
(1R,2R,4S,6R)-6-(((E)-3-(3,4-Diacetoxyphenyl)acryloyl)oxy)-4-((2-(4-(4-(5,7-dihydroxy-4-oxo-4H-chromen-2-yl)phenoxy)butanamido)ethyl)carbamoyl)cyclohexane-1,2,4-triyltriacetate (10b)Pale yellow foam solid (88.3 mg, yield 48%). 1H-NMR (400 MHz, CDCl3) δ: 7.65–7.51 (m, 3H), 7.37–7.35 (m, 3H), 7.25–7.13 (m, 1H), 6.77 (s, 2H), 6.49–6.21 (m, 3H), 5.64–5.55 (m, 3H), 5.13 (s, 1H), 3.96 (s, 2H), 3.76 (s, 2H), 3.43–3.31 (m, 3H), 2.80 (s, 1H), 2.62–2.42 (m, 3H), 2.30 (s, 6H), 2.23 (s, 3H), 2.10 (s, 3H), 1.99 (s, 5H), 1.87 (s, 3H); 13C-NMR (101 MHz, CDCl3) δ: 182.2, 174.6, 172.4, 172.3, 171.4, 170.3, 170.2, 169.9, 168.2, 168.0, 165.7, 163.8, 161.9, 157.7, 144.0, 143.8, 142.5, 132.8, 127.8, 126.6, 124.0, 122.9, 118.3, 114.7, 107.9, 106.6, 106.4, 106.0, 104.7, 103.4, 99.5, 94.4, 81.0, 77.4, 71.8, 68.1, 68.0, 67.7, 29.2, 25.6, 23.9, 22.9, 21.8, 21.0, 20.7, 20.6, 20.58; HR-MS: C47H48N2O19 +H+, Calcd 945.2924. Found 945.2912; C47H48N2O19 + Na+ Calcd 967.2743. Found 967.2750.
(1R,2R,4S,6R)-6-(((E)-3-(3,4-Diacetoxyphenyl)acryloyl)oxy)-4-((2-(10-(4-(5,7-dihydroxy-4-oxo-4H-chromen-2-yl)henoxy)decanamido)ethyl)carbamoyl)cyclohexane-1,2,4-triyltriacetate (10c)Pale yellow foam solid (70.0 mg, yield 31.3%). 1H-NMR (400 MHz, CDCl3) δ: 7.65 (d, J = 8.2 Hz, 2H), 7.50 (d, J = 15.8 Hz, 1H), 7.30–7.27 (m, 3H), 7.12 (d, J = 8.2 Hz,1H), 6.81 (d, J = 8.0 Hz, 2H), 6.57 (s, 1H), 6.40 (d, J = 16.2 Hz, 2H), 6.31–6.17 (m, 2H), 5.63–5.38 (m, 1H), 5.06 (dd, J = 9.8, 2.8 Hz, 1H), 3.85 (s, 2H), 3.31–3.21 (m, 3H), 2.73 (d, J = 15.0 Hz, 1H), 2.50 (dd, J = 38.6, 12.6 Hz, 2H), 2.32–2.17 (m, 7H), 2.12 (s, 4H), 2.01 (s, 3H), 1.90 (s, 3H), 1.66–1.64 (m, 2H), 1.53–1.51 (m, 2H), 1.36–1.10 (m, 12H); 13C-NMR (101 MHz, CDCl3) δ: 182.4, 177.0, 175.6, 171.5, 170.2, 170.0, 168.2, 168.0, 165.7, 164.2, 163.9, 162.2, 162.0, 157.9, 144.0, 143.8, 142.5, 132.8, 128.0, 126.6, 124.0, 123.0, 122.9, 118.3, 114.9, 104.7, 103.5, 99.6, 94.5, 80.9, 71.8, 68.3, 68.1, 67.1, 40.7, 39.1, 38.0, 36.4, 34.0, 30.9, 29.4, 29.2, 29.0, 25.9, 25.6, 24.9, 21.7, 21.0, 20.7, 20.6, 20.56; HR-MS: C53H60N2O19 + H+, Calcd 1029.3863. Found, 1029.3867; C53H60N2O19 + Na+, 1051.3682. Found 1051.3687.
