2025 Volume 50 Issue 1 Pages 91-101
2025 Volume 50 Issue 1 Pages 91-101
During angiogenesis, sprouting endothelial cells (ECs) migrate and eventually connect to target vessels to form new vessel branches. However, it remains unclear how these sprouting vessels migrate toward the target vessels in three-dimensional space. We performed in vivo imaging of the cerebral capillary network formation in zebrafish to investigate how sprouting tip cells migrate toward their targets. Of note, we found that tip cells reach the target vessels through two phases: a non-directional phase and a directional phase. In the non-directional phase, sprouting tip cells dynamically extend and retract their protrusions at the leading front and have less directionality in their movement. In contrast, once tip cells enter the directional phase, they migrate directly toward the anastomotic targets. Chemokine receptor Cxcr4a and its ligand Cxcl12b are important for the phase transition to the directional phase. In cxcr4a mutants, sprouting tip cells lose their directionality and tend to connect to nearby sprouting ECs, resulting in altered capillary network patterning. Furthermore, in wild-type (WT) larvae, local Ca2+ oscillations were detected in protrusions of tip cells, specifically in the non-directional phase, but almost disappeared in the directional phase as a result of the Cxcr4-dependent phase transition. Thus, this study provides evidence of a chemokine-induced phase transition in migrating tip cells, which is important for proper vascular network formation in the zebrafish brain.
Key words: angiogenesis, directional migration, live imaging, chemokine, Ca2+ dynamics, zebrafish

Graphical Abstract
The vascular network is crucial in vertebrates for delivering oxygen and nutrients to tissues throughout the body. In development to adulthood, branched structures of blood vessels form mainly by sprouting angiogenesis, which involves the emergence of new vessels from the pre-existing ones (Eilken and Adams, 2010; Herbert and Stainier, 2011; Huveneers and Phng, 2024). Angiogenesis consists of multiple steps (Betz et al., 2016). First, an endothelial cell (EC) is selected from a pre-existing vessel in response to angiogenic cues such as vascular endothelial growth factor (VEGF)-A to become a migratory tip cell. As the tip cell migrates outward from the parental vessel and trailing stalk cells follow, the sprout elongates in contact with pre-existing vessels (Gerhardt et al., 2003). Endothelial lumen forms and expands in the sprout (Gebala et al., 2016). Eventually, the sprouts connect with the target vessels via anastomosis. Behaviors of ECs at each step of angiogenesis have been extensively studied (Eilken and Adams, 2010; Hogan and Schulte-Merker, 2017). However, little is known about how the sprout (tip cell) migrates toward the anastomotic targets in three-dimensional space.
Tip cells migrate by extending membrane protrusions containing actin-rich filopodia and lamellipodia at the leading edge. Among them, filopodia are thought to function as sensors of guidance cues (Huveneers and Phng, 2024; Zakirov et al., 2021). VEGF-A is required for filopodia extension (Gerhardt et al., 2003) and, thereby, has been considered to guide tip cell migration during sprouting angiogenesis. Besides VEGF-A, chemokines can also act as guidance cues. Especially, the chemokine receptor CXCR4 and its ligand CXCL12 (also known as SDF-1) play an important role in EC migration during angiogenesis (Kiefer and Siekmann, 2011). In mice, loss of CXCR4 and CXCL12 show defects in vascular development of various organs, such as the kidney (Takabatake et al., 2009), lung (Chandrasekaran et al., 2022), and retina (Pitulescu et al., 2017). In zebrafish, Cxcr4a (an ortholog of CXCR4) is expressed in sprouting ECs and has a role in guiding the formation of the coronary artery (Harrison et al., 2015; Ivins et al., 2015) and eye vasculature during development (Hasan et al., 2017), and vascular plexus formation during fin regeneration (Harrison et al., 2015; Ivins et al., 2015). At earlier stages of zebrafish cerebral vascular formation (around 36 hours post fertilization (hpf)), Cxcl12b, expressed along the midline of the neural keel, immediately above the basilar artery (BA), guides the sprout to connect to the BA (Bussmann et al., 2011). However, it remains uncertain how such guidance cues regulate the migratory behavior of sprouting ECs toward the anastomotic targets.
In the present study, we performed in vivo time-lapse imaging of cerebral capillary network formation in zebrafish. We show that sprouting tip cells undergo two distinct phases: a non-directional phase and a directional phase, in which they exhibit different cellular behaviors. Cxcr4a and Cxcl12b are important for inducing the phase transition to the directional phase, thereby promoting directional migration toward the anastomotic target. Finally, we show that Cxcr4a-mediated directional migration is important for the formation of proper capillary network formation.
Zebrafish of the AB strain (Danio rerio) were maintained and bred in 28°C water (pH 7.25 and conductivity 500 μS) with a 14 h on/10 h off light cycle. Embryos and larvae were incubated at 28°C in E3 medium. All zebrafish husbandry was performed under standard conditions according to institutional (National Cerebral and Cardiovascular Center) and national (Japan) ethical and animal welfare regulations. The experiments using zebrafish were approved by the animal committee of the National Cerebral and Cardiovascular Center (No. 22054) and performed according to our institutional regulation.
