2025 Volume 73 Issue 4 Pages 382-387
2025 Volume 73 Issue 4 Pages 382-387
A squaramide organocatalyst was employed to efficiently promote asymmetric oxidative lactonization to construct spiro-fused 2-oxindoles in moderate-to-good yield and enantioselectivity (up to 81% enantiomeric excess (ee)). Herein, we report the first study accomplishing stereoselective oxidative cyclization from indole propionic acid using an organocatalyst, N-iodosuccinimide (NIS), and hydrogen peroxide under metal-free and mild reaction conditions.
Since List1) and MacMillan2) presented pioneering works, numerous organocatalysts and asymmetric reactions have been developed over the past two decades. Organocatalysts are powerful synthetic tools for constructing highly complex building blocks for synthesizing biologically active and medicinal compounds under low toxic and mild reaction conditions.3–8)
The disubstituted oxindoles comprising spiro-fused oxindoles have garnered significant attention as privileged scaffolds found in natural products, and pharmaceuticals. Furthermore, they are privileged building blocks9–15) (Fig. 1). These compounds are candidates for drug discovery because of their wide range of biological activities such as antituberculosis16) antitumor,17,18) and anti-human immunodeficiency virus (HIV) activities.12)

The unremitting studies in the past decade provide several efficient synthetic methodologies for constructing spiro-fused oxindoles.19–27) However, the discovery of a convenient approach to developing chiral spiro oxindole-γ-butyrolactones is in demand. Yoda et al. reported the efficient indium-catalyzed amide allylation of N-methyl isatin with high enantioselectivity21) (Chart 1a). The allylated adduct was smoothly cyclized to a lactone with the complete retention of stereochemistry. Glorius and colleagues discovered the stereoselective annulation of β,β-disubstituted enals with isatins by the dual catalysis of N-heterocyclic carbene and Brønsted acid. Thus, they constructed spirocyclic oxindoles bearing two highly congested contiguous quaternary carbon centers22) (Chart 1b). In these reported reactions, transition metals or complicated substrates were required to obtain spiro-lactone-fused oxindoles.

(a), (b) Previous Reports; (c) Our Challenges with the Asymmetric Synthesis of the 3-spiro-fused 2-oxindole
In this context, direct oxidative intramolecular cyclization reactions for constructing spiro-fused 2-oxindoles from indoles are recognized as important and practical procedures. Recently, some research groups developed an efficient catalytic system using iodide salt and an oxidant to yield spirocyclic lactone and ether containing oxindoles from indoles.28–30) However, the stereoselective synthesis of spiro oxindole-γ-butyrolactones from indoles is under development. Here, we report the first asymmetric synthesis of spiro fused 2-oxindoles via the organocatalyst/N-iodosuccinimide (NIS)/hydrogen peroxide-mediated oxidative cyclization of indole-3-propionic acids.
First, we examined several organocatalysts for the oxidative lactonization of 3-(1H-indol-3-yl) propanoic acid (1a) as a model substrate (Fig. 2, Table 1). The initial attempts using quinine C1, and organocatalysts C2 and C3 derived from quinine afforded (R)-3,4-dihydro-5H-spiro[furan-2,3′-indoline]-2′,5-dione (2a) in moderate yield with poor enantioselectivities (Entries 1–3). Organocatalyst C4, which had a thiourea motif was a poor catalyst (Entry 4). Squaramide catalysts C5–C7 bearing trifluoromethyl groups or t-butyl groups in the aniline ring smoothly promoted the reaction, affording the desired 3-spiro-fused 2-oxindole (2a) in moderate yields with moderate-to-good enantioselectivities (Entries 5–7). Thus, we determined that C5 was the most suitable for the oxidative cyclization of 1a.

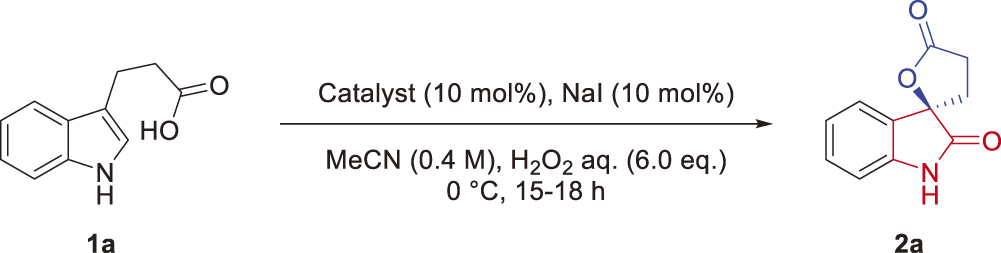 |
|||
|---|---|---|---|
| Entry | Catalyst | Yielda) (%) | Eeb) (%) |
| 1 | C1 | 32 | 27 |
| 2 | C2 | 74 | 1 |
| 3 | C3 | 62 | 3 |
| 4 | C4 | 68 | 6 |
| 5 | C5 | 58 | 75 |
| 6 | C6 | 70 | 48 |
| 7 | C7 | 60 | 66 |
a) Isolated yield. b) Determined by HPLC using a chiral stationary phase.