(1R,2R,4S,6R)-6-(((E)-3-(3,4-Diacetoxyphenyl)acryloyl)oxy)-4-((2-(11-(4-(5,7-dihydroxy-4-oxo-4H-chromen-2-yl)phenoxy)undecanamido)ethyl)carbamoyl)cyclohexane-1,2,4-triyltriacetate (10d)Pale yellow foam solid (80 mg, yield 35.2%). 1H-NMR (400 MHz, CDCl3) δ: 7.65 (d, J = 8.0 Hz, 2H), 7.51 (d, J = 15.8 Hz, 1H), 7.31–7.23 (m, 3H), 7.13 (d, J = 8.2 Hz, 1H), 6.81 (d, J = 8.2 Hz, 2H), 6.51 (s, 1H), 6.40 (d, J = 15.8 Hz, 2H), 6.32–6.18 (m, 2H), 5.56–5.45 (m, 2H), 5.14–4.94 (m, 1H), 3.84 (s, 2H), 3.33–3.22 (m, 3H), 2.86 (d, J = 30.5 Hz, 1H), 2.73 (d, J = 15.8 Hz, 1H), 2.50 (dd, J = 40.2, 13.1 Hz, 2H), 2.22 (s, 7H), 2.13 (s, 4H), 2.01 (s, 3H), 1.91 (s, 3H), 1.64 (s, 2H), 1.53 (d, J = 5.2 Hz, 2H), 1.40–1.11 (m, 12H); 13C-NMR (101 MHz, CDCl3) δ: 182.4, 175.5, 171.3, 170.2, 169.9, 168.1, 168.0, 165.6, 164.1, 162.9, 162.0, 157.9, 144.0, 143.8, 142.5, 132.9, 128.0, 126.6, 124.0, 122.9, 118.3, 114.9, 104.8, 103.7, 99.7, 94.4, 81.0, 71.8, 68.1, 67.0, 60.4, 40.8, 39.2, 38.1, 36.7, 36.4, 30.9, 29.2, 25.9, 25.6, 21.8, 21.0, 20.7, 20.6, 20.56; HR-MS: C54H62N2O19 + H+, Calcd 1043.4020. Found, 1043.4021.
The synthesis procedure of 11a–11d was described as above:
(1R,2R,4S,6R)-6-(((E)-3-(3,4-Diacetoxyphenyl)acryloyl)oxy)-4-((4-(2-(4-(5,7-dihydroxy-4-oxo-4H-chromen-2-yl)phenoxy)acetamido)butyl)carbamoyl)cyclohexane-1,2,4-triyl Triacetate (11a)Pale yellow foam solid (110.0 mg, yield 37.5%). 1H-NMR (400 MHz, CDCl3) δ: 12.71 (s, 1H), 9.53 (s, 1H), 7.68 (d, J = 8.2 Hz, 2H), 7.57 (d, J = 15.8 Hz, 1H), 7.40–7.32 (m, 2H), 7.23–7.19 (m, 1H), 7.04–6.82 (m, 3H), 6.59 (s, 1H), 6.39–6.29 (m, 4H), 5.63 (d, J = 2.8 Hz, 1H), 5.54 (td, J = 10.4, 4.6 Hz, 1H), 5.13 (dd, J = 10.2, 2.8 Hz, 1H), 4.49 (s, 2H), 3.34–3.26 (m, 2H), 3.19–3.15 (m, 1H), 2.98–2.83 (m, 1H), 2.66–2.45 (m, 3H), 2.30 (s, 6H), 2.18 (s, 3H), 2.08 (s, 3H), 2.01–1.95 (m, 4H), 1.63–1.45 (m, 4H), 1.34–1.28 (m, 2H); 13C-NMR (101 MHz, CDCl3) δ: 182.2, 170.6, 170.2, 169.9, 169.7, 168.2, 168.1, 165.8, 163.9, 163.2, 161.9, 159.9, 157.7, 144.1, 143.8, 142.5, 132.8, 128.0, 126.6, 124.6, 124.0, 122.9, 118.3, 115.1, 104.7, 104.1, 99.5, 98.7, 94.5, 81.1, 71.7, 68.1, 67.2, 50.6, 39.5, 38.9, 38.2, 30.8, 28.9, 28.7, 23.8, 21.7, 21.0, 20.7, 20.6, 20.57; HR-MS: C48H50N2O19 + H+, Calcd 959.3081. Found, 959.3016; C48H50N2O19 + Na+ Calcd 981.2900. Found 981.2802.