The following transgenic and mutant zebrafish lines were used for this study: Tg(kdrl:EGFP-CAAX,crya:EGFP)ubs47 (Heutschi et al., 2023), Tg(kdrl:EGFP)s843 (Jin et al., 2005), Tg(fli1:H2B-mCherry)ncv31 (Yokota et al., 2015), Tg(UAS:GCaMP7a) (Muto et al., 2013), Tg(kdrl:TagBFP)mu293 (Matsuoka et al., 2016), Cxcr4as421 (Schmid et al., 2013) and Tg(fli1:Gal4FF) (Herwig et al., 2011). Throughout the text, all Tg lines used in this study are simply described without their line numbers. For example, Tg(fli1:H2B-mCherry)ncv31 is abbreviated to Tg(fli1:H2B-mCherry).
The full locus deletion alleles ncv148 for cxcl12a and ncv149 for cxcl12b genes were generated by CRISPR-Cas9 techniques as described below.
Generation of full locus deletion mutants by CRISPR-Cas9 systemTo generate full locus deletion alleles of cxcl12a and cxcl12b, double guide RNAs (gRNAs) were designed upstream of the 5' UTR and downstream of the 3' UTR, flanking the region to be deleted (see Fig. 3A). crRNA sequences were designed using the CRISPR direct software (https://crispr.dbcls.jp) as follows: cxcl12a-5'-guide: 5'-AGTGTGAACTCTCCCACCGA-3', cxcl12a-3'-guide: 5'-GAAGACTTAAATTCACACCT-3', cxcl12b-5'-guide: 5'-ATGTTGAAGAATGCTATCGT-3', cxcl12b-3'-guide: 5'-TAGGCCAGGTTCTGTCCCGG-3'. crRNAs and tracrRNA were generated by IDT Inc, and the RNP complex was prepared according to the manufacturer’s instructions.
Embryos, injected with the RNP complex at the one-cell stage, were raised to adulthood and crossed with AB to identify full locus deletion founders. Screening for founders was conducted by genomic PCR and subsequent sequencing using the primer sets shown in Table S1. For the genotyping of the mutants, PCR analyses of genomic DNAs were routinely performed using the following three primer sets: cxcl12a(b)-5'-Fw and cxcl12a(b)-5'-Rev for WT allele, cxcl12a(b)-3'-Fw and cxcl12a(b)-3'-Rev for WT allele, cxcl12a(b)-5'-Fw and cxcl12a(b)-3'-Rev for full locus deletion allele. This allows us to distinguish between WT, Het, and Homo.
cxcr4a crispantsThe double-target CRISPR-mediated F0 knockout (Chiba et al., 2024) was generated by the sgRNAs (IDT Inc.) targeting the following sequences: 5′-GGACATCGGAGCCAACTTTG-3′, 5′- CTGCGAGCGCATATACCCGC-3′. The RNP complex was prepared according to the manufacturer’s instructions, except that 0.7 μl of each crRNA was used to prepare the gRNA solution. The RNP complex containing double gRNAs was injected into one-cell stage embryos. To test the efficiency of genome editing, genomic PCR was performed in individual larvae at 3 dpf using the following two primer sets: cxcr4a-target 1-Fw and cxcr4a-target 1-Rev, and cxcr4a-target 2-Fw and cxcr4a-target 2-Rev as shown in Fig. S5A. The PCR fragments from individual larvae were confirmed by gel electrophoresis and were analyzed using a microchip electrophoresis system with MCE202 MultiNA (Shimadzu) according to manufacturer’s instructions.
Chemical treatmentTg(kdrl:EGFP) larvae were treated with 1 μM ki8751, an inhibitor of Vegfr2, at 2 h after the start of confocal imaging.
Image acquisition and processingThe pigmentation of embryos and larvae was inhibited by treatment with 1-phenyl-2-thiourea (PTU) (Sigma-Aldrich). For confocal imaging, zebrafish embryos were dechorionated, anesthetized in 0.016% tricaine (Sigma-Aldrich) in E3 medium, and mounted in 1% low-melting agarose poured onto a 35-mm diameter glass-based dish (Asahi Techno Glass). Confocal imaging in Fig. 5D, S1A, S4A, S5E and Movie 3 and 4 was performed on Andor Dragonfly spinning disc confocal (Andor Technology Ltd) based on ECLIPSE FN1 upright microscope (Nikon), equipped with water-immersion LWD 16x/0.80 NA (Nikon), Zyla4.2 PLUS USB 3.0 sCMOS cameras (Andor Technology Ltd) and P-725.4 PIFOC piezo nano-positioner (Physik Instrumente) regulated with Fusion software (Andor Technology Ltd). Other confocal images were taken with a FluoView FV1200 or FV3000 confocal upright microscope system (Olympus) equipped with water-immersion XLUMPlan FL N 20x/1.00 NA (Olympus) and a multi-alkali or GaAsP photomultiplier tube regulated with FluoViewASW software (Olympus). The 405 nm, 473 nm, and 559 nm laser lines were used. Images were acquired sequentially to avoid cross-detection of the fluorescent signals.
All confocal images were processed and analyzed with IMARIS 9.5.1, 9.9.1, or 10.1.0 software (Oxford Instruments). In Fig. 1C, 4A, and 4B, and Movie 1, kdrl:TagBFP+ EC regions were extracted by the masking function to exclude non-specific signals. In Fig. 1D, the outline of the nucleus at each time point was automatically extracted.
