2018 Volume 65 Issue 1 Pages 1-11
2018 Volume 65 Issue 1 Pages 1-11
Frailty is a state of vulnerability and a consequence of cumulative decline in multiple physiological systems over a lifespan. The occurrence of frailty depends on deterioration in muscle and nerve function, declining cardiopulmonary reserve and loss of executive function. Diabetes mellitus (DM) often causes functional impairment in each of the above systems, thus leading to a loss of whole body homeostasis and deterioration in physical function. Inability of self-management in DM patients may also have considerable impact on the development of sarcopenia/frailty. Thus, there may be positive feedback between the progression of diabetic complications and frailty/sarcopenia. While various factors are involved in this process, insulin resistance or insulin depletion may be an important factor in the progression of frailty in diabetes patients since insulin is well known to be an anabolic hormone in muscle. Interestingly, in our study targeting elderly DM patients, low HbA1c was a significant and independent risk factor for frailty, as assessed using a broad sense frailty scale, the Clinical Frailty Scale (CSF), suggesting that reverse metabolism due to malnutrition in elderly type 2 DM patients might be involved. Therefore, an intervention that includes proper nutrition and exercise training may be essential for the prevention of frailty. The pathogenesis of frailty in DM patients is extensively discussed in this review.
Diabetes mellitus (DM) is characterized by chronic hyperglycemia and is associated with various complications including triopathy (retinopathy, neuropathy and nephropathy) and macrovascular complications. The proportion of elderly people (more than 65 years old) in the world is gradually increasing, constituting 5.1% of the world’s population in 1950 and 8.3% in 2015. The proportion of elderly people in Japan is the highest in the world at 26.7% in 2015 [1]. Accordingly, the number of elderly patients (65 years and older) with DM is currently 40% or more of all DM patients in Japan. Following this increase in elderly DM patients, dementia [2] and cancer [3] have recently been recognized as novel diabetic complications. While cognitive impairment itself in elderly DM patients could cause hypoglycemia, hypoglycemia is considered to have a negative effect on dementia in senile DM patients [2].
The development of DM in aged people stems from multiple factors including genetics, age-related mental and/or social problems and nutrition [4]. Both the insulin resistance and the insulin depletion characterize the pathology of elderly DM. With aging, sarcopenic obesity (sarcopenia and relative increase of visceral fat) and mitochondrial dysfunction are thought to cause the insulin resistance whereas gradual exhaustion of β-cell function is thought to cause decline of initial phase of insulin secretion [2, 4, 5]. DM has been associated with an increased risk of developing physical disability in older adults [5, 6]. DM is considered an independent risk factor in older people for fall and developing hip fractures [7]. Older individuals with DM have higher rates of premature death and of coexisting illnesses, such as hypertension, heart disease, cerebrovascular disease and stroke, than those without DM [8]. Likewise, older patients with DM are at greater risk for several common geriatric syndromes, such as polypharmacy, depression, cognitive impairment, urinary incontinence, injurious falls and persistent pain [9].
A common geriatric syndrome, frailty is a state of vulnerability and a consequence of cumulative decline in multiple physiological systems over a lifespan [10, 11]. It is strongly associated with adverse outcomes, including falls, disability, hospitalization, care home admission and mortality [10-14]. Thus, frailty in elderly people has become an important worldwide concern [11, 15]. DM is suggested to be linked with increased risk of frailty [16-18]. Currently, there are few comprehensive data on the risk factors related to the degree of frailty in elderly patients with type 2 DM. In this review, we discuss the pathogenesis and risk factors affecting the severity of frailty in elderly DM patients.