(1R,2R,4S,6R)-6-(((E)-3-(3,4-Diacetoxyphenyl)acryloyl)oxy)-4-((5-(2-(4-(5,7-dihydroxy-4-oxo-4H-chromen-2-yl)phenoxy)acetamido)pentyl)carbamoyl)cyclohexane-1,2,4-triyl Triacetate (11b)Pale yellow foam solid (70 mg, yield 23.6%). 1H-NMR (400 MHz, CDCl3) δ: 12.61 (s, 1H), 9.35 (s, 1H), 7.62 (d, J = 8.2 Hz, 2H), 7.51 (d, J = 15.8 Hz, 1H), 7.31–7.28 (m, 2H), 7.13 (d, J = 8.2 Hz, 1H), 6.83 (d, J = 7.6 Hz, 3H), 6.44 (s, 1H), 6.35–6.32 (m, 2H), 6.26–6.22 (m, 2H), 5.56 (d, J = 2.8 Hz, 1H), 5.46 (td, J = 10.4, 4.6 Hz, 1H), 5.05 (dd, J = 10.4, 2.8 Hz, 1H), 4.42 (s, 2H), 3.26–3.16 (m, 2H), 3.10–3.05 (m, 1H), 2.90–2.76 (m, 1H), 2.55–2.43 (m, 3H), 2.22 (s, 6H), 2.10 (s, 3H), 2.01 (s, 3H), 1.98–1.85 (m, 4H), 1.80–1.77 (m, 2H), 1.46–1.39 (m, 4H), 1.22–1.17 (m, 2H); 13C-NMR (101 MHz, CDCl3) δ: 182.2, 170.5, 170.2, 169.9, 169.7, 168.2, 168.1, 167.9, 144.1, 143.8, 142.5, 132.8, 128.1, 126.6, 124.7, 124.0, 122.9, 118.3, 115.1, 107.9, 104.8, 104.2, 99.6, 94.4, 81.2, 71.7, 68.1, 68.0, 67.2, 39.4, 38.8, 38.3, 30.7, 29.2, 29.1, 25.8, 25.6, 21.7, 21.0, 20.7, 20.6, 20.58; HR-MS: C49H52N2O19 + Na+ Calcd 995.3056. Found 995.3063.
(1R,2R,4S,6R)-6-(((E)-3-(3,4-Diacetoxyphenyl)acryloyl)oxy)-4-((6-(2-(4-(5,7-dihydroxy-4-oxo-4H-chromen-2-yl)phenoxy)acetamido)hexyl)carbamoyl)cyclohexane-1,2,4-triyltriacetate (11c)Pale yellow foam solid (100 mg, yield 50.2%). 1H-NMR (400 MHz, CDCl3) δ: 12.69 (s, 1H), 9.34 (s, 1H), 7.74 (d, J = 8.6 Hz, 2H), 7.59 (d, J = 16.0 Hz, 1H), 7.42–7.33 (m, 2H), 7.21 (d, J = 8.2 Hz, 1H), 6.94 (d, J = 8.6 Hz, 2H), 6.80 (s, 1H), 6.45–6.43 (m, 2H), 6.40–6.37 (m, 1H), 6.36–6.28 (m, 2H), 5.64 (d, J = 3.2 Hz, 1H), 5.57 (ddd, J = 19.6, 14.8, 3.6 Hz, 2H), 5.13 (dd, J = 10.2, 3.2 Hz, 1H), 4.52 (s, 2H), 3.41–3.08 (m, 4H), 2.93–2.80 (m, 1H), 2.67–2.44 (m, 2H), 2.34–2.30 (m, 8H), 2.18 (s, 3H), 2.08 (s, 3H), 2.05–1.91 (m, 4H), 1.58–1.39 (m, 4H), 1.26 (s, 6H); 13C-NMR (101 MHz, CDCl3) δ: 182.3, 170.4, 170.2, 169.9, 169.6, 168.2, 168.0, 167.9, 165.8, 163.9, 163.2, 162.0, 159.9, 157.7, 144.1, 143.8, 142.5, 132.8, 128.1, 126.6, 124.8, 124.0, 122.9, 118.3, 115.1, 104.8, 104.3, 99.7, 94.4, 81.2, 71.7, 68.1, 67.3, 67.2, 53.5, 39.8, 39.1, 38.3, 30.7, 29.2, 29.2, 28.6, 26.6, 21.7, 21.0, 20.7, 20.6, 20.58; HR-MS: C50H54N2O19 + H+, Calcd 987.3394. Found, 987.3395; C50H54N2O19 + Na+ Calcd 1009.3213. Found 1009.3211.
