2024 年 92 巻 2 号 p. 027008
2024 年 92 巻 2 号 p. 027008
Li-metal batteries employing Li-metal anodes with the highest capacity density have attracted considerable attention. However, the Li-metal deposition is often nonuniform, which reduces the cycle performance of Li-metal batteries. The three-dimensionally ordered macroporous polyimide (3DOM PI) separator has provided much better Li deposition/dissolution cycle performance owing to its unique and ordered pore structure, but its low mechanical strength is an obstacle to practical use. In this study, we added polybenzimidazole (PBI) to increase the mechanical strength of the 3DOM PI separator. Both the 3DOM PI and 3DOM PI+PBI separators with pore sizes of 300 and 100 nm were prepared to investigate the effect of pore size on the mechanical strength of the separator. The 3DOM PI+PBI separators exhibited higher tensile strength, higher thermal stability, better Li deposition/dissolution cycle performance, and higher charge–discharge performance of the Li-metal battery than the 3DOM PI separators. Here, it was suggested that the increase in the mechanical strength of the separator prevented the compression of the pores in the separator by external pressure and provided a more uniform Li+ ion flux inside the separator.
Li-ion batteries exhibit the highest energy density among rechargeable batteries in practical applications. Recently, next-generation batteries employing active materials with higher capacities have attracted attention. In particular, Li metal is the ultimate anode material owing to the highest theoretical capacity density of 3861 mAh g−1, which is 10 times larger than that of a graphite anode (372 mAh g−1).1 Furthermore, the Li-metal anode can provide a larger operation voltage of the cell owing to the lowest redox potential (−3.04 V vs. SHE) of Li metal. Therefore, Li-metal batteries employing Li-metal anodes have been widely studied.2–4 However, Li metal undergoes nonuniform deposition promoting a low coulombic efficiency and an internal short circuit resulting in a lower battery safety.
Various approaches have been investigated to solve these problems. The Li deposition morphology and the solid electrolyte interphase (SEI) layers were investigated most intensively.5–8 These studies reveal that a more stable and uniform SEI layer provides a higher coulombic efficiency owing to the suppression of electrolyte decomposition and the prevention of a large distribution of electrode reactions. Several studies have prepared more uniform and stable SEI layers by changing the type of electrolyte and using additives.9–15 In addition, the morphology of the deposited Li metal significantly affects the stabilization of the SEI layer. Owing to volume and morphology changes, SEI layers with low mechanical strength are physically damaged during the Li deposition/dissolution process. Large granular deposited Li prevents side reactions with electrolytes and damage to the SEI layer owing to volume changes.16,17 Another approach, such as the influence of external pressure on the cells, stabilizes the Li-metal anode.18–20 The application of external pressure to the cells resulted in the formation of dense Li-metal deposits and improved cycle performances.
By contrast, we focused on the Li+ ion flux in the separators to reduce reaction distribution in the electrodes. The separator contacts the Li-metal anode, strongly affecting Li deposition/dissolution behavior.21,22 The uniform deposition of Li metal was observed when the Li+ ion flux in the separator was uniform. However, conventional polyolefin separators have a nonuniform pore structure and they promote nonuniform reaction distribution in the electrode. To solve this problem, we have developed an ultrafine porous polyimide membrane with an inverse opal structure.23–26 This membrane has been used as a three-dimensionally ordered microporous polyimide (3DOM PI) separator, which has been applied to the Li-metal anode to improve cycle performance.27–32 The 3DOM PI separator provides better Li deposition/dissolution cycle performance owing to its unique and uniform pore structure. However, the mechanical strength of the 3DOM PI separator is still low, and this results in a compression of pores in the separator when external pressure is applied to cells.
