
- Issue 1 Pages 1-
- |<
- <
- 1
- >
- >|
-
Eriko Deguchi, Michiyuki Matsuda, Kenta TeraiArticle type: Reviews and Mini-reviews
2025 Volume 50 Issue 1 Pages 1-14
Published: 2025
Released on J-STAGE: January 22, 2025
JOURNAL OPEN ACCESS FULL-TEXT HTMLLive imaging techniques have revolutionized our understanding of paracrine signaling, a crucial form of cell-to-cell communication in biological processes. This review examines recent advances in visualizing and tracking paracrine factors through four key stages: secretion from producing cells, diffusion through extracellular space, binding to target cells, and activation of intracellular signaling within target cells. Paracrine factor secretion can be directly visualized by fluorescent protein tagging to ligand, or indirectly by visualizing the cleavage of the transmembrane pro-ligands or plasma membrane fusion of endosomes comprising the paracrine factors. Diffusion of paracrine factors has been studied using techniques such as fluorescence correlation spectroscopy (FCS), fluorescence recovery after photobleaching (FRAP), fluorescence decay after photoactivation (FDAP), and single-molecule tracking. Binding of paracrine factors to target cells has been visualized through various biosensors, including GPCR-activation-based (GRAB) sensors and Förster resonance energy transfer (FRET) probes for receptor tyrosine kinases. Finally, activation of intracellular signaling is monitored within the target cells by biosensors for second messengers, transcription factors, and so on. In addition to the imaging tools, the review also highlights emerging optogenetic and chemogenetic tools for triggering the release of paracrine factors, which is essential for associating the paracrine factor secretion to biological outcomes during the bioimaging of paracrine factor signaling.
Key words: paracrine signaling, live imaging, biosensors, optogenetics, chemogenetics
 Graphical Abstract Fullsize ImageView full abstractDownload PDF (4651K) Full view HTML
Graphical Abstract Fullsize ImageView full abstractDownload PDF (4651K) Full view HTML -
Zhang Weisheng, Jun Nakayama, Yukino Inomata, Shigeki Higashiyama, Tor ...Article type: Research Article
2025 Volume 50 Issue 1 Pages 15-24
Published: 2025
Released on J-STAGE: February 07, 2025
Advance online publication: December 18, 2024JOURNAL OPEN ACCESS FULL-TEXT HTML
Supplementary materialExtracellular signal-regulated kinase (ERK) regulates multiple cellular functions through distinct activation patterns. Genetically encoded fluorescent probes are instrumental in dissecting the ERK activity dynamics in living cells. Here we modified a previously reported Förster resonance energy transfer (FRET) probe for ERK, EKAREN5 by replacing its mTurquoise2 and YPet sequences with mTurquoise-GL and a synonymous codon variant of YPet, respectively. The modified biosensor, EKAREN5-gl, showed an increased sensitivity to EGF-induced ERK activation responding to a very low dose (20 pg/ml) of EGF stimulation. We quantitatively characterized two FRET-based ERK probes, EKAREN5 and EKAREN5-gl, and a subcellular kinase translocation-based probe, ERK-KTR. We found the three biosensors differently respond to EGF stimulations with different intensity, duration, and latency. Furthermore, we investigated how the minimal EGF-induced ERK activation affects the downstream transcription in HeLa cells by comprehensive transcriptional analysis. We found the minimal ERK activation leads to a distinct transcriptional pattern from those induced by higher ERK activations. Our study highlights the significance of sensitive fluorescent probes to understand cellular signal dynamics and the role of minimal ERK activation in regulating transcription.