(1R,2R,4S,6R)-6-(((E)-3-(3,4-Diacetoxyphenyl)acryloyl)oxy)-4-((11-(2-(4-(5,7-dihydroxy-4-oxo-4H-chromen-2-yl)phenoxy)acetamido)undecyl)carbamoyl)cyclohexane-1,2,4-triyltriacetate (11d)Pale yellow foam solid (110.0 mg, yield 51.2%). 1H-NMR (400 MHz, CDCl3) δ: 7.73 (d, J = 7.8 Hz, 2H), 7.52 (d, J = 16.0 Hz, 1H), 7.33–7.29 (m, 2H), 7.15 (d, J = 8.2 Hz, 1H), 6.92 (d, J = 8.3 Hz, 2H), 6.56–6.53 (m, 1H), 6.42 (d, J = 20.4 Hz, 2H), 6.33–6.16 (m, 3H), 5.62–5.41 (m, 2H), 5.06 (dd, J = 10.2, 3.6 Hz, 1H), 4.49 (s, 2H), 3.27–3.26 (m, 2H), 3.13–3.12 (m, 2H),2.89–2.73 (m, 1H), 2.55–2.44 (m, 2H), 2.23 (d, J = 2.8 Hz, 6H), 2.12 (s, 3H), 2.02 (s, 3H), 1.98–1.87 (m, 5H), 1.43–1.35 (m, 4H), 1.18–1.05 (m, 16H); 13C-NMR (101 MHz, CDCl3) δ: 182.3, 170.3, 170.1, 169.8, 169.5, 168.1, 167.9, 167.8, 167.0, 163.9, 163.3, 162.1, 159.9, 157.8, 144.2, 143.9, 142.5, 132.8, 128.2, 126.6, 125.0, 124.0, 122.9, 118.2, 115.2, 104.9, 104.4, 99.7, 94.5, 81.3, 71.7, 68.1, 67.3, 39.9, 39.2, 38.4, 30.7, 29.7, 29.5 (2C), 29.3 (2C), 29.2, 29.17, 26.8, 21.7, 21.0, 20.7, 20.6, 20.55; HR-MS: C55H64N2O19 + H+, Calcd 1057.4182. Found, 1057.4187.