To further optimize the reaction conditions, we examined the other reaction conditions of the asymmetric oxidative cyclization of 1a using organocatalyst C5 (Table 2). Additionally, H2O2⋅CO(NH2)2 was used to avoid the influence of water. First, a series of solvents were screened (Entries 1–8). Among the representative solvents used, CH3CN was determined to be the most appropriate. Next, several oxidants were tested in CH3CN (Entries 9–11). Oxone and cumene hydroperoxide (CHP) were not effective for this cyclization. When H2O2 (aq.) was used as the oxidant, the cyclized product (2a) was obtained in high enantioselectivity (Entry 11). Thereafter, the performances of other iodine sources such as KI, I2, tetra-n-butylammonium iodide (TBAI), and NIS, were evaluated (Entries 12–15). When the reaction was performed with KI, product (2a) was obtained in a moderate yield with good enantioselectivity (Entry 12). The reaction with I2 decreased the yield and enantioselectivity (Entry 13). Furthermore, the yield diminished when the reaction was carried out with TBAI (Entry 14). NIS slightly increased the yield and afforded high enantioselectivity (Entry 15). No cyclized product was observed in the reaction without an oxidizing agent (Entry 16).
 |
|||||
|---|---|---|---|---|---|
| Entry | Solvent | Iodine Source | Oxidant | Yielda) (%) | Eeb) (%) |
| 1 | Toluene | NaI | H2O2⋅CO(NH2)2 | 9 | 17 |
| 2 | CH2Cl2 | NaI | H2O2⋅CO(NH2)2 | 64 | 64 |
| 3 | EtOAc | NaI | H2O2⋅CO(NH2)2 | 65 | 62 |
| 4 | Acetone | NaI | H2O2⋅CO(NH2)2 | 26 | 64 |
| 5 | THF | NaI | H2O2⋅CO(NH2)2 | 37 | 24 |
| 6 | DMF | NaI | H2O2⋅CO(NH2)2 | 26 | 25 |
| 7 | MeOH | NaI | H2O2⋅CO(NH2)2 | 22 | 65 |
| 8 | CH3CN | NaI | H2O2⋅CO(NH2)2 | 77 | 65 |
| 9 | CH3CN | NaI | Oxone | 22 | 0 |
| 10 | CH3CN | NaI | CHP | 0 | – |
| 11 | CH3CN | NaI | H2O2 aq. | 58 | 75 |
| 12 | CH3CN | KI | H2O2 aq. | 57 | 75 |
| 13 | CH3CN | I2 | H2O2 aq. | 51 | 66 |
| 14 | CH3CN | TBAI | H2O2 aq. | 40 | 72 |
| 15 | CH3CN | NIS | H2O2 aq. | 66 | 75 |
| 16c) | CH3CN | NIS | None | 0 | – |
a) Isolated yield. b) Determined by HPLC using a chiral stationary phase. c) NIS (1.0 equiv) was used.
Under the optimized reaction conditions, the substrate scope of the asymmetric oxidative cyclization of various types of indole-3-propionic acids (1) was investigated (Chart 2). Methyl-group-substituted indole-3-propionic acid derivatives (1b–1e) were determined to be good substrates for this transformation. The reaction of 3-(7-methyl-1H-indol-3-yl) propanoic acid (1e) afforded a high yield with high enantioselectivity (81% ee). Substrates with methoxy groups afforded the products 2f and 2g in a slightly low yield. The reactions of indole-3-propionic acid derivatives (1h–1m) containing halogen groups, such as fluorine, chlorine, and bromine were slow, affording products (2h–2m) in moderate yields and enantioselectivities. The reactions using N-Me- and N-Bn-protected indoles (1n and 1o) furnished products 2n and 2o in low yield without enantioselectivity. The stereochemistry (R configuration) of 2a was determined by comparison with the reported data of chiral-phase HPLC retention time and optical rotation.20) The stereochemistry of the remaining products (2) was tentatively assigned by analogy.