In this study, we added polybenzimidazole (PBI) to increase the mechanical strength of the 3DOM PI separator. PBI is an engineering plastic, similar to PI, and has higher mechanical strength and thermal stability than PI. In the previous report, smaller pores in the 300–800 nm range have exhibited better Li deposition/dissolution and charge–discharge performances.29 In this study, pore sizes of 300 and 100 nm were prepared to make clear more detailed effects of pore size on the Li deposition/dissolution process. The physical properties of the four separators were investigated in terms of their tensile strength, thermal stability, and gas permeability. Subsequently, Li deposition/dissolution cycle tests were conducted to investigate the cycling performance of the Li-metal anodes. Finally, the charge–discharge cycle tests were performed using cells with a LiNi0.5Co0.2Mn0.3O2 (NCM523) cathode and a Li-metal anode. The effect of the addition of PBI and pore size was investigated by using a highly concentrated electrolyte. This electrolyte has provided a better cycling performance.31
The 3DOM PI and PI+PBI separators were prepared using a colloidal crystalline template method with mono-disperse silica particles. Figures 1a and 1b show the structural formula of PI and PBI. Polyamic acid (JVI-2002, JFE Chemical Corp.) was used as the precursor for the PI. PBI was purchased from SATO LIGHT INDUSTRIAL Co., Ltd. Mono-disperse silica spherical particles (Seahostar® KE-P, Nippon Shokubai Co., Ltd.) were used as template particles. The 3DOM PI separators contained 0 wt% PBI, and the 3DOM PI+PBI separators contained 10 wt% PBI. These materials were dispersed in N,N-dimethylacetamide (Wako Special Grade; Fujifilm Wako Pure Panaca Co., Ltd.) to prepare a slurry. The slurry was coated on PET films (PANA-PEEL®NP-75-A, Panac Co., Ltd.) and dried to remove the solvent. The precursor sheet was imidized by heating at 320 °C for 2 h. The remaining silica particles in the sheet were removed using a 10 wt% aqueous hydrofluoric acid solution. The resulting 3DOM PI and 3DOM PI+PBI separator were dried under vacuum at 120 °C for 48 h.
Structural formula of (a) PI and (b) PBI.
[LiFSI]1[EC]2 was prepared from Li bis(fluorosulfonyl)imide (LiFSI, KISHIDA CHEMICAL CO., Ltd.) and ethylene carbonate (EC, KISHIDA CHEMICAL CO., Ltd.). LiFSI and EC were mixed in a molar ratio of 1 : 2. The water contents of the prepared electrolytes were analyzed using the Karl Fischer titration method (Kyoto Electronic Manufacturing Co., Ltd., MKC-710). The water content was <50 ppm.
2.3 Characterization of the separators and Li-metal anodeThe morphologies of the separators and Li metal were observed using scanning electron microscopy (SEM) (JSM-6490A, JEOL Ltd.). The thermal stability of the separators was measured by thermogravimetric analysis (TGA) (DTG-60H, SHIMADZU Corp.) in air up to 600 °C with a heating rate of 5 °C/min. The mechanical strength and gas permeability of the separators were measured by using a tensile tester (Autograph AGS-X, SHIMADZU Corp.) and a Gurley permeabilities tester (No. 158 GURLEY TYPE DENSOMETER, TOYOSEIKI Co., Ltd.), respectively.
2.4 Transference number measurementThe cationic transference numbers t+ of the electrolyte in the separators were calculated using a frequency response analyzer built-in potentiostat/galvanostat (VMP-300, BioLogic Science Instruments) and the following Eq. 1,
| \begin{equation} t_{+} = i_{\text{s}}(\varDelta V - i_{0}R_{0})/i_{0}(\varDelta V - i_{\text{s}}R_{\text{s}}) \end{equation} | (1) |
where ΔV is the applied voltage, i is the current, and R is the sum of the charge transfer resistance and passivation film resistance. Subscripts 0 and s indicate the initial and steady-state values, respectively.33–36 The ΔV was set at 5 mV. The impedance measurement was performed using alternating current (AC) voltage with ±5 mV amplitude in the frequency ranges of 1 MHz to 50 mHz.
2.5 Ionic conductivity measurementThe ionic conductivities of the electrolytes in the separators were measured using the 2032-type coin cells with Au electrodes and a frequency response analyzer built-in potentiostat/galvanostat (VMP-300). Electrochemical impedance spectroscopy (EIS) was performed using AC voltage with ±5 mV amplitude in the frequency ranges of 100 kHz to 100 mHz. The ionic conductivity, σ (S cm−1), was determined using Eq. 2 as follows:
| \begin{equation} \sigma = d/(R_{\text{b}}S), \end{equation} | (2) |
where d denotes the separator thickness (cm), Rb is the bulk resistance (Ω) measured by EIS, and S is the electrode area (cm2). The measurements were performed in the temperature range of 20–60 °C to obtain Arrhenius plots of the ionic conductivity.