In vivo Ca2+ imaging of sprouting tip cellsFor long-term imaging for GCaMP7a signals in Fig. 4A and B, we performed 3D time-lapse imaging using an FV3000 confocal microscope every 5 min. Under these experimental conditions, we could capture GCaMP7a signals only when the region of interest was scanned and could not capture all local Ca2+ oscillations because the duration of each oscillation was around 20 sec. Therefore, to visualize local Ca2+ oscillations in Fig. S4A and S5E, we performed high-speed 3D time-lapse imaging using a Dragonfly spinning disc confocal microscope every 15 sec with a z-interval of 6 μm. Especially, to quantify the frequency of local Ca2+ oscillations in Fig. 4C, 3D images were taken every 15 sec for 10 min. Z-stack images were 3D volume-rendered and analyzed with IMARIS 9.5.1, 9.9.1, or 10.1.0 software (Oxford Instruments).
Quantitative analysesFor quantification of Fig. 1E, spots were manually created at the center of tip cell nucleus, the distal edge of the protrusion, and the target site of anastomotic vessel at each time point. The minimum distance from the spot of the target site to the spot of the nucleus or protrusion was measured at each time point.
For quantification of Fig. 1F and 2E, the velocity of tip cell was measured by tracking the nucleus as in Fig. 1E. The time-averaged velocity of each larva is shown as each dot. For quantification of Fig. 1G and 2F, the time-averaged protrusion number was measured and shown as each dot. In 13.3% of cases, as seen in Fig. S1B, directional migration stopped until the onset of stalk cell migration. Such cases were excluded from the quantitative analyses between the non-directional and directional phases as they were not typical directional phases.
For quantification of Fig. 2D, a spot was manually created at the center of tip cell nucleus at each time point. The minimum distance from the spot of the nucleus at the indicated time point to the spot of the nucleus at the end time point (at 12 h) was measured at each time point.
For quantification of Fig. 2B, 2C, 3E and 3F, the number of sprouting tip cells from the PHBC and the number of anastomoses from the PHBC to CtA were quantified from 73 hpf (3 dpf) to 121 hpf (5 dpf) for 48 h. Quantification was performed in the whole hindbrain.
For quantification of Fig. 5B and C, the number of anastomoses was quantified from 73 hpf (3 dpf) to 121 hpf (5 dpf) in the whole hindbrain. For quantification of Fig. 5E, the number of small loops less than 20 μm was counted in the CtA network of the whole hindbrain by setting a sphere with a radius of 20 μm using IMARIS software.
Data analysis and statisticsData were analyzed using GraphPad Prism software or Excel and were presented as mean ± s.d. Sample numbers and the statistical methods are indicated in figure legends.
To visualize how sprouting vessels migrate to their target vessels, we here focused on the formation of the hindbrain capillary network in zebrafish. In the hindbrain, the central arteries (CtAs) and cerebellar central arteries (CCtAs) form a capillary network (Fig. 1A) (Isogai et al., 2001), which creates a CNS-specific blood-brain barrier (Xie et al., 2010). Both the CtAs and CCtAs are derived from sprouting from the primordial hindbrain channels (PHBC) located bilaterally, and connect to the BA and the posterior communicating segment (PCS), respectively, at earlier stages of the cerebral vascular development (~1.5 days post fertilization (dpf)) (Bussmann et al., 2011; Chen et al., 2019; Isogai et al., 2001). For simplicity, the CtA and CCtA are hereafter referred to as the CtA. At later stages (after 3 dpf), sprouts from the PHBC connect to existing CtAs, thereby forming a capillary network of CtAs (Fig. 1B). Finally, CtA networks show different patterns in different individuals (Fig. S1A).

Sprouting tip cells undergo two phases, non-directional and directional phases, to reach the target vessels
(A) Representative confocal image of the brain of a Tg(kdrl:EGFP-CAAX) larva (7 dpf). Plasma membrane of endothelial cells (ECs) is labeled with EGFP-CAAX. Dorsal view, anterior to the top. The dotted line indicates the boundary between the hindbrain (posterior) and midbrain (anterior). (B) Time-sequential images of a Tg(kdrl:EGFP) larva (from 3 dpf). Elapsed time (h:min). kdrl:EGFP+ ECs are shown as green. ECs sprouting (arrowheads) from the primordial hindbrain channel (PHBC) eventually connect to the central artery (CtA) (circles). (C) Time-sequential images of a Tg(kdrl:TagBFP);Tg(fli1:H2B-mCherry) larva (from 3 dpf). kdrl:TagBFP+ ECs and fli1:H2B-mCherry+ EC nuclei are shown as cyan and magenta, respectively. Direction of protrusions is indicated by arrows. In the non-directional phase, tip cells sprouting from the PHBC dynamically retract (blue arrowhead) and extend (magenta arrowhead) their protrusions in various directions (arrows). Then, the nuclei of tip cells (asterisks) rapidly move toward the anastomotic targets, the CtAs, in the directional phase. (D) Tracking of the nucleus of the tip cell shown in (C). The outline of the nucleus is depicted every 0.5 h as different colors. (E) Quantitative analysis of the data shown in (C). Minimum distance between the anastomotic target and the tip cell nucleus (magenta) or the distal edge of the protrusion (green) at each time point until the connection to the target vessel (arrowhead). (F) Graph shows the time-averaged velocity of the tip cell nucleus in the non-directional and directional phases. Data are mean ± s.d. (n = 12 cells from 6 larvae). (G) Graph shows the time-averaged number of protrusions per tip cell throughout the non-directional and directional phases. Data are mean ± s.d. (n = 12 cells from 6 larvae). Scale bar: 10 μm. P values were determined by paired (F) or unpaired (G) two-tailed Studentʼs t-test. BA, basilar artery; PHBC, primordial hindbrain channel; CtA, central artery; CCtA, cerebellar central artery; PCS, posterior communicating segment; PMCtA, posterior metencephalic central artery.