Key words: fluorescent probe, ERK, FRET, KTR
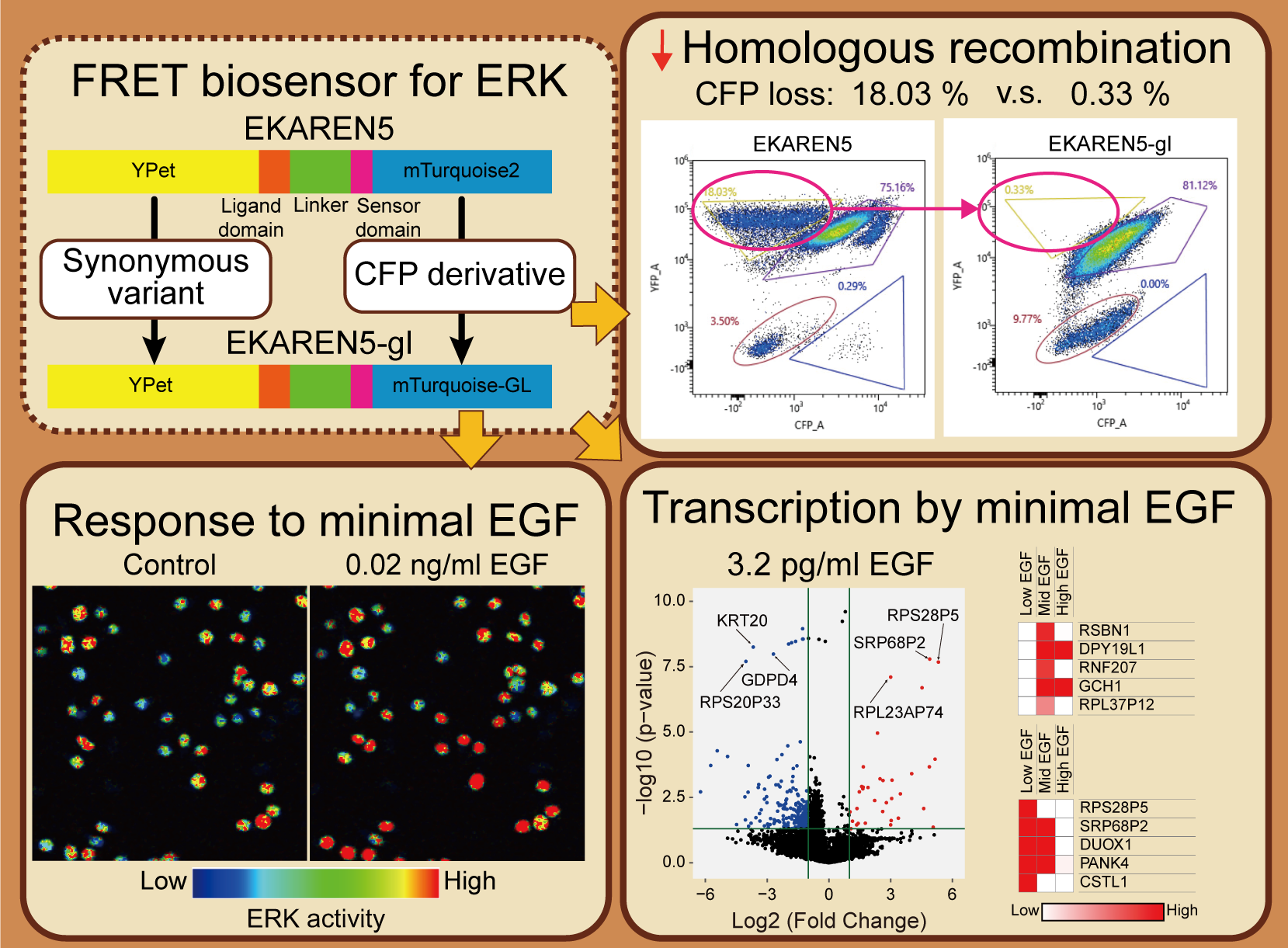 Graphical Abstract Fullsize ImageView full abstractDownload PDF (8864K) Full view HTML
Graphical Abstract Fullsize ImageView full abstractDownload PDF (8864K) Full view HTML -
Yuta Yonekawa, Kazuki Oikawa, Boldbaatar Bayarkhuu, Kizuna Kobayashi, ...Article type: Research Article
2025 Volume 50 Issue 1 Pages 25-39
Published: 2025
Released on J-STAGE: February 13, 2025
Advance online publication: December 27, 2024JOURNAL OPEN ACCESS FULL-TEXT HTML
Supplementary materialMembrane stiffness is essential for cell migration, tumorigenesis, and development; however, the physical properties of intracellular membrane are poorly characterized. In this study, we internalized 20 nm magnetic nanoparticles (MNPs) into MCF7 human breast cancer cells and applied a magnetic field. We investigated whether magnetic field could induce membrane damage of the early endosomes by analyzing the colocalization of MNPs with galectin 3 (Gal3), a cytosolic protein recruited to the lumen of damaged organelles. We first tried to apply magnetic field by electromagnet, and found a direct-current (DC) magnetic field for five minutes increased the colocalization of the MNPs with Gal3, suggesting that the magnetic field damaged the endosomal membrane. We used a neodymium magnet to apply longer and stronger static magnetic fields. The static magnetic field more than 50 mT for five minutes started to damage endosomes, while 100 mT was the most effective. Longer exposure or higher magnetic field strengths did not induce further membrane damage. We confirmed that a Gal3 positive compartment was also positive for the early endosome marker, EEA1, suggesting that the external magnetic field induced membrane damage in the early endosomes. Our results indicate that a static magnetic field can control the membrane damage in early endosomes using internalized MNPs.