While the concept of frailty and its practical definition may not be completely coincident [19], frailty has generally been defined in two ways, namely from either a physical or a combination of physical and psychosocial aspects. In 2001, Fried and colleagues [11] proposed their landmark definition of the frailty phenotype, which assessed frailty in the narrow sense by measuring five physical components (weight loss, exhaustion, weakness, slowness and reduced physical activity) [11]. They also defined a clinical frailty phenotype, which was identified by the presence of three or more of the five components [11]. Following this, Rockwood et al. [20] and Mitnitski et al. [21] proposed an accumulated deficits model with a broader definition of frailty based on a comprehensive geriatric assessment, which considered not only the physical aspects, but also the psychosocial aspects of frailty. These more broadly defined models of frailty are used for setting goals for diabetes medications in Europe and America [22-25]. One such broadly defined model, the Clinical Frailty Scale (CFS), originally developed by Rockwood K et al. [26], has been verified as a useful rapid assessment tool of frailty [23, 24] and adverse outcome prediction [27]. The original one [26] was modified by the same group and the information is available at http://geriatricresearch.medicine.dal.ca/clinical_frailty_scale.htm. Briefly, the CFS is scored from 1 (very fit) to 9 (terminally ill) based on clinical judgment [26]. An increase in the category number of the scale significantly increases the risk of death. CFS contains 9 stages (1 very fit, 2 well, 3 managing well, 4 vulnerable, 5 mildly frail, 6 moderately frail, 7 severely frail, 8 very severely frail and 9 terminally ill).
Sarcopenia is characterized by the progressive and generalized loss of skeletal muscle mass and strength that occurs with advancing age [28]. Sarcopenia is the most common potential cause of the physical aspect of frailty and is often used as a surrogate for frailty. The degree of sarcopenia thus creates periodicity of frailty [29]. The physical phenotype of the frail elderly consists of clinical symptoms that can be linked to sarcopenia such as weight loss, exhaustion, weakness, slowness and reduced physical activity [11]. The measurement of gait speed is recommended as a screening tool for the detection of sarcopenia [28, 29]. The 2010 European Working Group on Sarcopenia in Older People defined three stages of this process [28]: presarcopenia as simply loss of muscle mass, sarcopenia as muscle loss that occurs in conjunction with loss of strength or physical performance, and severe sarcopenia as muscle loss with both strength and physical performance loss. Sarcopenia and frailty may share a similar pathway for multiple pathologic processes in older people, and DM may promote their development as discussed in the next section.
In a systemic review, the reported prevalence of frailty in the community varied enormously (range 4.0–59.1%) and the overall weighted prevalence of frailty was 10.7% [30]. When screened for in a population of people with DM, the prevalence of frailty also varied and was reported to be between 5–48% [31]. Several studies have shown that older DM patients were more likely to be frail than their non-DM counterparts [19-21, 32-35]. These studies also reported that DM patients with frailty had a higher mortality rate than non-frail DM patients, with the presence of frailty an independent risk factor for mortality. The German ESTHER study and the Whitehall II Prospective Study demonstrated that the prevalence of frailty was 3–5 fold higher in elderly patients with DM than in those without DM [32].
The presence of frailty/sarcopenia depends on deterioration in muscle and nerve function, declining cardiopulmonary reserve and loss of executive function. DM tends to cause impairment in each of these systems, thus leading to the loss of homeostasis and vulnerability to various stressors. General loss of self-management ability in DM patients [36] may have some impact on the development of sarcopenia/frailty. Moreover, there may be positive feedback between DM and frailty/sarcopenia, as frailty is more common in DM patients while DM is more progressive in the frail elderly. Insulin is known to increase the rate of protein synthesis and decrease protein degradation in muscle [37]. Thus, in patients with type 2 DM, insulin resistance may lead to impairment of muscle strength and performance [38].
Frailty/sarcopenia in DM and non-DM patients are produced by several other components, including hormones, inflammation, neurologic factors, nutrition and activity components. From a nutritional aspect, many older people do not have sufficient dietary intakes or protein intakes, resulting in a reduction in lean body mass and increased functional disability by sarcopenia/frailty [39, 40]. Serum albumin levels and frailty have an inverse relationship in older people [41], and hypoalbuminemia is also induced by inflammation along with chronic disease [42]. The current recommended intake of dietary protein is 0.8 g/kg/day, but 40% of people aged over 70 years did not meet this requirement [43]. A low protein diet (protein intake below the above value) resulted in a significant decline in muscle mass and strength in older women [44]. Further, older people taking the recommended allowance of dietary protein showed a negative nitrogen balance, suggesting a necessity for a higher protein intake than the recommended allowance to maintain their skeletal muscle [45]. Community-dwelling older subjects with DM are at risk of malnutrition compared with those without DM, suggesting a causal relationship between malnutrition and functional decline in DM patients [46]. Subclinical deficiencies in vitamin B12 have been often found in type 2 DM patients, especially those taking metformin [47]. Vitamin B12 deficiency is well known to cause neurogenic disorders including bathyanesthesia and muscle weakness, thus increasing the likelihood of falling over [47].