2.6 Li deposition/dissolution test of Li/Li symmetric cellsThe Li/Li symmetric cells were constructed in a glovebox filled with Ar gas using Li foil on the Cu current collector (100 µm Li/10 µm Cu, Honjo Metal Co., Ltd.), [LiFSI]1[EC]2 electrolyte, and 3DOM PI or PI+PBI separator with pouch cells.
Li deposition/dissolution tests were performed using Li/Li symmetric cells at a current density of 10 mA cm−2 for 30 min at 30 °C using charge–discharge test equipment (TOSCAT-3100, TOYO SYSTEM Co., Ltd.). This current density was employed. The higher current density generally provides a more uniform Li deposition/dissolution behavior and better cycle performance. The cell impedances for Li/Li symmetric cells were measured using a frequency response analyzer built-in potentiostat/galvanostat (VMP-300) with AC voltage with ±5 mV amplitude in the frequency ranges of 1 MHz to 50 mHz.
2.7 Charge–discharge test of full cellsLiNi0.5Mn0.2Co0.3O2 (NCM523)/Li full cells were constructed in a glovebox filled with Ar gas. NCM523 electrode sheet (bcaf-ncm523ss, MTI Corp.) with 2 mAh cm−2 was used as the cathode. Li foil on the Cu current collector (20 µm Li/10 µm Cu, Honjo Metal Co., Ltd.) was used as the anode. [LiFSI]1[EC]2 was used as an electrolyte. 3DOM PI or PI+PBI separator was used in the pouch cell with NCM523 and Li.
The charge–discharge test of NCM523/Li full cells was performed in the voltage range of 2.5–4.3 V with constant current-constant voltage mode for charging and constant current mode for discharging at 30 °C using charge–discharge test equipment (TOSCAT-3100, TOYO SYSTEM Co., Ltd.). The test was performed at 0.1 C rate for the initial three cycles and then at 0.2 C rate after the 4th cycle. The rest for the 48 hours was included after the 70th cycle in this cycle test.
Figures 2a–2d show the SEM images of the surface morphology of the separators. All the separators had uniform pore structures and controlled pore sizes of approximately 300 or 100 nm, depending on the template particle size from SEM images. Figures 2e–2h show SEM images of the cross sections of the separators. Highly uniform and a controlled pore size was confirmed. Porosities of 3DOM PI (300 nm), PI (100 nm), PI+PBI (300 nm), and PI+PBI (100 nm) were 77.9 %, 76.2 %, 74.2 %, and 74.0 %, respectively, as listed in Table 1. The 3DOM PI separators exhibit higher porosities than a theoretical value of hexagonal closed-packed structures (74.0 %) owing to a volume shrinkage of PI during the preparation process. This volume change was suppressed by the addition of PBI, leading to the theoretical value of porosity. Gurley numbers of 3DOM PI (300 nm), PI (100 nm), PI+PBI (300 nm), and PI+PBI (100 nm) were 45.8, 138.8, 88.0, and 166.5 s 100 cm−3, respectively, as listed in Table 1. Gas permeability was higher for the 3DOM PI separators than for the 3DOM PI+PBI ones, and for the 3DOM separators with 300 nm pores than for those with 100 nm pores. The Gurley numbers depended on the size of the connecting pores, suggesting that the suppression of shrinkage during the separator preparation process by the addition of PBI resulted in a smaller connecting pore size.

SEM images of (a–d) surface and (e–h) cross section of 3DOM PI and 3DOM PI+PBI separators.
| Thickness /µm |
Porosity /% |
Gurley number/s 100 cm−3 |
Li+ transference number |
|
|---|---|---|---|---|
| [LiFSI]1[EC]2 | — | — | — | 0.57 |
| 3DOM PI (300 nm) | 20 | 77.9 | 45.8 | 0.60 |
| 3DOM PI (100 nm) | 20 | 76.2 | 138.8 | 0.62 |
| 3DOM PI+PBI (300 nm) |
20 | 74.2 | 88.0 | 0.77 |
| 3DOM PI+PBI (100 nm) |
20 | 74.0 | 166.5 | 0.78 |
TGA was performed to investigate the effect of the addition of PBI on the thermal stability of the 3DOM separators, as shown in Fig. 3. The weights of 3DOM PI (300 nm), PI (100 nm), PI+PBI (300 nm), and PI+PBI (100 nm) notably decreased at 470, 420, 500, and 490 °C, respectively, because of the combustion of PI. The residual weight at 600 °C was approximately 10 wt% for the 3DOM PI+PBI separator and was attributed to PBI. These results suggest that the addition of PBI improved the thermal stability of the 3DOM separator. Furthermore, the weight loss of the 3DOM PI separator from room temperature may be due to the desorption of absorbed water and DMAc, indicating that the 3DOM PI separator must be sufficiently dried before use.