Key words: magnetic nanoparticles, endosomes, membrane damage, organelle
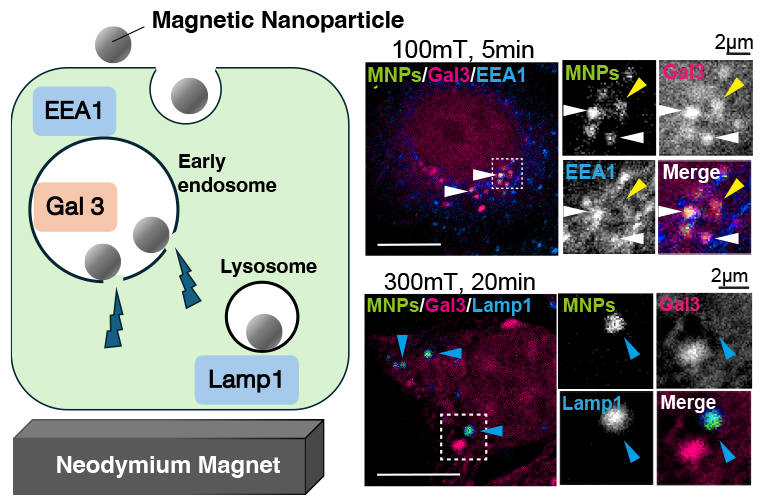 Graphical Abstract Fullsize ImageView full abstractDownload PDF (15421K) Full view HTML
Graphical Abstract Fullsize ImageView full abstractDownload PDF (15421K) Full view HTML -
Misaki Sagawa, Kazuhiro Oiwa, Hiroaki Kojima, Ken’ya Furuta, Keitaro S ...Article type: Research Article
2025 Volume 50 Issue 1 Pages 41-51
Published: 2025
Released on J-STAGE: February 18, 2025
Advance online publication: January 08, 2025JOURNAL OPEN ACCESS FULL-TEXT HTML
Supplementary materialThe motility of biological molecular motors has typically been analyzed by in vitro reconstitution systems using motors isolated and purified from organs or expressed in cultured cells. The behavior of biomolecular motors within cells has frequently been reported to be inconsistent with that observed in reconstituted systems in vitro. Although this discrepancy has been attributed to differences in ionic strength and intracellular crowding, understanding how such parameters affect the motility of motors remains challenging. In this report, we investigated the impact of intracellular crowding in vitro on the mechanical properties of kinesin under a high ionic strength that is comparable to the cytoplasm. Initially, we characterized viscosity in a cell by using a kinesin motor lacking the cargo-binding domain. We then used polyethylene glycol to create a viscous environment in vitro comparable to the intracellular environment. Our results showed that kinesin frequently dissociated from microtubules under high ionic strength conditions. However, under conditions of both high ionic strength and crowding with polymers, the processive movement of kinesin persisted and increased in frequency. This setting reproduces the significant variations in the mechanical properties of motors measured in the intracellular environment and suggests a mechanism whereby kinesin maintains motility under the high ionic strengths found in cells.