Movie 1
Non-directional and directional migration of a sprouting tip cell from the primordial hindbrain channels (PHBC) toward the central artery (CtA).
Time-lapse recording in the hindbrain of a Tg(kdrl:TagBFP);Tg(fli1:H2B-mCherry) larva (from 3 dpf). Endothelial cells (ECs) and EC nuclei are labeled with TagBFP (cyan) and H2B-mCherry (magenta), respectively. Elapsed time (h:min). Dorsal view, anterior to the top.
Download VideoAs a model of capillary network formation, we carefully observed how sprouts from the PHBC connect to the CtAs by time-lapse imaging. Our imaging analyses have revealed that sprouting ECs undergo two distinct phases before connecting to the target vessels (Fig. 1C–G). Initially, sprouting tip cells extended and retracted multiple protrusions (average number = 2.05, Fig. 1G) in various directions (Fig. 1C) and had less directionality toward the anastomotic targets (Fig. 1C–E, Movie 1). We here define this phase as a “non-directional phase.” After this phase, the tip cells rapidly migrated toward the target vessels, the CtAs (Fig. 1C–F), while their protrusions were mostly directed to their targets (Fig. 1C: yellow arrows, Movie 1). We define this phase as a “directional phase.” Our quantification analyses revealed that the average migration velocity of tip cell nuclei was significantly faster in the directional phase than in the non-directional phase (Fig. 1F). In addition, the time-averaged number of protrusions became significantly less in the directional phase (average number = 1.34, Fig. 1G). Therefore, our results demonstrate that during the formation of the zebrafish cerebral capillary network, sprouting tip cells undergo two distinct phases that exhibit different cellular behavior.
We also found that, in 13.3% of the cases, directional migration of tip cells stopped before arrival to the anastomotic targets. In such cases, directional migration restarted after stalk cells migrate out of the parental vessel (Fig. S1B). These results suggest that coordinated migration of tip and stalk cells is also important for the directional migration of angiogenic sprouts.
Cxcr4a is required for directional migration to anastomotic targetsNext, we examined which extracellular stimuli regulate the transition from the non-directional to directional phase. VEGF-A is a known guidance cue for sprouting ECs (Gerhardt et al., 2003). When we suppressed VEGF-A/VEGFR2 signaling by the treatment with ki8751, an inhibitor of Vegfr2 (also known as Kdrl in zebrafish) (Yokota et al., 2015), the initial tip cell sprouting from the PHBC was completely blocked, and budding sprouts were retracted to the PHBC (Fig. S2). These results suggest that VEGF is important for the initial tip cell sprouting from the PHBC. However, considering that VEGF-A/VEGFR2 signaling regulates multiple aspects of angiogenesis, including tip cell selection, sprouting, migration, and proliferation (Koch and Claesson-Welsh, 2012; Lohela et al., 2009), it is technically difficult to segregate the function of VEGF-A as a guidance cue.
CXCL12/CXCR4 signaling is another guidance cue for ECs during angiogenesis (Kiefer and Siekmann, 2011). To examine the role of the CXCL12-CXCR4 axis, we performed time-lapse imaging of cxcr4a mutants, focusing on the connection of tip cells from the PHBC to the CtA. In cxcr4a mutants, tip cells sprouted from the PHBC and extended protrusions similarly to WT (Fig. 2A). The number of tip cells sprouting from the PHBC in cxcr4a mutants was also comparable to WT (Fig. 2B). These results suggest that tip cells acquire angiogenic capacity even in cxcr4a mutants, presumably by VEGF-A/VEGFR2 signaling. However, most of these sprouting ECs could not connect to the CtAs in cxcr4a mutants (Fig. 2A and C). In these mutants, tip cells repeatedly extended and retracted their protrusions in various directions (Fig. 2A), but lost directionality in their movement (Fig. 2A and D, Movie 2). In the tip cells of cxcr4a mutant larvae, the velocity and the number of protrusions are comparable to those in the non-directional phase of WT (Fig. 2E and F), suggesting that tip cells in the cxcr4a mutants persistently retain the cellular characteristics of the non-directional phase. Therefore, our results indicate that Cxcr4a is important for the transition from the non-directional phase to the directional phase.