Low-grade inflammation elicited by proinflammatory factors (e.g. interleukin-1, interleukin-6, tumor necrosis factor-α, interferon-γ) may have an influence on both the aging process as well as development of DM [48-50]. Age-related alterations in various hormonal levels are also associated with frailty/sarcopenia. The insulin-like growth factor (IGF)-1 and type 1 IGF receptor (IGF1r) axes are well known to be linked with frailty via influences on muscle strength, bone strength and mobility [38]. IGF-1 has been shown to be lower in those with DM [51] and to play a role in the protein synthesis of muscle. Testosterone also increases protein synthesis and satellite cell proliferation in muscle, thus producing increased muscle mass [51]. Many meta-analyses have revealed that 30–50% of middle-aged and elderly men with type 2 DM show decreased blood testosterone levels. In a prospective study, men with relatively high blood testosterone were reported to show a 42% risk reduction for future type 2 DM [52]. A similar relationship has been observed between metabolic syndrome and serum testosterone levels, including from our study [53, 54]. When the intrinsic serum testosterone value of young healthy adults was lowered by the administration of a gonadotropin-releasing hormone analogue, an increase in body fat percentage and a decrease in resting energy consumption were observed [55]. Likewise, male androgen receptor knockout mice exhibited late onset visceral fat obesity due to decreased energy expenditure [56]. These results suggest that a decrease in testosterone concentration with aging and diabetes causes fat accumulation and muscle reduction in men. Vitamin D levels are also known to be relatively lower in those with DM, and studies in animals and humans suggest that vitamin D deficiency may contribute to β-cell dysfunction, insulin resistance and inflammation that may result in type 2 DM [57]. The overall schematic representation of pathophysiological relationship between sarcopenia/frailty and Type 2 DM was summarized in Fig. 1.

Schematic representation of the pathophysiological relationship between sarcopenia/frailty and diabetes.
↓, decreased; CVD, cardiovascular diseases; OHA, oral hypoglycemic agent
We enrolled 132 elderly patients with type 2 DM (the mean age, HbA1c and body mass index were 78.3 ± 8.0 years old, 7.0 ± 1.0%, and 23.0 ± 4.4 kg/m2, respectively), categorized the patients as having frailty or not using CFS and attempted to identify the risk factors of frailty [58]. Patients with a CFS score of 1–4 and 5–9 were defined as non-frail and frail, respectively. Nineteen cases of insulin therapy, 44 cases of sulfonylureas or glinide and 69 cases of other type 2 DM medications were observed, but there were no significant differences in the type or number of type 2 DM medications used between the frail and non-frail groups. Multiple regression analysis revealed that advanced age, low levels of albumin, high density lipoprotein cholesterol, systolic blood pressure, HbA1c, total cholesterol and body weight were regarded as strong risk factors for CFS, with age and albumin the strongest. HbA1c was a risk factor for frailty; independent of hemoglobin. Malnutrition in elderly type 2 DM patients may thus contribute to frailty. The malnutrition may be a constitutional symptom or stem from strict diet control that is self-guided or guided by their doctor. HbA1c level was not a U-shaped risk for frailty, suggesting that relatively good glycemic control may be a risk for frailty in elderly type 2 DM patients [58].
Regarding the effect of older age in patients with type 2 DM, a U-shaped risk of disease was associated with blood glucose levels or HbA1c values i.e. not only high but also low levels were associated with dementia [59], stroke [60], fall [61] and increased mortality [2, 62] (Fig. 2). In a retrospective cohort study of elderly type 2 DM patients (n = 71,092) with an average age of 71 years, the relation between HbA1c value and mortality at the start of the observation showed a U-shaped curve in the 60th, 70th and 80th [2]. Namely, mortality rates at HbA1c levels of 6% and 7% were significantly lower [2]. It should be noted that hypoglycemia and/or the type of medication in type 2 DM patients might be important contributing factors for the above incidences [2, 59-62]. Indeed, Schwartz et al. reported that the risk of fall in 446 older diabetic patients (mean age 73.6 years) was 4-fold higher in an insulin treatment group with HbA1c values less than 6% than in those with HbA1c values more than 8% [61]. However, a U-shaped association between mortality and HbA1c values has also been reported in non-diabetic adults [63], suggesting that low HbA1c itself may be a risk factor for increased mortality, independent of diabetic treatment and/or hypoglycemia.