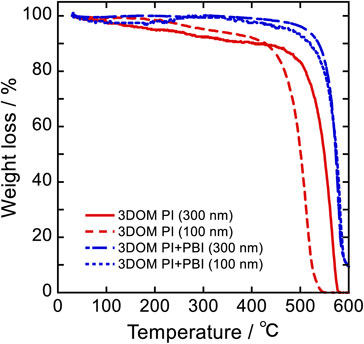
TGA curves of 3DOM PI and 3DOM PI+PBI separators.
The effect of pore size and the addition of PBI on the tensile strength of the separators were investigated, as shown in Fig. 4. The tensile strengths of 3DOM PI (300 nm), PI (100 nm), PI+PBI (300 nm), and PI+PBI (100 nm) were 9.1, 8.0, 20.6, and 15.3 MPa, respectively. The displacements were 4.3, 3.3, 6.4, and 5.1 mm, respectively. The 3DOM PI+PBI separators exhibited higher tensile strengths and larger displacements than the 3DOM PI separators. The 3DOM separators with 300 nm pores exhibited higher tensile strength and larger displacement than those with 100 nm pores owing to the larger average thickness of the 3DOM framework.
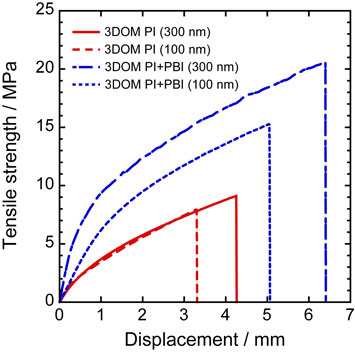
Stress–strain curves of 3DOM PI and 3DOM PI+PBI separators.
Figure 5 shows the Arrhenius plots of ionic conductivity of the separators with [LiFSI]1[EC]2, evaluated using EIS measurement at various temperatures. Ionic conductivities at 30 °C of highly concentrated electrolyte without separators, with 3DOM PI (300 nm), 3DOM PI (100 nm), 3DOM PI+PBI (300 nm), and 3DOM PI+PBI (100 nm), were 1.59, 0.80, 0.54, 0.45, and 0.39 mS cm−1, respectively. Ionic conductivities were higher for the 3DOM separators with 300 nm pores than for those with 100 nm pores, and for the 3DOM PI separators than for the 3DOM PI+PBI separators. These results are consistent with the notion that the Gurley number is strongly related to ionic conductivity.22,37 The activation energies of highly concentrated electrolyte without separators, with 3DOM PI (300 nm), 3DOM PI (100 nm), 3DOM PI+PBI (300 nm), and 3DOM PI+PBI (100 nm), were 29.7, 21.8, 17.2, 16.4, and 15.9 kJ mol−1, respectively. The different activation energies suggest different ion conduction mechanisms. The Li+ transference numbers of those separators were 0.57, 0.60, 0.62, 0.77, and 0.78, respectively, as listed in Table 1, and increased with decreasing activation energy. This also suggests that the interaction between the highly concentrated electrolyte and 3DOM separators influences the ion conduction mechanisms. However, a previous study for a conventional electrolyte (1 M LiPF6/EC) without separators and with 3DOM PI separators reported the same slope of the Arrhenius plots.29 From these results, it is confirmed that the interaction between the electrolyte and the 3DOM PI separator is greatly affected by the types of electrolytes. PI may interact with Li+ ions, which affect the formation of contact ion pair (CIP) and aggregate (AGG), Li+ transference numbers, and ion conductive mechanisms of the highly concentrated electrolyte in the separators. The addition of PBI may influence the interaction between Li+ ions and the polymer matrix, resulting in ion conduction mechanisms.

Arrhenius plots of [LiFSI]1[EC]2 electrolytes with 3DOM PI and 3DOM PI+PBI separators.