a) Reaction conditions: 1 (0.20 mmol), NIS (0.02 mmol), H2O2 aq. (6.0 equiv), MeCN (0.5 mL). Isolated yield. % enantiomeric excess (ee) was determined by HPLC using a chiral stationary phase.
In conclusion, the NIS/hydrogen peroxide/organocatalyst C5 system provided efficient asymmetric access to spiro-heterocyclic oxindoles from indole-3-propionic acids. Furthermore, the applications of the obtained 3-spiro-fused 2-oxindoles are currently being investigated in our laboratory.
1H-NMR and 13C-NMR spectra were recorded on a Bruker Avance III Nanobay 400 MHz spectrometer (400 MHz for 1H-NMR, 100 MHz for 13C-NMR). The chemical shifts were expressed in ppm downfield from tetramethylsilane (δ=0.00) as an internal standard and CDCl3 (δ=77.16), CD3OD (δ=49.00). Mass spectra were recorded by an electrospray ionization-time of flight (ESI-TOF) mass spectrometer (Xevo G2-XS QTof). Melting points were obtained with Yanaco MP-J3. For TLC analyses, Merck precoated TLC plates (silica gel 60F254) were used. Flash column chromatography was performed on neutral silica gel (Kanto Silica gel 60N, 40–50µm) HPLC analysis was conducted using Shimadzu LC-20AT coupled diode array-detector SPD-M20A and chiral column of DAICEL ChiralPak IA (4.6 × 250 mm), DAICEL ChiralPak IC (4.6 × 250 mm), DAICEL ChiralPak IG (4.6 × 250 mm). 2a–o are known compounds that exhibited spectroscopic data identical to those reported in the literature.28,30)
A typical procedure of the asymmetric oxidative cyclization of 1a using organocatalyst C5 is as follows: To a stirred solution of 3-(1H-indol-3-yl) propanoic acid (1a, 37.8 mg, 0.200 mmol), NIS (4.50 mg, 20.0 μmol) and organocatalyst C5 (12.6 mg, 20.0 μmol) in MeCN (0.5 mL), and 35% H2O2(aq.) (103µL, 1.20 mol) were added at 0°C. After stirring at 0°C for 16h, the reaction mixture was diluted with EtOAc (15 mL) and successively washed with 10% Na2S2O3(aq.) (15 mL), sat. NaHCO3(aq.) (15 mL) and brine. The organic layer was dried over Na2SO4, filtered, and evaporated. The obtained residue was purified by flash column chromatography on silica gel with a 5 : 1–3 : 1 mixture of hexane and EtOAc to afford (R)-3,4-dihydro-5H-spiro[furan-2,3′-indoline]-2′,5-dione (2a) (26.8 mg, 0.132 mmol, 66%, 75% ee) as a white solid. 1H-NMR (CDCl3, 400 MHz) δ: 8.57 (brs, 1H), 7.36–7.32 (m, 2H), 7.12 (t, J=7.6 Hz, 1H), 6.94 (d, J=7.9 Hz, 1H), 3.18 (dt, J=17.8, 10.1 Hz, 1H), 2.79 (ddd, J=17.7, 9.6, 2.9Hz, 1H), 2.63 (ddd, J = 13.2, 9.8, 3.0Hz, 1H), 2.48 (dt, J = 13.3, 10.2 Hz, 1H); 13C-NMR (CDCl3, 100 MHz) δ: 176.8, 176.5, 141.2, 131.3, 126.8, 124.6, 123.7, 111.1, 82.9, 31.4, 28.3; high resolution (HR)MS (ESI-TOF) m/z: [M+Na]+ Calcd for C11H9NO3Na+, 226.0475; Found, 226.0474. The enantiomeric excess of the product was determined by chiral HPLC analysis using a DAICEL ChiralPak IC, Hexane/i-PrOH = 80/20, 1.0 mL/min), λ = 254 nm: tminor = 25.2 min, tmajor = 28.0 min.