The synthesis procedure of 12a–12d was described as above:
(1R,2R,4S,6R)-6-(((E)-3-(3,4-Diacetoxyphenyl)acryloyl)oxy)-4-((2-(2-(2-(4-(5,7-dihydroxy-4-oxo-4H-chromen-2-yl)phenoxy)acetamido)ethoxy)ethyl)carbamoyl)cyclohexane-1,2,4-triyltriacetate (12a)Pale yellow foam solid (65 mg, yield 44.2%). 1H-NMR (400 MHz, CDCl3) δ: 12.73 (s, 1H), 7.66 (d, J = 8.2 Hz, 2H), 7.57 (d, J = 15.8 Hz, 1H), 7.38–7.36 (m, 3H), 7.21 (d, J = 8.2 Hz, 1H), 6.99 (s, 1H), 6.89 (d, J = 8.2 Hz, 2H), 6.39–6.30 (m, 4H), 5.74–5.49 (m, 2H), 5.15 (d, J = 7.4 Hz, 1H), 4.55 (s, 2H), 3.57–3.55 (m, 7H), 3.31 (s, 1H), 2.89 (d, J = 15.8 Hz, 1H), 2.61 (dd, J = 41.0, 13.6 Hz, 2H), 2.30 (s, 6H), 2.18 (s, 3H), 2.08 (s, 3H), 2.05–1.94 (m, 4H); 13C-NMR (100 MHz, CDCl3) δ: 182.2, 170.7, 170.2, 169.9, 169.7, 168.3, 168.2, 168.1, 163.9, 163.0, 161.7, 160.0, 157.7, 144.3, 143.9, 142.5, 132.7, 128.0, 126.7, 124.4, 124.1, 123.0, 118.1, 115.1, 104.8, 104.1, 99.6, 94.6, 81.1, 71.6, 69.7, 69.4, 68.1, 67.5, 67.3, 39.7, 39.1, 38.4, 30.7, 21.6, 21.0, 20.7, 20.64, 20.60; HR-MS: C47H48N2O20 + H+, Calcd: 961.2897. Found, 961.2881; C47H48N2O20 + Na+, Calcd 983.2693. Found, 983.2697.
(1R,2R,4S,6R)-6-(((E)-3-(3,4-Diacetoxyphenyl)acryloyl)oxy)-4-((2-(2-(2-(2-(4-(5,7-dihydroxy-4-oxo-4H-chromen-2-yl)phenoxy)acetamido)ethoxy)ethoxy)ethyl)carbamoyl)cyclohexane-1,2,4-triyl Triacetate (12b)Pale yellow foam solid (104.0 mg, yield 52%). 1H-NMR (400 MHz, CDCl3) δ: 12.73 (s, 1H), 9.12 (s, 1H), 7.72 (d, J = 8.0 Hz, 2H), 7.57 (d, J = 15.9 Hz, 1H), 7.38–7.35 (m, 2H), 7.24–7.15 (m, 2H), 6.91 (d, J = 7.8 Hz, 2H), 6.74 (s, 1H), 6.44–6.30 (m, 4H), 5.64–5.54 (m, 2H), 5.13 (d, J = 7.6 Hz, 1H), 4.52 (s, 2H), 3.62–3.52 (m, 12H), 2.87 (d, J = 15.8 Hz, 1H), 2.58 (dd, J = 40.0, 13.4 Hz, 3H), 2.30 (s, 6H), 2.19 (s, 3H), 2.09 (s, 3H), 2.00 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ: 182.2, 170.6, 170.2, 169.9, 169.6, 168.2, 168.1, 168.0, 165.8, 163.6, 163.1, 162.0, 159.8, 157.7, 144.1, 143.8, 142.5, 132.8, 128.0, 126.6, 124.7, 124.0, 122.9, 118.2, 115.1, 104.9, 104.2, 99.5, 94.4, 81.1, 71.7, 70.2, 70.1, 69.5, 68.1, 67.2, 39.5, 38.9, 38.2, 30.8, 21.6, 21.0, 20.7, 20.64, 20.60; HR-MS: C49H52N2O21 + H+, Calcd 1005.3135. Found, 1005.3134; C49H52N2O21 + Na+, Calcd 1027.2955. Found, 1027.2954.