Cxcr4a is important for directional migration to anastomotic targets
(A) Time-sequential images of Tg(kdrl:EGFP) WT and cxcr4as421 sibling larvae (from 3 dpf). Asterisks indicate tip cell nuclei. In cxcr4as421 larvae, tip cells sprouting from the PHBC repeatedly extend (magenta arrowheads) and retract (blue arrowhead) their protrusions in various directions (arrows). They do not migrate toward the target vessel. (B) Graph shows the number of tip cells sprouting from the PHBC in WT and cxcr4as421 sibling larvae (from 73 hpf to 121 hpf). Data are mean ± s.d. (WT, n = 8 larvae; cxcr4as421, n = 8 larvae). Each dot represents an individual larva in this graph. (C) Graph shows the number of connections of tip cells sprouting from the PHBC to the CtAs or CCtAs in WT and cxcr4as421 sibling larvae (from 73 hpf to 121 hpf). Data are mean ± s.d. (WT, n = 8 larvae; cxcr4as421, n = 8 larvae). (D) Quantitative analysis of the data shown in (A). Minimum distance between the position of the tip cell nucleus at each time point and the position of the nucleus at 12 h in WT and cxcr4as421 sibling larvae. Connection to the target CtA occurs around 12 h in this WT larva as shown in (A). (E) Graph shows the time-averaged velocity of the tip cell nucleus in WT (non-directional and directional phases) and cxcr4as421 sibling larvae. Data are mean ± s.d. (WT, n = 6 cells in 4 larvae; cxcr4as421, n = 6 cells in 4 larvae). (F) Graph shows the time-averaged number of protrusions per tip cell in WT (non-directional and directional phases) and cxcr4as421 sibling larvae. Data are mean ± s.d. (WT, n = 6 cells in 4 larvae; cxcr4as421, n = 6 cells in 4 larvae). Scale bar: 10 μm. P values were determined by two-tailed Studentʼs t-test (B, C) and one-way ANOVA with Tukey’s test (E, F).
Movie 2
Non-directional migration of a sprouting tip cell from the PHBC in the cxcr4a mutant.
Time-lapse recording in the hindbrain of a Tg(kdrl:EGFP) homozygous cxcr4a mutant (from 3 dpf). ECs are labeled with EGFP. Elapsed time (h:min). Dorsal view, anterior to the top.
Download VideoNext, we investigated the guidance cues for the directional EC migration. As orthologues of CXCL12, zebrafish have Cxcl12a and Cxcl12b, both of which act as ligands for Cxcr4a in a context-dependent manner (Bussmann et al., 2011; Xu et al., 2014). To examine the roles of Cxcl12a and Cxcl12b, we analyzed the effects of loss of these ligands. Here, to avoid genetic compensation (El-Brolosy et al., 2019), we established full locus deletion fish of cxcl12a and cxcl12b (Fig. 3A). All exons are deleted in these mutant alleles (Fig. 3B, C, and S3). In both cxcl12a mutants and cxcl12b mutants, tip cells sprouted normally from the PHBC, in numbers comparable to WT (Fig. 3D and E). These sprouting tip cells were normally connected to the CtAs in cxcl12a mutants as in WT (Fig. 3D and F). In contrast, in cxcl12b mutants, about half of tip cells did not connect to the CtA, and persistently extended and retracted their protrusions (Fig. 3D and F). Thus, cxcl12b mutant larvae but not cxcl12a mutants phenocopied cxcr4a mutants, although the effects were slightly milder. These results strongly suggest that Cxcl12b acts as a guidance cue to control directional migration toward anastomotic targets during cerebral capillary network formation.

Cxcl12b but not Cxcl12a is responsible for directional migration to anastomotic targets
(A) Schematic representation for generating full locus deletion alleles in cxcl12a and cxcl12b. Double guide RNAs (gRNAs) (yellow) were designed upstream of the 5' UTR and downstream of the 3' UTR, flanking all exons. (B,C) Images of gels showing full locus deletions in cxcr12ancv148 (B) and cxcr12bncv149 homozygous mutants (C). Primer sites are shown in (A). (D) Time-sequential images of Tg(kdrl:EGFP) WT, cxcr12ancv148, and cxcr12bncv149 larvae (from 3 dpf). Asterisks indicate tip cell nuclei. In WT and cxcr12ancv148 larvae, tip cells sprouting from the PHBC finally undergo directional migration to the target CtAs (yellow arrows). In contrast, in cxcr12bncv149 larvae, tip cells sprouting from the PHBC repeatedly extend (magenta arrowhead) and retract (blue arrowhead) their protrusions while extending them in various directions (arrows), and do not migrate toward the target. (E) Graph shows the number of tip cells sprouting from the PHBC in WT, cxcr12ancv148, and cxcr12bncv149 larvae (from 73 hpf to 121 hpf). Data are mean ± s.d. (WT, n = 14 larvae; cxcr12ancv148, n = 7 larvae; cxcr12bncv149 , n = 7 larvae). (F) Graph shows the number of connections of tip cells sprouting from the PHBC to the CtAs or CCtAs in WT, cxcr12ancv148, and cxcr12bncv149 larvae (from 73 hpf to 121 hpf). Data are mean ± s.d. (WT, n = 14 larvae; cxcr12ancv148, n = 7 larvae; cxcr12bncv149 , n = 7 larvae). Scale bar: 10 μm. P values were determined by one-way ANOVA with Tukey’s test.
We previously showed that intracellular Ca2+ dynamics reflect angiogenic capacity in tip cells sprouting from the dorsal aorta (DA) (Yokota et al., 2015). To investigate the Ca2+ dynamics during non-directional and directional migration of tip cells, we conducted in vivo Ca2+ imaging by expressing GCaMP7a (GFP-based Ca2+ probe) in ECs. Ca2+ oscillations that occur throughout the cell were dominant in tip cells sprouting from the DA in the trunk (Yokota et al., 2015). In contrast, in tip cells sprouting from the PHBC, in addition to the whole cell Ca2+ oscillations (see Fig. S4A), local Ca2+ oscillations were predominantly detected in protrusions at the leading front (Fig. 4A and S4A, Movie 3). Of note, these local Ca2+ oscillations were detected mainly in the non-directional phase and mostly disappeared after the transition to the directional phase (Fig. 4A and S4A, Movie 4). We quantified these local Ca2+ oscillations and found that oscillation frequency was markedly reduced in the directional phase (Fig. 4C).