Key words: kinesin motility, molecular crowding, ionic strength, intracellular transport, processivity of molecular motors
View full abstractDownload PDF (4867K) Full view HTML -
Zhichao Wu, Nuo Chen, Daisuke TakaoArticle type: Reviews and Mini-reviews
2025 Volume 50 Issue 1 Pages 53-63
Published: 2025
Released on J-STAGE: February 18, 2025
Advance online publication: January 10, 2025JOURNAL OPEN ACCESS FULL-TEXT HTMLThe process of mammalian myogenesis is fundamental to understanding muscle development and holds broad relevance across multiple fields, from developmental biology to regenerative medicine. This review highlights two key aspects: myoblast proliferation and the role of cilia in this process. Myoblasts, as muscle precursor cells, must undergo tightly regulated cycles of proliferation and differentiation to ensure proper muscle growth and function. Recent research has uncovered an essential role for primary cilia, hair-like sensory organelles on the cell surface, in modulating signaling pathways crucial to myogenesis. Cilium-mediated signaling appears to regulate various stages of myogenesis, including the control of myoblast differentiation. Furthermore, primary cilia undergo multiple cycles of formation and disassembly during myogenesis, presumably enabling detailed, context-dependent regulation of their functions. In particular, the regulation of myoblast proliferation through cell cycle control by primary cilia is an important topic that requires further investigation. By examining the interactions between primary cilia and myoblasts, this review aims to provide new insights into the molecular and cellular mechanisms driving muscle development, with potential applications for understanding muscle-related diseases and advancing therapeutic strategies. Additionally, advancements in imaging and image analysis technologies have become indispensable for studying these processes at the cellular level. This review also addresses these technological advancements and current challenges.
Key words: myogenesis, myoblast, proliferation, cilia, imaging
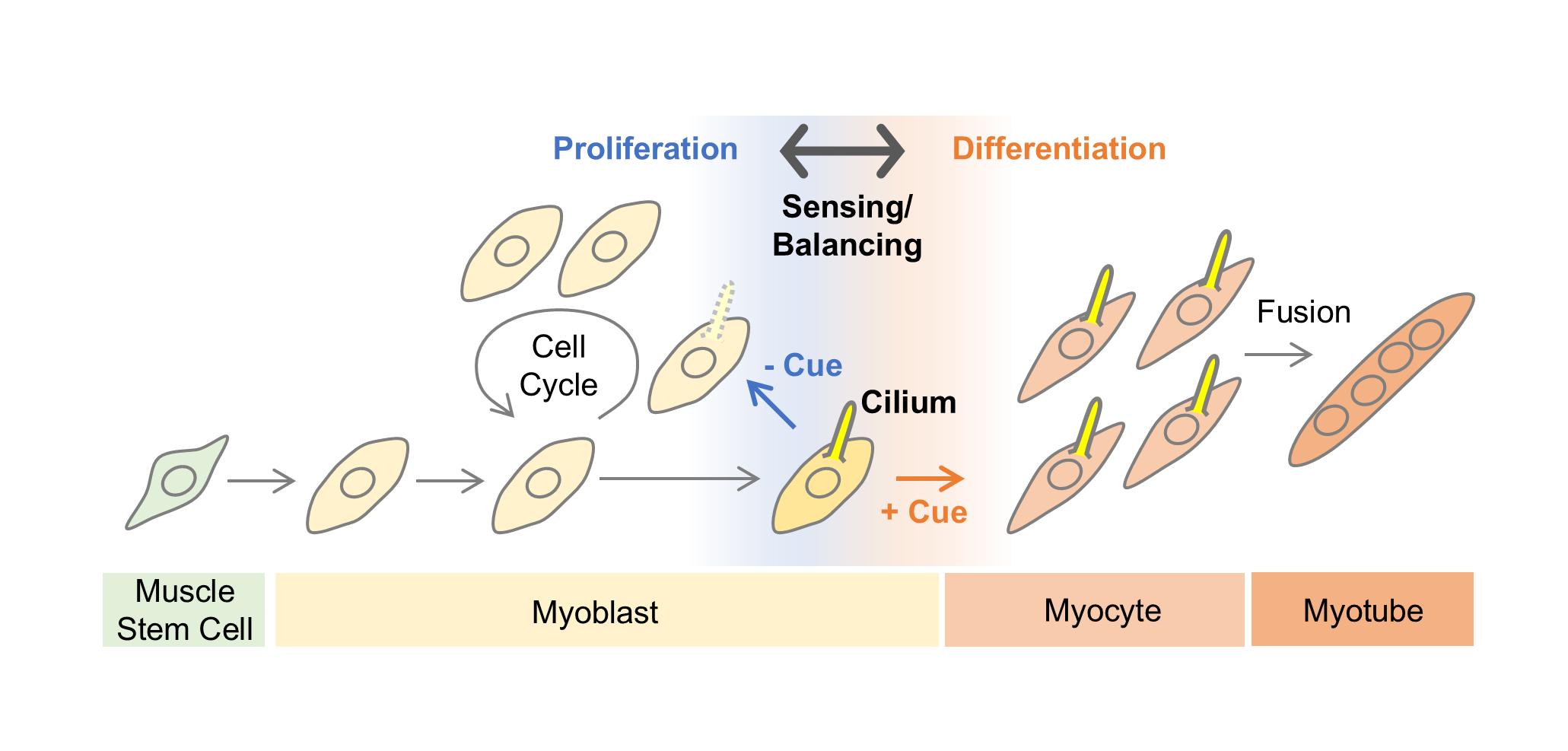 Graphical Abstract Fullsize ImageView full abstractDownload PDF (2227K) Full view HTML
Graphical Abstract Fullsize ImageView full abstractDownload PDF (2227K) Full view HTML -
Yusuke Yasuda, Tomoka Yoshida, Mahiro Oue, Masaya Sengiku, Tokiro Ishi ...Article type: Research Article
2025 Volume 50 Issue 1 Pages 65-76
Published: 2025
Released on J-STAGE: March 12, 2025