1H-NMR (CDCl3, 400 MHz) δ: 8.23 (brs, 1H), 7.80 (t, J = 7.8 Hz, 1H), 6.88 (d, J = 7.8 Hz, 1H), 6.75 (d, J = 7.7 Hz, 1H), 3.22 (dt, J = 17.6, 10.6 Hz, 1H), 2.77 (ddd, J = 17.6, 9.8, 2.5 Hz, 1H), 2.69–2.52 (m, 2H), 2.34 (s, 3H); 13C-NMR (CD3OD, 100 MHz) δ: 177.4, 177.0, 142.0, 136.2, 130.6, 125.0, 124.0, 108.0, 83.9, 28.3, 27.3, 16.0. The enantiomeric excess of the product was determined by chiral HPLC analysis using a DAICEL ChiralPak IC, Hexane/i-PrOH = 70/30, 1.0 mL/min), λ = 254 nm: tminor = 14.3 min, tmajor = 17.9 min.
1H-NMR (CDCl3, 400 MHz) δ: 8.29 (brs, 1H), 7.16 (s, 1H), 7.13 (d, J = 8.0 Hz, 1H), 6.81 (d, J = 7.9 Hz, 1H), 3.17 (ddd, J = 17.7, 10.5, 9.8 Hz, 1H), 2.78 (ddd, J = 17.6, 9.6, 3.1 Hz, 1H), 2.65–2.58 (m, 1H), 2.50–2.42 (m, 1H), 2.33 (s, 3H); 13C-NMR (CD3OD, 100 MHz) δ: 178.8, 178.2, 140.8, 134.2, 132.5, 128.5, 126.3, 111.4, 84.7, 32.0, 29.1, 21.0. The enantiomeric excess of the product was determined by chiral HPLC analysis using a DAICEL ChiralPak IC, Hexane/i-PrOH = 80/20, 1.0 mL/min), λ = 254 nm: tminor = 26.3 min, tmajor = 32.0 min.
1H-NMR (CDCl3, 400 MHz) δ: 8.48 (brs, 1H), 7.22 (d, J = 7.7 Hz, 1H), 6.91 (d, J = 7.7 Hz, 1H), 3.17 (dt, J = 17.8, 10.4 Hz, 1H), 2.77 (ddd, J = 17.6, 9.6, 3.0 Hz, 1H), 2.63–2.57 (m, 1H), 2.49–2.41 (m, 1H), 2.36 (s, 3H); 13C-NMR (CD3OD, 100 MHz) δ: 177.4, 177.0, 142.1, 141.6, 124.13, 124.08, 123.4, 111.0, 83.3, 30.5, 27.8, 20.5. The enantiomeric excess of the product was determined by chiral HPLC analysis using a DAICEL ChiralPak IC, Hexane/i-PrOH = 85/15, 1.0 mL/min), λ = 254 nm: tminor = 48.5 min, tmajor = 51.8 min.
1H-NMR (CDCl3, 400 MHz) δ: 8.26 (brs, 1H), 7.17 (t, J = 7.3 Hz, 2H), 7.04 (t, J = 7.6 Hz, 1H), 3.17 (ddd, J = 17.6, 10.6, 9.8 Hz, 1H), 2.78 (ddd, J = 17.6, 9.6, 3.0 Hz, 1H), 2.65–2.59 (m, 1H), 2.50–2.44 (m, 1H), 2.27 (s, 3H); 13C-NMR (CD3OD, 100 MHz) δ: 177.4, 177.1, 140.4, 132.1, 126.7, 122.9, 121.6, 120.2, 83.5, 30.7, 27.7, 15.1. The enantiomeric excess of the product was determined by chiral HPLC analysis using a DAICEL ChiralPak IC, Hexane/i-PrOH = 80/20, 1.0 mL/min), λ = 254 nm: tminor = 29.1 min, tmajor = 37.3 min.
1H-NMR (CD3OD, 400 MHz) δ: 7.31 (t, J = 8.2 Hz, 1H), 6.73 (d, J = 8.5 Hz, 1H), 6.55 (dd, J = 7.8, 0.6 Hz, 1H), 3.87 (s, 3H), 2.93–2.88 (m, 2H), 2.65–2.58 (m, 1H), 2.50 (dt, J = 13.4, 8.6 Hz, 1H); 13C-NMR (CD3OD, 100 MHz) δ: 177.8, 176.5, 157.0, 143.0, 132.4, 113.4, 105.9, 103.5, 83.3, 54.7, 28.0, 27.6. The enantiomeric excess of the product was determined by chiral HPLC analysis using a DAICEL ChiralPak IC, Hexane/i-PrOH = 80/20, 1.0 mL/min), λ = 254 nm: tminor = 45.1 min, tmajor = 68.4 min.