Figures 6a–6d show the voltage profiles of Li/Li symmetric cells during the Li deposition/dissolution cycles. The capacity for the Li deposition/dissolution cycle was 5 mA h cm−2 and the current density was 10 mA cm−2. All cells employing the 3DOM separators exhibited stable Li deposition/dissolution behavior over 500 cycles. The 3DOM PI (300 nm) separator provided a polarization of 30 mV for up to 200 cycles and 50 mV after 200 cycles. The 3DOM PI (100 nm) separator provided a polarization of 60–80 mV. The 3DOM PI+PBI (300 nm) and 3DOM PI+PBI (100 nm) separators provided a polarization of 30 and 25 mV, respectively. The 3DOM PI+PBI separators exhibited lower polarization and better polarization stability than the 3DOM PI separators.

Voltage profiles of Li/Li symmetric cells employing (a) 3DOM PI (300 nm), (b) 3DOM PI (100 nm), (c) 3DOM PI+PBI (300 nm), and (d) 3DOM PI+PBI (100 nm) separators measured at 10 mA cm−2 for 30 min.
Figures 7a–7d show the Cole–Cole plots of the Li/Li symmetric cells after the 1st, 50th, and 100th cycles. Capacitive semicircles were observed in all the plots. In the 3DOM PI separators, the bulk resistance at the 1st cycle was lower in the separator with 300 nm pores than in the one with 100 nm pores, consistent with their ionic conductivity. In these separators, the bulk resistances at the 50th and 100th cycles were higher than those at the 1st cycle. The 3DOM PI+PBI separators exhibited better bulk and interfacial resistances stability than the 3DOM PI separators. Furthermore, the bulk resistances almost unchanged up to 100 cycles. The separator with 300 nm pores exhibited slightly higher bulk and interfacial resistances than those with 100 nm pores owing to the effect of the deposited Li surface area.

Cole–Cole plots during Li deposition/dissolution cycles in Li/Li symmetric cells employing (a) 3DOM PI (300 nm), (b) 3DOM PI (100 nm), (c) 3DOM PI+PBI (300 nm), and (d) 3DOM PI+PBI (100 nm) separators after the 1st, 50th, and 100th cycles.
After 100 cycles of the Li deposition/dissolution test, the cells were disassembled, and the Li metal was observed by SEM. Figures 8a–8d show the SEM images of the surface of the deposited Li metal. Granular Li particles were observed on the Li surface of all the cells. The 3DOM separators with 300 nm pores provided a slightly larger granular Li than those with 100 nm pores. This suggests that the pore size of the 3DOM separators affects the size of the deposited Li. Furthermore, the 3DOM PI+PBI separators provided a larger granular Li than the 3DOM PI separators.

SEM images of deposited Li metal surface in Li/Li symmetric cells employing (a) 3DOM PI (300 nm), (b) 3DOM PI (100 nm), (c) 3DOM PI+PBI (300 nm), and (d) 3DOM PI+PBI (100 nm) separators after the 100th cycle.
The addition of PBI to the 3DOM PI separators resulted in lower polarization and bulk resistances in Li dissolution/deposition tests. The increase in the mechanical strength of the separators prevented compression of the pores in the separators by external pressure, leading to a preservation of ionic conduction pathways. This contributed to the improving apparent ionic conductivity of the electrolyte inside the separator and a more uniform reaction distribution. Furthermore, the pore size of separators affects the size of deposited Li.29 The larger granular Li with smaller surface area enable stable polarization and internal resistance owing to the suppression of side reactions between the deposited Li and the electrolyte.
3.3 Charge–discharge performanceCharge–discharge cycle performance tests of NCM523/Li full cells were conducted to investigate the effect of the cathode on the charge–discharge behaviors. Figures 9a and 9b show the charge–discharge curves at the 4th and 100th cycles, respectively, and Figs. 10a and 10b show the discharge capacities and coulombic efficiencies of the charge–discharge cycle performance tests. The charge–discharge curves of the cells employing 3DOM PI (300 nm), PI (100 nm), PI+PBI (300 nm), and PI+PBI (100 nm) at the 4th cycle exhibited nearly the same behavior and their discharge capacities were 151.6, 155.2, 152.5, and 155.1 mAh g−1, respectively. Polarization of these cells in the charge–discharge curves at the 100th cycle was larger than those at the 4th cycle. The discharge capacities at the 100th cycle were 106.2, 118.3, 128.9, and 144.7 mAh g−1, respectively. The 3DOM PI+PBI separators exhibited better cycle performance than the 3DOM PI separators. This suggests that the addition of PBI improves the mechanical strength of the separator and suppresses the compression of pores in the separator, providing a more uniform ion flux inside the separator and reaction distribution on the electrodes. Furthermore, the 3DOM PI+PBI (100 nm) separator provided the best cycling performance for the full cells. Separators with smaller pore sizes may provide a more uniform reaction distribution. The average coulombic efficiencies of the cells with 3DOM PI (300 nm), PI (100 nm), PI+PBI (300 nm), and PI+PBI (100 nm) between the 4th and 100th cycles were 99.08, 99.25, 99.32, and 99.36 %, respectively. The coulombic efficiency was higher in the 3DOM PI+PBI separator than in the 3DOM PI separator, again indicating that more uniform reaction distribution on the electrode and suppressed side reactions with the electrolyte. The discharge capacity degradation started after the rest in the 70th cycle. The capacity degradation after the 70th cycle could be attributed to electrolyte depletion, which was expected from the coulombic efficiency.