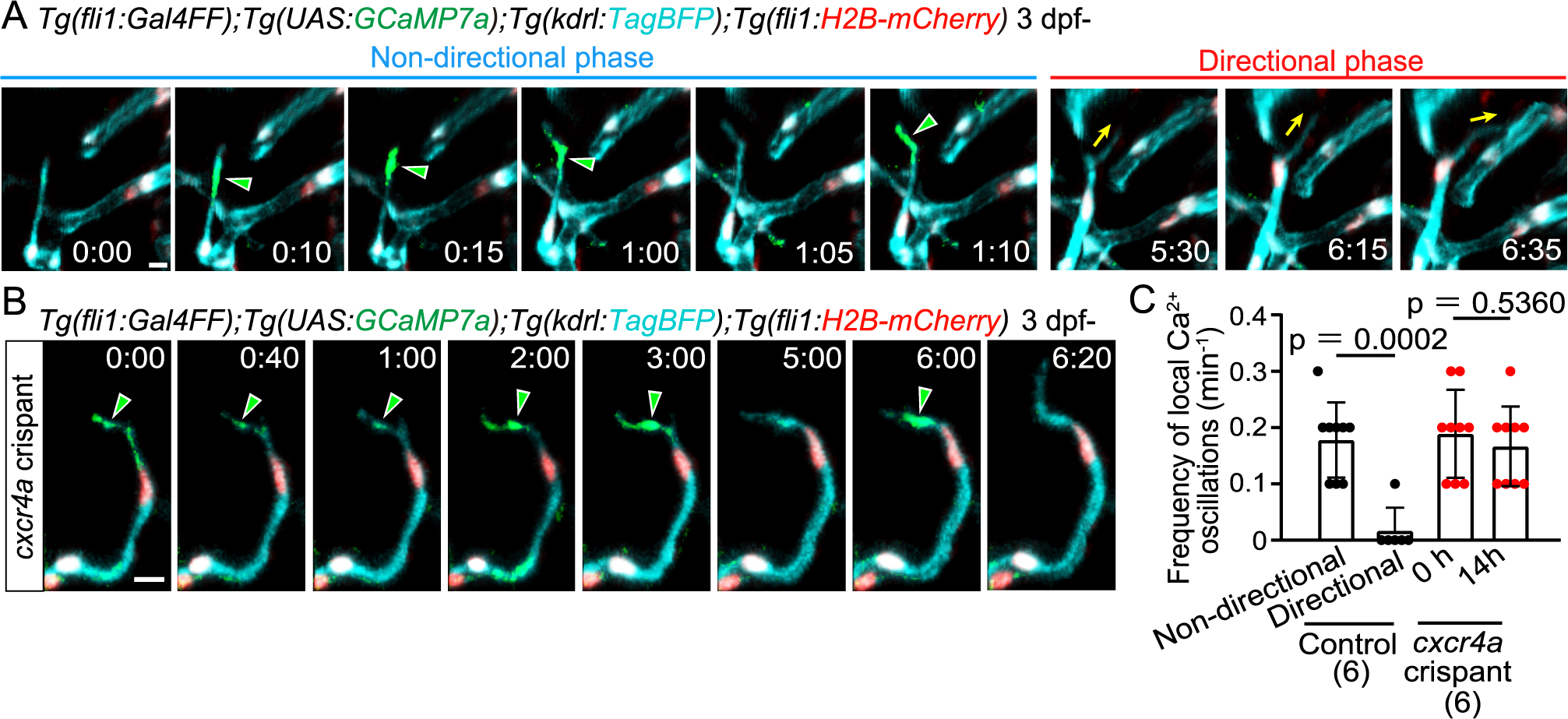
Local Ca2+ oscillations occur specifically in the non-directional phase
(A) Time-sequential images of a Tg(fli1:Gal4FF);Tg(UAS:GCaMP7a);Tg(kdrl:TagBFP);Tg(fli1:H2B-mC) larva (3 dpf) after tip cell sprouting from the PHBC. Elapsed time (h:min). Local Ca2+ signals (green arrowheads) are detected in the leading front of tip cells in the non-directional phase, but rarely in the directional phase. (B) Time-sequential images of a Tg(fli1:Gal4FF);Tg(UAS:GCaMP7a);Tg(kdrl:TagBFP);Tg(fli1:H2B-mC) cxcr4a crispant larva (F0 injected larva) (3 dpf) after tip cell sprouting from the PHBC. Local Ca2+ signals are maintained in the leading front of tip cells (green arrowheads) that do not head to the target vessels. (C) Graph shows the frequency of local Ca2+ oscillations in the leading front of tip cells in control and cxcr4a crispants (3–4 dpf). Data are mean ± s.d. (WT: n = 9 cells in the non-directional phase, n = 6 cells in the directional phase in 6 larvae; cxcr4as421: n = 9 cells (at 0 h), 9 cells (at 14 h) in 6 larvae). Scale bar: 10 μm. P value was determined by unpaired two-tailed Studentʼs t-test.