Advance online publication: January 23, 2025JOURNAL OPEN ACCESS FULL-TEXT HTMLNewly synthesized proteins destined for the secretory pathway are folded and assembled in the endoplasmic reticulum (ER) and then transported to the Golgi apparatus via COPII vesicles, which are normally 60–90 nm. COPII vesicles must accordingly be enlarged to accommodate proteins larger than 90 nm, such as long-chain collagen. Key molecules involved in this enlargement are Tango1 and Tango1-like (Tali), which are transmembrane proteins in the ER encoded by the MIA3 and MIA2 genes, respectively. Interestingly, two splicing variants are expressed from each of these two genes: Tango1L and Tango1S from the MIA3 gene, and Tali and cTAGE5 from the MIA2 gene. Here, we constructed Tango1L-knockout (KO), Tango1S-KO, Tali-KO, and cTAGE5-KO separately in medaka fish, a vertebrate model organism, and characterized them. Results showed that only Tango1L-KO conferred a lethal phenotype to medaka fish. Only Tango1L-KO medaka fish exhibited a shorter tail than wild-type (WT) fish and showed the defects in the export of type II collagen from the ER, contrary to the previous reports analyzing Tango1-KO or Tali-KO mice and the results of knockdown experiments in human cultured cells. Medaka fish may employ a simpler system than mammals for the export of large molecules from the ER.
Key words: intracellular transport, COPII vesicles, enlargement, endoplasmic reticulum, Golgi apparatus
 Graphical Abstract Fullsize ImageView full abstractDownload PDF (10668K) Full view HTML
Graphical Abstract Fullsize ImageView full abstractDownload PDF (10668K) Full view HTML -
Sachiya Nakashima, Aika Toyama, Hironori Sugiyama, Kazuhiro Aoki, Yuhe ...Article type: Reviews and Mini-reviews
2025 Volume 50 Issue 1 Pages 77-90
Published: 2025
Released on J-STAGE: April 09, 2025
Advance online publication: March 05, 2025JOURNAL OPEN ACCESS FULL-TEXT HTMLCyclin-dependent kinases (CDKs) orchestrate cell cycle progression through precise temporal control of substrate phosphorylation. While traditional biochemical approaches and phosphoproteomics have provided valuable insights into CDK-mediated regulation, these methods require cell population analyses and cannot capture real-time dynamics in individual cells. The recent development of fluorescent biosensors has revolutionized our ability to monitor CDK activity in living cells with unprecedented temporal and spatial resolution. Here, we comprehensively review genetically encoded fluorescent biosensors for measuring CDK activity. The two major modes of action in CDK activity biosensors—FRET-based and translocation-based biosensors—enable researchers to select appropriate tools for their specific experimental objectives. These biosensors have revealed precise spatiotemporal CDK activity dynamics across diverse model systems, including yeast, cultured mammalian cells, worms, flies, frog egg extract, fish, and mice. Such technological advances are transforming our understanding of quantitative principles underlying cell cycle control and opening new avenues for investigating cell cycle regulation in various biological contexts.