1H-NMR (CD3OD, 400 MHz) δ: 7.10 (d, J = 2.5 Hz, 1H), 6.91 (dd, J = 8.5, 2.6 Hz, 1H), 6.83 (d, J = 8.5 Hz, 1H), 3.78 (s, 3H), 3.08–2.99 (m, 1H), 2.81 (ddd, J = 17.7, 7.9, 5.0 Hz, 1H), 2.56–2.51 (m, 2H); 13C-NMR (CD3OD, 100 MHz) δ: 177.4, 176.8, 156.6, 134.9, 128.2, 115.8, 110.9, 83.6, 54.9, 30.6, 27.7. The enantiomeric excess of the product was determined by chiral HPLC analysis using a DAICEL ChiralPak IC, Hexane/i-PrOH = 80/20, 1.0 mL/min), λ = 254 nm: tminor = 35.9 min, tmajor = 39.2 min.
1H-NMR (CD3OD, 400 MHz) δ: 7.31 (dd, J = 7.9, 2.6 Hz, 1H), 7.10 (td, J = 9.0, 2.7 Hz, 1H), 6.90 (dd, J = 8.6, 4.2 Hz, 1H), 3.08–2.98 (m, 1H), 2.81 (ddd, J = 17.7, 9.0, 4.0 Hz, 1H), 2.61–2.48 (m, 2H); 13C-NMR (CD3OD, 100 MHz) δ: 177.0, 176.7, 159.4 (q, JC-F = 240.5 Hz), 138.0, 128.7 (q, JC-F = 8.1 Hz), 117.1 (q, JC-F = 23.8 Hz), 112.2 (q, JC-F = 25.6 Hz), 111.3 (q, JC-F = 8.0 Hz), 83.1, 30.5, 27.5. The enantiomeric excess of the product was determined by chiral HPLC analysis using a DAICEL ChiralPak IC, Hexane/i-PrOH = 70/30, 1.0 mL/min), λ = 254 nm: tminor = 12.5 min, tmajor = 10.4 min.
1H-NMR (CD3OD, 400 MHz) δ: 7.46 (dd, J = 8.3, 5.3 Hz, 1H), 6.81 (ddd, J = 9.7, 8.3, 2.2 Hz, 1H), 6.69 (dd, J = 8.9, 2.3 Hz, 1H), 3.08–2.99 (m, 1H), 2.80 (ddd, J = 17.7, 8.6, 4.3 Hz, 1H), 2.60–2.48 (m, 2H); 13C-NMR (CD3OD, 100 MHz) δ: 177.1, 176.9, 164.6 (q, JC-F = 247.1 Hz), 144.0 (q, JC-F = 12.4 Hz), 126.2 (q, JC-F = 10.4 Hz), 122.8 (q, JC-F = 3.0 Hz), 109.0 (q, JC-F = 23.2 Hz), 98.6 (q, JC-F = 27.8 Hz), 82.6, 30.4, 27.7. The enantiomeric excess of the product was determined by chiral HPLC analysis using a DAICEL ChiralPak IC, Hexane/i-PrOH = 70/30, 1.0 mL/min), λ = 254 nm: tminor = 9.4 min, tmajor = 10.8 min.
1H-NMR (CD3OD, 400 MHz) δ: 7.30–7.28 (m, 1H), 7.20–7.07 (m, 2H), 3.08–2.99 (m, 1H), 3.04 (dt, J = 17.7, 9.9 Hz, 1H), 2.82 (ddd, J = 17.7, 9.3, 3.8 Hz, 1H), 2.63–2.49 (m, 2H); 13C-NMR (CD3OD, 100 MHz) δ: 177.0, 176.2, 148.3, 145.9, 129.9 (q, JC-F = 3.5 Hz), 123.8 (q, JC-F = 6.0 Hz), 120.2 (q, JC-F = 3.5 Hz), 117.6 (q, JC-F = 17.6 Hz), 82.9, 30.6, 27.5. The enantiomeric excess of the product was determined by chiral HPLC analysis using a DAICEL ChiralPak IC, Hexane/i-PrOH = 70/30, 1.0 mL/min), λ = 254 nm: tminor = 11.6 min, tmajor = 27.7 min.
