2023 年 91 巻 11 号 p. 112010
2023 年 91 巻 11 号 p. 112010
Anodic formation of N,O-acetals using amides or carbamates as starting materials, commonly referred to as Shono oxidation, has been an iconic reaction in the field of synthetic organic electrochemistry. However, it is, in principle, not stereoselective and the commonly used neighboring-carbonyl-group participation is not usually effective. Herein, we demonstrate that the use of alkylidene protective groups is extremely effective in controlling the stereoselectivity. Although the detailed mechanism remains an open question, under our reaction conditions, the conformation restriction effect is dominant rather than the steric hindrance effect.

Stemming from the pioneering work of Shono and Matsumura in 1975,1 an anodic formation of N,O-acetals using amides or carbamates as starting materials, commonly referred to as Shono oxidation,2–7 has been an iconic reaction in the field of synthetic organic electrochemistry.8–18 An anodic oxidation of amides or carbamates generates N-acyliminium ions, which are then trapped by alcohol solvents to give the corresponding N,O-acetals (Scheme 1). From a modern viewpoint, this reaction provides a useful path to C–H activation that accesses N-α functionalized amines. The obtained N,O-acetals can regenerate N-acyliminium ions under nonelectrochemical Lewis acidic conditions in the presence of various nucleophiles to enable the N-α substitutions. When an anodic oxidation is performed in the absence of alcohol solvents or nucleophiles at low temperatures, the N-acyliminium ions can be accumulated as a “cation pool”,19 which is then trapped by various nucleophiles, even those with high electron density (low oxidation potential). The cation-pool method has expanded the synthetic utility of the Shono-type oxidation process and has been one of the symbolic procedures in this field.20,21

Shono-type oxidations.
With respect to the mechanism, the trapping by alcohol solvents or nucleophiles is, in principle, not stereoselective because attacks from both the upper and lower surfaces of N-acyliminium ions are possible (Fig. 1). A classical yet effective methodology to control the stereoselectivity is neighboring-carbonyl-group participation, where one surface is blocked and nucleophilic attack from the other surface is facilitated. This methodology has been widely studied using oxocarbenium ions (presumably because five- and six-membered ring systems are important structural motifs in nucleic acids and carbohydrates) to enable the stereoselective synthesis of substituted tetrahydrofuran and tetrahydropyran derivatives.22 However, the participation of neighboring carbonyl groups does not adequately explain the stereoselectivity of the nucleophilic attacks on oxocarbenium ions. Through systematic studies, Woerpel proposed that electronic effects also strongly influence the stereoselectivity.23–27 In addition, Matsuda and Ichikawa demonstrated that alkylidene protective groups are powerful tools to control the stereoselectivity through steric-hindrance effects.28–32 Although such a mechanistic aspect has been studied extensively for oxocarbenium ions, that for N-acyliminium ions is less well understood.

Stereoselectivity of the nucleophilic attacks.
We have been developing Shono-type oxidations to access N-α functionalized prolines and prolinols.33–41 An anodic oxidation of proline and prolinol derivatives generates N-acyliminium ions, which are then trapped by various nucleophiles to enable the N-α substitutions. However, the trapping is not usually stereoselective even when the neighboring carbonyl groups participate; thus, establishing a general methodology to control the stereoselectivity remains a challenge. During the course of our study, we found that the use of a pentylidene protective group is also effective to control the stereoselectivity of the hydroxylation of N-acyliminium ion derived from the prolinol derivative,42 as was previously reported for oxocarbenium ions. We thus questioned whether the use of an alkylidene protective group could generalize the steric hindrance effects in Shono oxidations. Described herein are the stereoselective Shono oxidations enabled by the use of alkylidene protective groups.
The present work began with the synthesis of a series of prolinol derivatives with different protective groups (1–3). Methoxycarbonyl (Moc) and acetyl (Ac) groups are commonly used to protect amines and primary alcohols. Secondary alcohols are protected with Ac (1), pentylidene (2), or methylidene (3) and are used for Shono oxidations in methanol solvent (see the Supporting Information for details). For the prolinol derivatives, the stereoselectivity is expressed by β/α: the trapping of N-acyliminium ions by alcohol solvents or nucleophiles from the “upper” surface gives the β-anomer, and that by alcohol solvents or nucleophiles from the “lower” surface gives the α-anomer (Fig. 2).