Key words: CDK, FRET, cell cycle, live imaging, biosensor
 Graphical Abstract Fullsize ImageView full abstractDownload PDF (4320K) Full view HTML
Graphical Abstract Fullsize ImageView full abstractDownload PDF (4320K) Full view HTML -
Chemokine induces phase transition from non-directional to directional migration during angiogenesisNing Gui, Keisuke Sako, Moe Fukumoto, Naoki Mochizuki, Hiroyuki Nakaji ...Article type: Research Article
2025 Volume 50 Issue 1 Pages 91-101
Published: 2025
Released on J-STAGE: April 16, 2025
Advance online publication: March 13, 2025JOURNAL OPEN ACCESS FULL-TEXT HTML
Supplementary materialDuring angiogenesis, sprouting endothelial cells (ECs) migrate and eventually connect to target vessels to form new vessel branches. However, it remains unclear how these sprouting vessels migrate toward the target vessels in three-dimensional space. We performed in vivo imaging of the cerebral capillary network formation in zebrafish to investigate how sprouting tip cells migrate toward their targets. Of note, we found that tip cells reach the target vessels through two phases: a non-directional phase and a directional phase. In the non-directional phase, sprouting tip cells dynamically extend and retract their protrusions at the leading front and have less directionality in their movement. In contrast, once tip cells enter the directional phase, they migrate directly toward the anastomotic targets. Chemokine receptor Cxcr4a and its ligand Cxcl12b are important for the phase transition to the directional phase. In cxcr4a mutants, sprouting tip cells lose their directionality and tend to connect to nearby sprouting ECs, resulting in altered capillary network patterning. Furthermore, in wild-type (WT) larvae, local Ca2+ oscillations were detected in protrusions of tip cells, specifically in the non-directional phase, but almost disappeared in the directional phase as a result of the Cxcr4-dependent phase transition. Thus, this study provides evidence of a chemokine-induced phase transition in migrating tip cells, which is important for proper vascular network formation in the zebrafish brain.
Key words: angiogenesis, directional migration, live imaging, chemokine, Ca2+ dynamics, zebrafish
 Graphical Abstract Fullsize ImageView full abstractDownload PDF (7087K) Full view HTML
Graphical Abstract Fullsize ImageView full abstractDownload PDF (7087K) Full view HTML -
Li Wang, Yanan Li, Yuxin He, Yuchen Fang, Hitomi Mimuro, Adam C. Midgl ...Article type: Research Article
2025 Volume 50 Issue 1 Pages 103-113
Published: 2025
Released on J-STAGE: April 18, 2025
Advance online publication: March 08, 2025JOURNAL OPEN ACCESS FULL-TEXT HTML
Supplementary materialMacropinocytosis, a type of large-scale endocytosis process, is induced in macrophages by extracellular stimuli, including lipopolysaccharide (LPS). In addition to uptake function, emerging evidence supports a link between macropinocytosis and LPS-induced signal transduction. Following LPS stimulation, membrane ruffles are induced to form cup-like structures known as macropinocytic cups, a necessary precursory step for macropinocytosis. We have recently shown that Akt is activated at the cups and is an upstream regulator of the Iκ-B/NF-κB pathway implicated in the production of IL-1α and IL-6. Here, we further investigated the molecular mechanisms and show that the macropinocytic cups also regulated the Ras/Mek/Erk/c-Fos pathway to modulate IL-1β expression independently of the Akt pathway. In addition, we observed that the cup-dependent Akt pathway downregulated the expression of IL-10, in which the activation of the Erk pathway was critical. Taken together, we propose that macropinocytic cups separately modulate the Akt and Erk pathways in cytokine expression.
Key words: macropinocytosis, Erk, IL-1β, IL-10
 Graphical Abstract Fullsize ImageView full abstractDownload PDF (6096K) Full view HTML
Graphical Abstract Fullsize ImageView full abstractDownload PDF (6096K) Full view HTML -
Tsumugi Shoji, Kanako Sato, Ayumi Shinojima, Shogo Koide, Ruri Shindo, ...Article type: Research Article
2025 Volume 50 Issue 1 Pages 115-124
Published: 2025
Released on J-STAGE: May 20, 2025
Advance online publication: April 19, 2025JOURNAL OPEN ACCESS FULL-TEXT HTMLStimulator of interferon genes (STING) triggers the type I interferon and inflammatory responses against a variety of DNA pathogens, which is essential to limiting viral infection and replication. STING activates the downstream kinase TBK1 at the trans-Golgi network (TGN) and is degraded at lysosomes through a process called lysosomal microautophagy. Impaired STING targeting to lysosomes results in the prolonged inflammatory signal, which may be associated with a variety of neurodegenerative and autoinflammatory diseases. Thus, development of methods to quantify STING degradation helps understand the mechanism of lysosomal microautophagy and its related diseases. Here we report a quantitative method to monitor STING degradation with two luciferases, firefly luciferase (FLuc) and Nanoluciferase (NLuc). The expression plasmid is composed of FLuc, a P2A self-cleavage site, and NLuc-tagged STING. FLuc intensity reflects the total amount of translated protein, serving as an internal control, while NLuc intensity corresponds to the amount of STING. Comparison of the NLuc/FLuc ratios at different time points after STING stimulation revealed the kinetics of decay of STING levels in live cells. This method should provide a useful complement to western blotting and fluorescence-activated cell sorter (FACS) analysis presently used to monitor STING degradation.