1H-NMR (CD3OD, 400 MHz) δ: 7.52–7.51 (m, 1H), 7.36–7.33 (m, 1H), 6.92–6.89 (m, 1H), 3.02 (dt, J = 17.7, 10.0 Hz, 1H), 2.81 (ddd, J = 17.7, 8.7, 4.3 Hz, 1H), 2.60–2.49 (m, 2H); 13C-NMR (CD3OD, 100 MHz) δ: 177.0, 176.4, 140.8, 130.7, 128.9, 128.1, 124.9, 111.5, 82.8, 30.4, 27.5. The enantiomeric excess of the product was determined by chiral HPLC analysis using a DAICEL ChiralPak IG, Hexane/i-PrOH = 90/10, 0.5 mL/min), λ = 254 nm: tminor = 43.9 min, tmajor = 46.2 min.
1H-NMR (CD3OD, 400 MHz) δ: 7.28–7.22 (m, 2H), 6.92 (dd, J = 7.0, 1.7 Hz, 1H), 3.01–2.99 (m, 1H), 2.92–2.79 (m, 2H), 2.57–2.49 (m, 1H); 13C-NMR (CD3OD, 100 MHz) δ: 177.1, 176.1, 144.2, 132.4, 126.5, 125.4, 119.2, 109.7, 83.5, 27.4, 27.0. The enantiomeric excess of the product was determined by chiral HPLC analysis using a DAICEL ChiralPak IC, Hexane/i-PrOH = 70/30, 1.0 mL/min), λ = 254 nm: tminor = 11.1 min, tmajor = 15.7 min.
1H-NMR (CD3OD, 400 MHz) δ: 7.65–7.64 (m, 1H), 7.51–7.47 (m, 1H), 6.86 (d, J = 8.3 Hz, 1H), 3.02 (dt, J = 17.4, 10.0 Hz, 1H), 2.81 (ddd, J = 17.7, 8.7, 4.3 Hz, 1H), 2.57–2.51 (m, 2H); 13C-NMR (CD3OD, 100 MHz) δ: 178.4, 177.7, 142.7, 135.0, 130.7, 129.1, 116.4, 113.4, 84.1, 31.8, 28.9. The enantiomeric excess of the product was determined by chiral HPLC analysis using a DAICEL ChiralPak IA, Hexane/i-PrOH = 90/10, 1.0 mL/min), λ = 254 nm: tminor = 20.7 min, tmajor = 22.8 min.
1H-NMR (CDCl3, 400 MHz) δ: 7.43–7.35 (m, 2H), 7.16–7.12 (m, 1H), 6.87 (d, J = 7.8 Hz, 1H), 3.25–3.16 (m, 1H), 3.20 (s, 3H), 2.77 (ddd, J = 17.6, 9.5, 3.1 Hz, 1H), 2.58 (ddd, J = 17.6, 9.5, 3.1 Hz, 1H), 2.46 (ddd, J = 13.3, 10.6, 9.5 Hz, 1H). 13C-NMR (CD3OD, 100 MHz) δ: 178.6, 176.2, 145.2, 132.4, 127.9, 125.4, 124.8, 110.4, 84.2, 31.9, 29.1, 26.6. The enantiomeric excess of the product was determined by chiral HPLC analysis using a DAICEL ChiralPak IC, Hexane/i-PrOH = 70/30, 1.0 mL/min), λ = 254 nm: t = 43.1 min, t = 48.3 min.
1′-Benzyl-3,4-dihydro-5H-spiro[furan-2,3′-indoline]-2′,5-dione (2o)1H-NMR (CDCl3, 400 MHz) δ: 7.37–7.27 (m, 7H), 7.10 (t, J = 7.1 Hz, 1H), 6.74 (d, J = 7.9 Hz, 1H), 4.88 (s, 2H), 3.25 (ddd, J = 17.6, 10.6, 9.8 Hz, 1H), 2.84–2.76 (m, 1H), 2.63 (ddd, J = 13.2, 9.8, 3.1 Hz, 1H), 2.50 (ddd, J = 13.3, 10.7, 9.6 Hz, 1H). 13C-NMR (CDCl3, 100 MHz) δ: 176.1, 174.4, 143.0, 134.9, 131.1, 129.0, 128.0, 127.2, 126.4, 124.3, 123.6, 109.9, 82.3, 43.9, 31.5, 28.3. The enantiomeric excess of the product was determined by chiral HPLC analysis using a DAICEL ChiralPak IC, Hexane/i-PrOH = 70/30, 1.0 mL/min), λ = 254 nm: t = 23.7 min, t = 29.6 min.
The authors declare no conflict of interest.