Prolinol derivatives used in this work.
Anodic oxidation of the Ac-protected prolinol (1) gave the methoxylated product (4) in 75 % yield, with negligible stereoselectivity of β/α = 44/56, suggesting that the neighboring-carbonyl-group participation was not effective, in accordance with our previous reports (Table 1). However, when the pentylidene-protected prolinol (2) was used instead, the synthesis outcome dramatically changed. The anodic oxidation gave the methoxylated product (5) in 98 % yield, with extremely high stereoselectivity of β/α = >99/1, suggesting that the use of the pentylidene protective group is effective for stereocontrol of not only the hydroxylation but also the methoxylation of the N-acyliminium ion derived from the prolinol derivative.
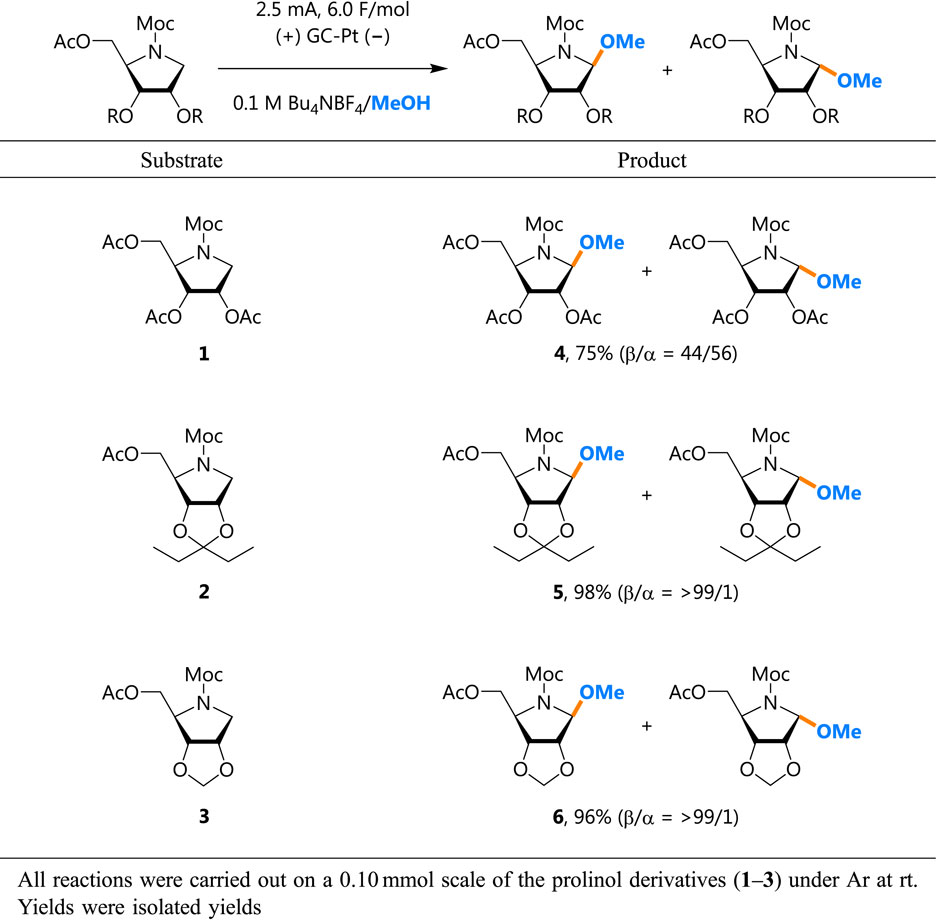
In a previous report on oxocarbenium ions, Matsuda and Ichikawa speculated that the stereocontrol function of the pentylidene protective group is twofold: restricting the conformation of the oxocarbenium ions and blocking the “lower” surface via a steric hindrance effect provided by diethyl groups. Therefore, we expected a methylidene protective group to be less effective than a pentylidene protective group in the stereocontrol function because the steric hindrance effects would be weaker. To our surprise, however, the anodic oxidation of the methylidene-protected prolinol (3) gave the methoxylated products (6) in 96 % yields, with extremely high stereoselectivities of β/α = >99/1. This result suggests that the conformation restriction effect is dominant rather than the steric hindrance effect in the stereoselectivity under our reaction conditions.
In conclusion, we have demonstrated that stereoselective Shono oxidations were made possible by the use of alkylidene protective groups. The anodic oxidations of alkylidene-protected prolinols gave the corresponding methoxylated products in high yields and with extremely high stereoselectivities. Not only pentylidene but also methylidene group was found to be effective in controlling the stereoselectivity. Although the detailed mechanism remains an open question, the conformation restriction effect would be dominant rather than the steric hindrance effect under our reaction conditions. Further experimental and theoretical studies focusing on stereoselective Shono oxidations are underway in our laboratory.
This work was supported in part by JSPS KAKENHI Grant No. 22K05450 (to Y. O.), 23KJ0870 (to H. M.), and by the TEPCO Memorial Foundation (to Y. O.).
The data that support the findings of this study are openly available under the terms of the designated Creative Commons License in J-STAGE Data at https://doi.org/10.50892/data.electrochemistry.24172950.
Shinnosuke Akahane: Formal analysis (Equal), Investigation (Equal), Methodology (Lead)
Haruka Morizumi: Formal analysis (Equal), Investigation (Equal)
Yoshikazu Kitano: Supervision (Equal), Writing – review & editing (Equal)
Yohei Okada: Conceptualization (Lead), Funding acquisition (Lead), Supervision (Equal), Writing – original draft (Lead), Writing – review & editing (Equal)
The authors declare no conflict of interest in the manuscript.
Japan Society for the Promotion of Science: 22K05450
Japan Society for the Promotion of Science: 23KJ0870
TEPCO Memorial Foundation
Y. Okada: ECSJ Active Member