(1R,2R,4S,6R)-6-(((E)-3-(3,4-Diacetoxyphenyl)acryloyl)oxy)-4-((1-(4-(5,7-dihydroxy-4-oxo-4H-chromen-2-yl)phenoxy)-2-oxo-6,9,12-trioxa-3-azatetradecan-14-yl)carbamoyl)cyclohexane-1,2,4-triyltriacetate (12c)Pale yellow foam solid (67.0 mg, yield 43.7%). 1H-NMR (400 MHz, CDCl3) δ: 12.72 (s, 1H), 9.14 (s, 1H), 7.73 (d, J = 7.6 Hz, 2H), 7.58 (d, J = 16.0 Hz, 1H), 7.39–7.35 (m, 2H), 7.25–7.11 (m, 2H), 6.92 (d, J = 7.4 Hz, 2H), 6.78 (s, 1H), 6.52–6.23 (m, 4H), 5.73–5.47 (m, 2H), 5.12 (d, J = 7.4 Hz, 1H), 4.53 (s, 2H), 3.73–3.43 (m, 15H), 3.37 (s, 1H), 2.86 (d, J = 15.8 Hz, 1H), 2.57 (dd, J = 41.0, 14.2 Hz, 3H), 2.31 (s, 6H), 2.18 (s, 3H), 2.08 (s, 3H), 1.99 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ: 182.2, 170.6, 170.2, 169.8, 169.6, 168.2, 168.0, 167.9, 165.8, 163.6, 163.1, 162.1, 159.8, 157.7, 144.0, 143.8, 142.5, 132.8, 128.0, 126.6, 124.7, 124.0, 122.9, 118.3, 115.1, 104.9, 104.3, 99.6, 94.3, 81.0, 71.8, 70.4, 70.2, 70.1, 69.6, 68.1, 67.2, 67.1, 39.6, 39.0, 38.2, 30.8, 21.6, 21.0, 20.7, 20.64, 20.6; HR-MS: C51H56N2O22 + Na+, Calcd 1071.3217. Found, 1071.3214.
(1R,2R,4S,6R)-6-(((E)-3-(3,4-Diacetoxyphenyl)acryloyl)oxy)-4-((1-(4-(5,7-dihydroxy-4-oxo-4H-chromen-2-yl)phenoxy)-2-oxo-6,9,12,15-tetraoxa-3-azaheptadecan-17-yl)carbamoyl)cyclohexane-1,2,4-triyltriacetate (12d)Pale yellow foam solid (48.0 mg, yield 30.0%). 1H-NMR (400 MHz, CDCl3) δ: 12.72 (s, 1H), 9.12 (s, 1H), 7.76 (d, J = 5.0 Hz, 2H), 7.58 (d, J = 15.8 Hz, 1H), 7.36 (s, 2H), 7.23 (s, 2H), 6.95 (d, J = 4.8 Hz, 2H), 6.85 (s, 1H), 6.55–6.23 (m, 4H), 5.64–5.57 (m, 2H), 5.11 (d, J = 9.8 Hz, 1H), 4.54 (s, 2H), 3.72–3.45 (m, 19H), 3.36 (s, 1H), 2.86 (d, J = 15.2 Hz, 1H), 2.68–2.48 (m, 3H), 2.31 (s, 6H), 2.19 (s, 3H), 2.09 (s, 3H), 1.99 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ: 182.2, 170.6, 170.2, 169.8, 169.6, 168.2, 168.0, 167.8, 165.7, 163.6, 163.2, 162.1, 159.9, 157.7, 144.0, 143.8, 142.5, 132.8, 128.1, 126.6, 124.8, 124.0, 123.3, 122.9, 118.3, 115.2, 105.0, 104.4, 99.6, 94.3, 81.0, 71.8, 70.4, 70.2, 70.15, 69.7, 68.1, 67.3, 67.1, 39.6, 39.0, 38.3, 30.8, 21.6, 21.0, 20.7, 20.64, 20.60; HR-MS: C53H60N2O23 + Na+, Calcd 1115.3479. Found, 1115.3481.
In Vitro XOD Inhibitory Activity ScreeningDifferent concentrations (0, 6.25, 12.5, 25, 50, and 100 µM) of CA conjugates and the positive control allopurinol (Innochem, Beijing, China) were added to a 67-mM phosphate buffer solution (pH = 7.4) containing XOD (Sigma-Aldrich, Wuhan, China, activity of 20 nM, 5 mU/mL). The mixture was preincubated at 37 °C for 15 min, 50 mM xanthine (Innochem, Beijing, China) was added, and the absorbance at 295 nm was immediately measured once every minute, for a total of 30 min. The percentage of inhibition rate (%) was calculated using the following formula, and the drug concentration required to achieve 50% inhibition rate was calculated as the IC50 value:
 |
Dimethyl sulfoxide (DMSO) was used to dissolve the test compound and allopurinol; hence, DMSO was used as the negative control.