Movie 3
Ca2+ imaging of a sprouting tip cell in the non-directional phase.
Time-lapse recording in the hindbrain of a Tg(fli1:Gal4FF);Tg(UAS:GCaMP7a);Tg(kdrl:TagBFP) larva during the non-directional phase (3 dpf). Elapsed time (sec). Local Ca2+ oscillations (yellow) are detected in the leading front of tip cell sprouting from the PHBC. Dorsal view, anterior to the top.
Download VideoMovie 4
Ca2+ imaging of a sprouting tip cell in the directional phase.
Time-lapse recording in the hindbrain of a Tg(fli1:Gal4FF);Tg(UAS:GCaMP7a);Tg(kdrl:TagBFP) larva during the directional phase (3 dpf). Elapsed time (sec). Local Ca2+ oscillations (yellow) are not detected in the leading front of tip cell. Dorsal view, anterior to the top.
Download VideoNext, to investigate whether these changes in calcium dynamics are related to the Cxcr4a-dependent phase transition, we examined the effect of loss of Cxcr4a on the local calcium dynamics. We observed cxcr4a F0 knockout zebrafish injected with cxcr4a gRNAs (called cxcr4a crispants) (Fig. S5A). Microchip electrophoresis analyses confirmed that the cxcr4a genome was efficiently edited in the cxcr4a crispants (Fig. S5B S5C). As in the cxcr4a mutants, in cxcr4a crispants, sprouting tip cells extended and retracted their protrusions, and have less directionality toward the anastomotic targets (Fig. S5D). These larvae persistently exhibited local Ca2+ oscillations in their extending and retracting protrusions (Fig. 4B, and S5E). These results indicate that the Cxcr4a-dependent phase transition causes the disappearance of local Ca2+ oscillations in tip cells undergoing directional migration.
Previously, similar local Ca2+ oscillations were reported in retracting protrusions of tip cells (Liu et al., 2020). In our analysis, local Ca2+ oscillations were observed not only in retracting protrusions but also in extending protrusions (Fig. S4B). These results suggest that local Ca2+ oscillations might be involved in the dynamic extension and retraction of unstable tip cell protrusions.
Cerebral capillary network patterning becomes altered in cxcr4a mutantsFinally, to delineate the role of Cxcr4a-mediated directional migration, the phenotype of cxcr4a mutants in cerebral capillary network formation was carefully examined. In WT, sprouts from the PHBC anastomosed with the CtAs (Fig. 1B and C), whereas in cxcr4a mutants, most sprouts failed to connect with the CtAs and remained as sprouts (Fig. 2A and C). We observed that some of these sprouts connected with the other sprouts from the nearby PHBC, although this did not occur in WT (Fig. 5A and B). In the cxcr4a mutants, there was increased sprouting from CtAs (Fig. 5A and C). These sprouts anastomosed with nearby sprouting ECs rather than with lumenized vessels (Fig. 5A and C). Thus, in the cxcr4a mutants, anastomoses from sprouts to vessels, as seen in the WT (see Fig. 1B and C), were markedly reduced, whereas anastomoses from sprouts to sprouts were enhanced. As a result, more small vessel loops were formed in the cerebral capillaries in cxcr4a mutant (Fig. 5D and E). Thus, cerebral capillary network pattering becomes altered in cxcr4a mutants. These results suggest that Cxcr4a-mediated directional migration is important for the formation of proper capillary network formation.

Cerebral capillary network patterning becomes altered in cxcr4as421 mutants
(A) Time-sequential images of Tg(kdrl:EGFP) cxcr4as421 mutant larva (from 3 dpf). Upper: a tip cell sprout from the PHBC (magenta arrowheads) connects with another tip cell sprout from the PHBC nearby (orange arrowheads). Lower: a tip cell ectopically sprouts from the CtA (magenta arrowheads) and is often connected with another tip cell sprouting from the nearby CtA (orange arrowheads). (B) Graph shows the number of tip cell sprouts from the PHBC anastomosing with other sprouts from the PHBC (sprout to sprout, orange bar) and the number of tip cell sprouts from the PHBC anastomosing with the PHBC vessel (sprout to vessel, green bar) in WT and cxcr4as421 sibling larvae (from 73 hpf to 121 hpf). Data are mean ± s.d. (WT, n = 8 larvae; cxcr4as421, n = 8 larvae). (C) Graph shows the number of CtA sprouts anastomosing with other CtA sprouts (sprout to sprout, orange bar) or with CtA vessels (sprout to vessel, green bar) in WT and cxcr4as421 sibling larvae (from 73 hpf to 121 hpf). Data are mean ± s.d. (WT, n = 8 larvae; cxcr4as421, n = 8 larvae). (D) Tg(kdrl:EGFP) WT and cxcr4as421 sibling larvae (7 dpf). Small vessel loops (dotted circles) are increased in cxcr4as421 mutants. (E) Quantitative analysis of the data shown in (D). The number of loops (less than 20 μm) in the CtA capillary network in the hindbrain of WT and cxcr4as421 sibling larvae. Data are mean ± s.d. (WT, n = 6 larvae; cxcr4as421, n = 6 larvae). Scale bar: 10 μm. P value was determined by unpaired two-tailed Studentʼs t-test.