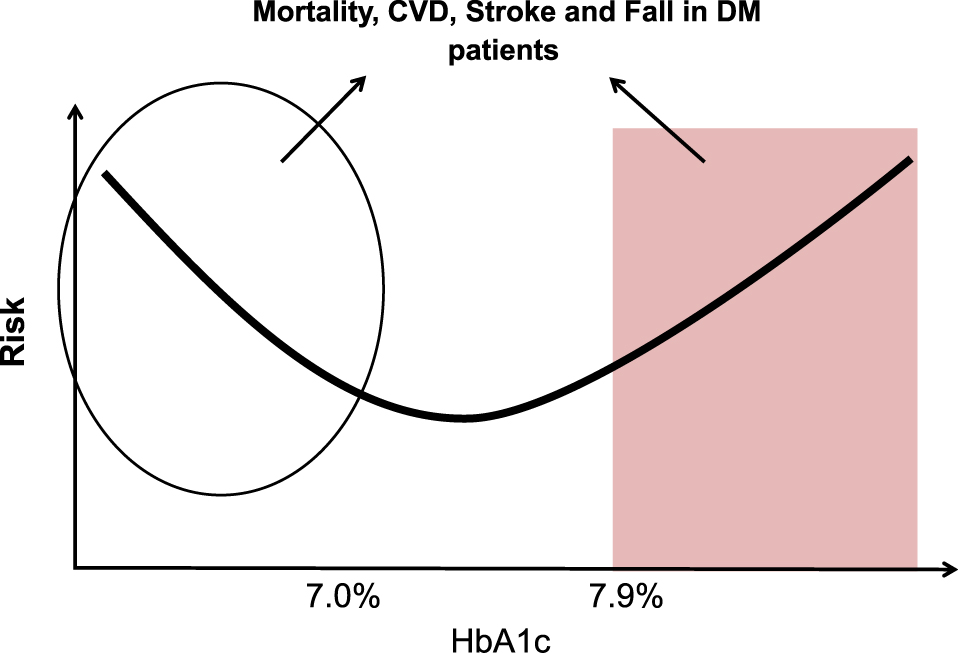
Schematic representation of relationship between HbA1c levels and various diseases or events.
A U-shaped risk of disease was associated with blood glucose levels or HbA1c values i.e. not only high but also low levels were associated with dementia [59], stroke [60], fall [61] and increased mortality [2, 62]. However, a U-shaped association between mortality and HbA1c values has also been reported in non-diabetic adults [63], suggesting that low HbA1c itself may be a risk factor for increased mortality, independent of diabetic treatment and/or hypoglycemia. The circle and the box indicate areas of relatively lower and higher HbA1c, respectively, thus indicating that lower and higher HbA1c may be associated with various events including frailty in elderly DM patients. The approximate range of low or high HbA1c values indicated in the figure is schematic representation of various reports. The actual high or low HbA1c values were varied by the difference of the subjects and events [2, 59-63].
Several studies suggest that high blood glucose is more strongly associated with increased frailty risk than low blood glucose based on a narrowly defined frailty scale [16, 17], suggesting a J-shaped association of HbA1c with incidence of frailty. However, in a recent study, a U-shaped relationship between glucose and frailty (as evaluated by a narrowly defined frailty scale) was reported, with glucose levels lower than 8.8 mmol/L and higher than 10 mmol/L associated with increased risk of frailty and levels about 9.4 mmol/L associated with the lowest risk [64]. This study [64] was the first to show an association between low HbA1c and frailty in elderly type 2 DM patients using a narrowly defined frailty scale. In our study [58], a U-shaped risk between HbA1c and frailty was not observed; low HbA1c was similarly associated with frailty in elderly type 2 DM patients using the broadly defined frailty scale, CSF. Pilotto et al. [25] demonstrated a U-shaped risk between blood glucose levels and frailty as measured with the multiple prognostic index (MPI), another broadly defined frailty scale. The subtle difference among these studies [16, 17, 58, 64] may be the kind of frailty scale used i.e. a narrowly defined (physical based) scale such as Fried’s measurement or a broadly defined scale like the CFS or MPI. In Japan, the relationship between frailty and glucose control has not been well investigated except in our study [58] though there have been several reports on related conditions including associations between higher HbA1c values and increased risk of retinopathy [65] and mortality [66] and a U-shaped relationship between HbA1c values and risk of stroke [60].