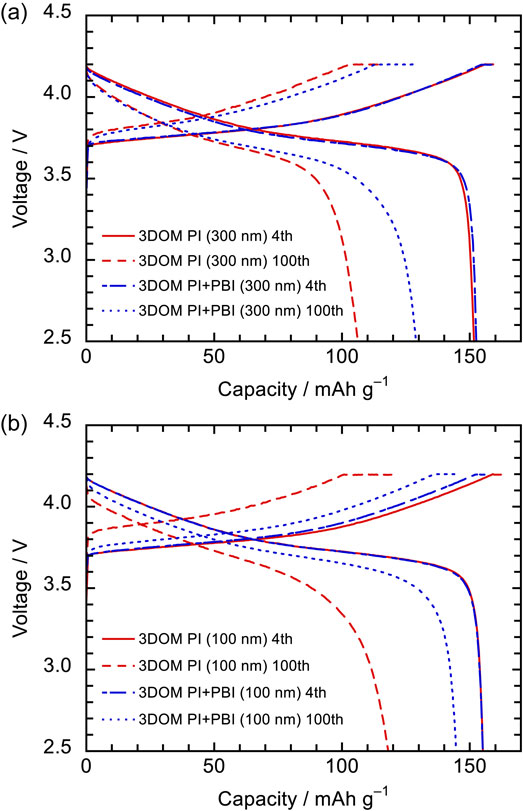
Charge–discharge curves of NCM523/Li full cells employing 3DOM PI and 3DOM PI+PBI separators with a pore size of (a) 300 nm and (b) 100 nm at 4th and 100th cycles.

Discharge capacity and coulombic efficiency of NCM523/Li full cells employing 3DOM PI and 3DOM PI+PBI separators with a pore size of (a) 300 nm and (b) 100 nm.
PBI was added to a 3DOM PI separator to increase its mechanical strength. Four types of separators were prepared using two materials, PI and PI+10 wt% PBI, and two types of template particles, 300 and 100 nm, and their physical and electrochemical properties are also investigated. The 3DOM PI separator exhibited a higher porosity and Gurley number, while the 3DOM PI+PBI separator exhibited higher tensile strength and thermal stability. Furthermore, the 3DOM PI+PBI separator showed a more stable Li deposition/dissolution and charge–discharge cycle performance of the full cell than the 3DOM PI separator. An increase in the mechanical strength of the separator prevented the compression of pores in the separator by external pressure, and provided a more uniform reaction distribution in the electrodes. The pore size of the 3DOM PI+PBI separators also affected the size of granular Li. The 3DOM PI+PBI (100 nm) separator provided smaller granular Li but a more uniform electrode reaction distribution, which resulted in better charge–discharge cycle performance of the full cell.
This work was supported by JST ALCA-SPRING Grant Number JPMJAL1301.
Yuma Shimbori: Conceptualization (Equal), Data curation (Lead), Formal analysis (Lead), Investigation (Supporting), Writing – original draft (Lead), Writing – review & editing (Lead)
Shinnosuke Ooga: Conceptualization (Lead), Data curation (Equal), Formal analysis (Equal), Investigation (Lead)
Koichi Kajihara: Project administration (Supporting), Writing – original draft (Equal), Writing – review & editing (Equal)
Kiyoshi Kanamura: Funding acquisition (Lead), Methodology (Equal), Project administration (Lead), Writing – original draft (Equal), Writing – review & editing (Equal)
The authors declare no conflict of interest in the manuscript.
Advanced Low Carbon Technology Research and Development Program: JPMJAL1301
Y. Shimbori: ECSJ Student Member
K. Kanamura: ECSJ Fellow