In this paper, we found that tip cells reach the anastomotic targets through two distinct phases: the non-directional and directional phases. Whereas tip cells have less directionality in their movement in the non-directional phase, they migrate directly toward the anastomotic targets in the directional phase. Chemokine signaling Cxcl12b and Cxcr4a induce a phase transition from the non-directional phase to the directional phase. In cxcr4a mutants, sprouting tip cells lose their directionality and tend to connect to nearby sprouting ECs in the cerebral capillary formation. In contrast, Cxcr4a is dispensable for the formation of the intersegmental vessels (ISVs) in the zebrafish trunk (Hasan et al., 2017). In the ISV formation, sprouting ECs always run through a defined space between each pair of somites and eventually anastomose with neighboring sprouts on the dorsal side (Childs et al., 2002; Isogai et al., 2003). Therefore, Cxcr4a may not be required for angiogenesis through a pre-determined pathway. In this case, the location of Vegfa, a key driver of angiogenesis, and soluble Flt1 (sFlt1) and Semaphorin, which negatively act on VEGF-A signaling, determines the defined vascular patterns of the ISVs (Krueger et al., 2011; Yokota et al., 2015; Zygmunt et al., 2011). In contrast, in many other vascular beds, such as skin (Kam et al., 2023), heart (Harrison et al., 2015), retina (Gerhardt et al., 2003), and brain (Blinder et al., 2010), broad vascular patterns such as large arteries and veins are preserved, but the detailed branching patterns of capillaries differ among individuals. Because CXCR4/Cxcr4a mutant ECs generally show defective migration in such organs (Cavallero et al., 2015; Ivins et al., 2015; Li et al., 2013; Pitulescu et al., 2017), it is reasonable to speculate that zebrafish Cxcr4a and mammalian CXCR4 may commonly regulate directional migration of tip cells to form a capillary network without a defined pattern.
Our results here strongly suggest that Cxcl12b is a ligand for Cxcr4a to induce directional migration to anastomotic target. At earlier stages of zebrafish cerebral vascular formation (around 1.5 dpf), ECs sprout from the PHBC and connect to the centrally located BA to form the arch-shaped CtAs (Bussmann et al., 2011). At this stage, cxcl12b is expressed along the midline of the neural keel, immediately above the BA, and plays an essential role in targeting EC sprouts to reach the BA (Bussmann et al., 2011). On the other hand, cxcl12b-expressing cells have not been identified during the formation of the cerebral capillary network (3–5 dpf) when the PHBC-CtA connection occurs. If cxcl12b-expressing cells can be visualized, it is expected to reveal how anastomotic targets are determined to establish the proper cerebral capillary network in zebrafish.
cxcr4a mutants have been shown to have abnormal EC migration in various vascular beds (Bussmann et al., 2011; Harrison et al., 2015; Hasan et al., 2017; Xu et al., 2014). Our detailed time-lapse analyses of cxcr4a mutants revealed that Cxcr4a is dispensable for tip cell sprouting, including filopodia formation, but is required for directional migration toward the anastomotic target. On the other hand, our results and previous reports indicate that the initial tip cell sprouting is controlled by Vegfa/Vegfr2 signaling (Fig. S2) (Ferrara, 2009; Lohela et al., 2009; Yokota et al., 2015). In summary, in sprouting angiogenesis in cerebral capillaries, Vegfa/Vegfr2 signaling first induces tip cell sprouting from the parental vessels. After sprouting, tip cells are angiogenic but have less directionality in the non-directional phase. Once tip cells enter the directional phase by Cxcr4a/Cxcl12b signaling, they migrate directly toward the anastomotic targets and finally produce the new vessel connections. Thus, we propose that Vegfa/Vegfr2-dependent tip cell sprouting and Cxcl12b/Cxcr4a-dependent tip cell targeting are distinct processes in sprouting angiogenesis.
This work was supported in part by the grants: JSPS KAKENHI (No. 17K08560 to H.N.; No. 19H01022 to N.M.), the JST (No. JPMJPF2018 to N.M. and H.N.), the JST [Moonshot R&D–MILLENNIA Program] (No. JPMJMS2024-3 to N.M. and H.N.), AMED (No. JP23gm6710017 to H.N.), Takeda Science Foundation (to H.N. and N.M.), the SENSHIN Medical Research Foundation (to H.N.), the Kao Foundation for Research on Health Science (to H.N.), a Grant for Basic Science Research Projects from the Sumitomo Foundation (to H.N.), the Ichiro Kanehara Foundation (to H.N.).
The authors declare no competing interests.
We thank Didier Stainier (Max Planck Institute for Heart and Lung Research, Germany) for cxcr4as421, Etienne Schmelzer and Heinz-Georg Belting (University of Basel, Switzerland) for Tg(kdrl:EGFP-CAAX,crya:EGFP)ubs47, and Akira Muto and Koichi Kawakami (National Institute of Genetics) for Tg(UAS:GCaMP7a) zebrafish. We are grateful to Arndt Siekmann (University of Pennsylvania) for helpful advice. We are grateful to M. Sone, T. Satoh, S. Toyoshima, and E. Hanimura for technical assistance.
endothelial cell
VEGFvascular endothelial growth factor
WTwild-type
sgRNAsingle guide RNA
crRNACRISPR RNA
RNPribonucleoprotein