Higher glucose levels or HbA1c may contribute to an increased risk of frailty through several potential mechanisms. The most important factor may be insulin resistance or insulin depletion associated with elderly DM [2, 4, 5, 67], since insulin exerts its anabolic effect on muscle [37]. In addition, hyperglycemia has an additive or independent effect on microvascular complications [68], possibly leading to the impairment of multiple organs associated with frailty [69]. In this mechanism, glucose-mediated cellular oxidative stress [70] and chronic inflammation [71-73] might be involved in the process of frailty development. Hyperglycemia also causes skeletal muscle mitochondrial dysfunction [74], which may partly explain muscle weakness and poor muscle quality in elderly DM patients [75]. Poor muscle quality in patients with type 2 DM becomes even poorer with longer duration of DM and higher levels of HbA1c [2]. Interestingly, higher HbA1c levels are reported to be associated with greater walking difficulties [76]. Kalyami et al. has reported that the difficulty in self-reported walking or performance based on lower extremity function is greater in persons with HbA1c ≥8% compared with persons with <5.5% at baseline [16]. These findings seem to be a plausible explanation of the relationship between hyperglycemia and frailty. While poor glycemic control may cause frailty, the disability caused by frailty may further enhance the poor glycemic control.
However, the mechanism for the elevated risks of frailty with relatively lower glucose levels or lower HbA1c levels in elderly DM could not be simply explained by the underlying mechanism between DM and frailty. Regardless of consciousness or unconsciousness in elderly DM patients, malnutrition or insufficient dietary intake seems to be the most plausible reason, which may link frailty with low HbA1c levels. The above condition may be further modified or enhanced by the presence of diabetic complications, medications including hypoglycemic agents and coexisting geriatric syndrome including cognitive impairment as already discussed. On the other hand, general disability caused by frailty may enhance malnutrition or insufficient dietary intake in elederly DM.
While previous studies or our study that have focused on the relationship between glycemic control and frailty are cohort studies [16, 17, 25, 64] or a cross-sectional study [58], it is difficult to completely rule out the effect of duration or changes of glycemic control or the HbA1c level on the severity of frailty in any study. Nevertheless, these findings are important for the control of elderly DM patients.
In the United Kingdom Prospective Diabetes Study (UKPDS), which excluded subjects over the age of 65 years, intensive glycemic control reduced microvascular complications [77]. Similar findings were reported from the Kumamoto study, targeting Japanese type 2 DM patients with a mean age of approximately 50 years [78]. Thus, it is accepted that microvascular complications in DM can be prevented by lowering HbA1c. Unexpectedly, tight glycemic control did not reduce all-cause mortality in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) and the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trials, which included older people [79, 80]. It is possible that the different outcomes of the above studies might be affected by the age of the subjects enrolled. The study involving older people may have produced negative results because of aging-associated contradictory metabolism.
As already stated, both good and bad (U-shaped curve) HbA1c levels [64] or low levels of HbA1c in patients with type 2 DM [58] are associated with frailty. Thus, traditional risk factors for metabolic syndrome and/or cardiovascular disease such as high blood glucose, obesity, high cholesterol and hypertension in middle age may shift from being unfavorable to favorable in old age. This kind of shift has been called a metabolic shift or reverse metabolism [81, 82]. Reverse metabolism has been shown in several studies targeting people aged ≥85 years, whereby hypertension, high cholesterol and high blood glucose did not predict risk of cardiovascular mortality [83]; rather, lower levels of body mass index, diastolic blood pressure and total cholesterol and high density lipoprotein cholesterol predicted total mortality [82]. The reverse metabolic syndrome is probably attributable to malnutrition and/or chronic disorders [84].