Key words: innate immunity, STING, membrane traffic, lysosomal degradation, luciferase
 Graphical Abstract Fullsize ImageView full abstractDownload PDF (4981K) Full view HTML
Graphical Abstract Fullsize ImageView full abstractDownload PDF (4981K) Full view HTML -
Serina Kita, Tokiro Ishikawa, Kazutoshi MoriArticle type: Research Article
2025 Volume 50 Issue 1 Pages 125-133
Published: 2025
Released on J-STAGE: June 03, 2025
Advance online publication: May 13, 2025JOURNAL OPEN ACCESS FULL-TEXT HTMLThe accumulation of unfolded or misfolded proteins in the endoplasmic reticulum (ER) activates the unfolded protein response (UPR) to maintain the homeostasis of the ER. The UPR consists of the IRE1, PERK and ATF6 pathways in vertebrates. Knockout of the IRE1 and PERK pathways causes defects in liver and pancreatic β cells, respectively, in mice, whereas knockout of the ATF6 pathway causes very early embryonic lethality in mice and medaka fish, a vertebrate model organism. We previously showed that ATF6 knockout in medaka causes a defect in the development of the notochord—the notochord becomes shorter—but that transient overexpression of the ER chaperone BiP via microinjection of BiP mRNA into one-cell stage embryos of these ATF6 knockout rescues this defect. Here, we microinjected mRNA encoding various ER chaperones and found that GRP94, calreticulin and calnexin also partially rescued this defect. Thus, BiP/GRP94 and calreticulin/calnexin greatly contribute to the development of the notochord by controlling the quality of collagens and N-glycosylated proteins (such as laminin and fibrillin), respectively, which have been confirmed necessary for the formation of the notochord in zebrafish.
Key words: endoplasmic reticulum, protein folding, molecular chaperone, collagen, glycoprotein
View full abstractDownload PDF (4060K) Full view HTML -
Shogo Koide, Eisuke Yumoto, Jun Nakayama, Shigeki Higashiyama, Yoshihi ...Article type: Reviews and Mini-reviews
2025 Volume 50 Issue 1 Pages 135-144
Published: 2025
Released on J-STAGE: June 07, 2025
Advance online publication: May 14, 2025JOURNAL OPEN ACCESS FULL-TEXT HTMLStimulator of interferon genes (STING) is an endoplasmic reticulum (ER)-localized transmembrane protein. STING induces type I interferon and inflammatory responses against a variety of double-stranded DNA (dsDNA) viruses, which is critical for limiting their infection and replication. In certain settings where self-DNAs (genomic or mitochondrial DNA) emerge in the cytosol or when intracellular membrane traffic is impaired, STING becomes activated and triggers inflammation, which may contribute to the pathogenesis of various autoinflammatory and neurodegenerative diseases, including COPA syndrome and Parkinson’s disease. The human STING gene exhibits genetic heterogeneity with R232, HAQ (R71H-G230A-R293Q), and H232 being the most common variants, along with population stratification. A very recent study has shown that HAQ, not R232 or H232, mediates complete clinical protection in the pathogenesis of COPA syndrome. These results reveal, for the first time, the distinct activities of the major variants in the context of the pathogenesis of autoinflammatory diseases. Besides these major variants, there exist minor pathogenic STING variants that cause an autoinflammatory disease called STING-associated vasculopathy with onset in infancy (SAVI). This review summarizes recent insights into human STING variants and their inflammatory activities.
Key words: innate immunity, STING variants, COPA syndrome, membrane traffic, the Golgi
 Graphical Abstract Fullsize ImageView full abstractDownload PDF (4575K) Full view HTML
Graphical Abstract Fullsize ImageView full abstractDownload PDF (4575K) Full view HTML
- |<
- <
- 1
- >
- >|