NO Production AssaysRAW264.7 cells were cultured in a 96-well plate at a density of 5 × 104 cells per well with a final volume of 200 µL. The cells were then incubated with various CA conjugates at a concentration of 10 µg/mL for 2 h. Next, LPS (1 µg/mL, Sigma-Aldrich, U.S.A.) was added and incubated for another 24 h. The production of NO was quantified by measuring the accumulation of nitrites in the culture medium using the colorimetric Griess reaction, with minor adjustments. Then 100 µL of supernatant medium, was combined with an equal volume of Griess A (0.1% (w/v) N-(1-naphthyl)-ethylenediamine dihydrochloride) and B (1% (w/v) sulfanilamide containing 5% (w/v) H3PO4) (1 : 1) at room temperature for 10 min in the dark. The absorbance was promptly measured at a wavelength of 540 nm.
MTT AssayRAW264.7 cells were seeded in a 96-well plate in triplicate at a density of 5 × 104 cells per well, with a final volume of 200 µL. The cells were then incubated with or without CA conjugates for 2 h. Next, LPS (1 µg/mL, Sigma-Aldrich) was added and incubated for another 24 h, followed by the addition of 20 µL of MTT (5 mg/mL) to each well and incubation for 4 h at 37 °C. After removing the supernatant, 200 µL of DMSO was added to facilitate the dissolution of formazan crystals. The absorbance of each well was measured at a wavelength of 492 nm using a microplate reader (Tecan Infinite 200 Pro, Switzerland).
RNA Isolation and Quantitative Real-Time PCR (qRTPCR) AnalysisTotal RNA was isolated from RAW264.7 cells treated with different concentrations of CA conjugates in the presence of LPS (1 µg/mL) and ATP (Sigma-Aldrich, 5 mM) using Trizol reagent (Life Technologies, 15596018). One microgram of total RNA was subjected to synthesize cDNA using PrimeScript™ First-Strand cDNA Synthesis kit (TaKaRa, Beijing, China). The real-time PCR was performed using qTOWER 3G (Analytic Jena, Jena, Germany) with 2XM5 HiPer SYBR Premix Es Taq kit. The reactions of the Samples were then run in triplicate and Internal β-actin gene was used as a reference. Relative expression differences of PCR results were calculated by the comparative cycle threshold method. PCR was performed with specific primers NLRP3, IL-1β, and TNF-α (Table 2).
| Primer name | Primer sequence |
|---|---|
| β-Actin-F | AAATCGTGCGTGACATCAAAGA |
| β-Actin-R | GCCATCTCCTGCTCGAAGTC |
| IL-1β-F | TGCCACCTTTTGACAGTGATG |
| IL-1β-R | AAGGTCCACGGGAAAGACAC |
| TNF-α-F | CGTCAGCCGATTTGCTATCT |
| TNF-α-R | CGGACTCCGCAAAGTCTAAG |
| NLRP3-F | AAGGCTTGTGTGGGACCAAA |
| NLRP3-R | AGGAGGGGCAGGAGTAAGAG |
RAW264.7 cells (2 × 106/well) were cultured in a 6-well plate overnight and then treated with various concentrations (6.25, 25, and 100 µM) of 10d and Isoliquiritigenin (ISL, Innochem, Beijing, China, 25 µM) for 1 h, followed by stimulation with LPS (1 µg/mL) for 5 h and ATP (5 mM) for another 1 h. Cell culture supernatants were collected and added into ELISA plates for the determination of IL-1β levels (BIOSTER, Wuhan, China) according to the manufacturer’s instructions. Each sample was tested in triplicate.