Importantly, it has been reported in the UKPDS targeting elderly type 2 DM patients (60–80 years) that the higher the age of DM onset, the smaller the effect of glycemic control on the extension of life expectancy [85]. In addition, the effect of glycemic control on the extension of life expectancy in elderly DM patients was notably decreased as the number of disease complications increased [85]. Therefore, the glycemic targets of elderly patients with DM should be flexible and determined upon careful consideration of the condition of individual patients.
The results of a prospective large-scale clinical intervention study on elderly diabetes mellitus (J-EDIT: Japanese Elderly Diabetes Invention Trial) conducted in Japan clarified that the most significant contributing factor to the reduction in death and cardiovascular disease occurrence in elderly DM patients was not strict glucose control, but mild blood glucose management with total management of cardiovascular risks [60]. The Japan Diabetes Society/Japan Geriatrics Society Joint Committee recently published a consensus statement regarding the glycemic targets of elderly patients with diabetes [86]. In this statement, a lower limit for the glycemic target was proposed to ensure safer glycemic control in those who are likely to be at risk of severe hypoglycemia. This consensus should be kept in mind not only with regard to hypoglycemic risk but also susceptibility to severe frailty from low HbA1c levels.
Fig. 1 summarizes the overall schematic representation of the pathophysiological relationship between glycemic control and frailty in type 2 DM.
Sarcopenia is the most important target for the management of frailty in patients with DM [87]. Exercise (mainly resistance training) in combination with nutritional intervention (adequate protein and energy intake) is currently a kind of standard method for prevention and treatment of sarcopenia [88]. Both aerobic and resistance exercise training have been shown to prevent the decline in muscle mass and strength with age [89]. Improvements in muscle strength can be achieved with one resistance exercise training per week [90]. Although older adults who exercise may have additional protein requirements, studies investigating whether nutritional supplementation in combination with resistance exercise can augment muscle strength and mass have yielded inconsistent results [91]. Although pharmacologic agents to prevent or treat sarcopenia are not essentially available to date, some trials have been done. One direction under investigation is the development of selective androgen receptor modulators (SARMs), a group of synthetic compounds that bind to androgen receptors on many cell surfaces and specifically activate or inhibit selective functions of the androgen receptor in a tissue-specific manner. This selective activation/inhibition could encourage muscle growth while at the same time prevent some of the unwanted aspects of hormone therapy, such as prostate growth in men, and minimize the virilization effects on women. It is expected that SARMs will be used in the treatment of sarcopenia, but they must not adversely cause cardiovascular risk or prostate stimulation. Currently, there are several SARMs in clinical trials [92]. One such example is Ostarine, a SARM developed to help with muscle wasting. In a double-blind clinical study of 120 healthy elderly people aged 60 years or older who took part in the 2-week treatment, the administration of 3 mg Ostarine showed a significant improvement of lean body mass and physical ability by step stair climbing, compared to the placebo treatment group [93]. Inhibition of myostatin, which negatively regulates skeletal muscle growth, may be another promising strategy for the treatment of muscle atrophic disorders, such as muscular dystrophy, cachexia and sarcopenia. In this respect, myostatin inhibitory peptides have been developed although they are still at an experimental level [94]. The area of drug development targeting sarcopenia/frailty may be very promising in the aging society.
While recent studies in elderly type 2 DM patients have shown a J-shaped or U-shaped relationship of HbA1c with frailty, only low HbA1c is associated with frailty in our study. This difference may be due to the type of frailty scale used, i.e. a narrowly defined (physical based) scale or a broadly defined scale. Nevertheless, strict glycemic control for some elderly DM patients may cause a poor prognosis for mortality, cardiovascular events, dementia and frailty because of malnutrition, hypoglycemia and other unknown mechanisms.
The authors essentially have no conflict of interest regarding this study. T. Yanase was supported financially in his research by MSD K.K.; Sanofi K.K.; Takeda Pharmaceutical Co., Ltd.; Daiichi Sankyo Company Ltd.; Sumitomo Dainippon Pharma Co., Ltd.; Sanwa Chemistry Co., Ltd.; Eli Lilly Japan K.K.; Novo Nordisk Pharma Ltd.; Novartis Pharma K.K.; Kowa Company Ltd.; Boehringer Ingelheim GmbH; and Fujifilm Pharma Co., Ltd.
We thank Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.