Western BlottingRAW264.7 cells (2 × 106/well) were cultured in a 6-well plate overnight and then pretreated with different concentrations (6.25, 12.5, 25, 50, and 100 µM) of 10d and ISL (25 µM) for 1 h, followed by stimulation with LPS (1 µg/mL) for 5 h and incubation with ATP (5 mM) for an additional 1 h. The cells were harvested and lysed in a radio immunoprecipitation assay (RIPA) lysis buffer (Solarbio, Beijing, China) on ice for 30 min, and the supernatant was collected. Proteins were quantified using the enhanced bicinchoninic acid (BCA) protein assay kit (Elabscience, Wuhan, China), and equal amounts of protein (40 µg) were separated via 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, CA, U.S.A.). The membranes were blocked with 5% skim milk at room temperature for 1 h and then incubated overnight at 4 °C with primary antibodies specific for NLRP3 (ABclonal, Wuhan, China), caspase-1 (ABclonal), and IL-1β (Elabscience, Wuhan, China), and iNOS (ABclonal), COX2 (Bioworld Technology, Inc, MN, U.S.A.), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Bioworld Technology, Inc, MN, U.S.A.), followed by washing with TBST and incubation for an additional 1 h with goat anti-rabbit immunoglobulin G (lgG)/HRP (Bioss, Beijing, China). Then, the membranes were analyzed using the Super ECL Plus kit (US Everbright Inc., Suzhou, China) for imaging with ChemiScope 3000 mini (Clinx, Shanghai, China).
Protein Structure Prediction and Molecular DockingThe structure of the XOD protein was downloaded from the PDB database (PDB code: 3NVY), the structure of NLRP3 was predicted using AlphaFold2. Firstly, UCSF Chimera was used to add hydrogens, and the AMBER14SB force field was employed to calculate the atomic charges of the protein.38,39) The three-dimensional structure of compound 10d was generated using the open-source cheminformatics software package RDKit, the AM1-BCC model was employed to assign partial charges to the low-energy conformations of 10d, AutoDock 4.2 software was employed for Molecular docking. The SiteMap software was utilized to predict the binding sites of the ligands, the predicted binding sites were designated as the docking center, with a box size set to a cube measuring 22.5 Å on each side. The spacing searching parameter was set to 0.375 Å, the maximum limit for searching conformations was set as 10000, and the Lamarckian Genetic Algorithm (LGA) was employed for conformation sampling and scoring.40–42)
Statistical AnalysisParallel experiments were repeated three times, Statistical analysis comparing LPS-stimulated cells to controls was performed using the two-sided unpaired t-test. To compare the effect of different concentrations of CA conjugates in LPS-stimulated cells, one-way ANOVA followed by a Dunnett’s multiple comparison test was used. The statistical tests were applied using GraphPad Prism, version 8.0.1 (GraphPad Software, San Diego, CA, U.S.A.).
In this study, 12 novel derivatives of Chlorogenic acid connected with apigenin through various linkers were synthesized, the inhibitory activities of all synthesized CA conjugates on XOD were also assessed. The conjugates 10c and 10d exhibited remarkable inhibitory activity on XOD (with IC50 values of 30.74 and 22.42 µM, respectively). Subsequently, the selected compounds (10 and 12 series) also showed good anti-inflammatory activities. The optimal compound 10d was chosen for further NLRP3 inflammasome and related proinflammatory cytokine inhibition analysis, 10d exhibited significant inhibitory activity on NLRP3 release as well as effectively reduced the secretion of IL-1β and TNF-α, with comparable or superior performance to that of the positive control ISL. In summary, based on its dual inhibitory effects on XOD and NLRP3, the CA conjugate 10d is a promising therapeutic agent for acute gout treatment. Further study on the other aspect of CA conjugate is still in progress in our laboratory.
This work was supported by Grants from the Sub project of National Key Research and Development Program of China (2023YFC3503904), Natural Science Foundation of China (Nos. 22107039, 32260250), the Project of Jiangxi Province (No. 20225BCJ23030) and the Project of Jiangxi Academy of Sciences (Nos. 2021YSBG22012, 2020-YYB-18, and 2023YSBG21022).
The authors declare no conflict of interest.
This article contains supplementary materials.