Abstract
We compared treatment satisfaction between daily dipeptidyl peptidase-4 (DPP-4) inhibitors and a weekly DPP-4 inhibitor in patients with type 2 diabetes. The study was a 12-week, open-label, randomized, multicenter, controlled trial. Participants were Japanese patients with type 2 diabetes who had received daily DPP-4 inhibitors for more than 3 months. Patients were randomly assigned to a treatment cohort: (1) a group that continued taking daily DPP-4 inhibitors (daily group); or (2) a group that switched from daily DPP-4 inhibitors to a weekly DPP-4 inhibitor, trelagliptin (weekly group). The primary outcome was the change in treatment satisfaction levels from baseline to 12 weeks between the two groups, according to Diabetes Treatment Satisfaction Questionnaire (DTSQ) and Diabetes Therapy-Related Quality of Life (DTR-QOL) questionnaire scores. The changes in glycemic control and body weight were also assessed. Of 49 patients initially enrolled in the study, 47 completed the study. The change in DTSQ scores in the weekly group was not significantly different from that in the daily group. However, the improvements in total score and subscale domains 1 and 2 in the DTR-QOL analysis, which relate to burden on social/daily activities and anxiety/dissatisfaction with treatment, were significantly greater in the weekly group than the daily group (p = 0.048, 0.013 and 0.045, respectively). Mean changes in glycated hemoglobin levels and body weight were comparable between the groups. Switching from daily DPP-4 inhibitors to a weekly DPP-4 inhibitor, trelagliptin, could partially improve treatment satisfaction levels in patients with type 2 diabetes without affecting glycemic control.
THE ULTIMATE GOAL OF TREATMENT for patients with diabetes mellitus is to ensure quality of life (QOL) and a long lifespan similar to those of healthy people by preventing the onset or progression of diabetes-related complications. To achieve this goal, intensive metabolic controls, especially adequate glycemic control, are necessary [1]. Therefore, it is important for patients to maintain a favorable lifestyle and continue the necessary treatment with good medication adherence over a long period time. Patients who are dissatisfied with their treatment are less likely to adhere to that treatment [2, 3], and non-adherence to treatments for type 2 diabetes can result in poor glycemic control [4-7], which increases the risk of complications leading to disease deterioration.
Therefore, a subjective assessment of patients’ treatment satisfaction or QOL is an important factor in assessing diabetes treatment [8]. The Diabetes Treatment Satisfaction Questionnaire (DTSQ) is widely used to quantitatively evaluate treatment satisfaction in patients with diabetes. Another tool, the Diabetes Therapy-Related Quality of Life (DTR-QOL) questionnaire, was recently developed and can be used to evaluate the influence of diabetes treatment on patient QOL with good reliability and validity [9]. The DTR-QOL consists of 4 domains with 29 items: “burden on social activities and daily activities” (13 items), “anxiety and dissatisfaction with treatment” (8 items), “hypoglycemia” (4 items), and “satisfaction with treatment” (4 items) [10, 11].
Compared with conventional agents, such as sulfonylureas and insulin injection therapy, DPP-4 inhibitors are associated with a lower incidence of hypoglycemia because they enhance insulin secretion in a glucose-dependent manner [12]. DPP-4 inhibitors are the most frequently used oral hypoglycemic drugs among the patients with type 2 diabetes in Japan who are treated with hypoglycemic drugs [13]. Seven types of Once- or twice-daily DPP-4 inhibitors are in use in Japan. A weekly DPP-4 inhibitor, trelaglipin, was approved for the first time in Japan in May 2015. The DPP-4 inhibitory activity of trelaglipin continues for up to 168 hours after administration. Studies have shown that trelagliptin improves blood glucose levels as effectively as DPP-4 inhibitors taken orally once a day [14], and the frequency of side effect occurrence was also comparable with daily DPP-4 inhibitors. This weekly DPP-4 inhibitor has the potential to reduce the medication burden of patients, which could improve their QOL. However, no studies have yet investigated the difference in treatment satisfaction levels between daily and weekly DPP-4 inhibitors among patients with diabetes.
To elucidate whether a weekly DPP-4 inhibitor, trelagliptin, could improve satisfaction levels or QOL in patients with type 2 diabetes, we conducted a 12-week, open-label, randomized, multicenter trial using two evaluation methods, the DTSQ and the DTR-QOL.
Materials and Methods
Study population
Participants were Japanese patients with type 2 diabetes who had received daily DPP-4 inhibitors for more than 3 months, who were recruited via four medical institutions (Hokkaido University Hospital, Manda Memorial Hospital, Kurihara Clinic and Aoki Clinic). The inclusion criteria were as follows: outpatient with type 2 diabetes; aged 20–80 years; and a total number of medications other than daily DPP-4 inhibitor of 1–6. The exclusion criteria were: type 1 diabetes; pre- or post-surgery; and patients with malignant tumor, severe infection, severe trauma, pregnancy, severe liver dysfunction (aspartate aminotransferase or alanine aminotransferase >100 U/L), or hypersensitivity to the drugs used in this study. During the study period, diet and exercise were maintained as usual, and change to the dose and frequency of all medications other than DPP-4 inhibitors were prohibited. Adverse effects, such as hypoglycemia and gastrointestinal symptoms, were monitored during the trial. Hypoglycemia was defined as blood glucose <70 mg/dL or the presence of hypoglycemic symptoms.
Protocol
This was a 12-week, multicenter, open-label, prospective, randomized controlled trial. After consent was provided, participants were randomly assigned to either continue taking daily DPP-4 inhibitors (daily group) or to switch from daily DPP-4 inhibitors to a weekly DPP-4 inhibitor, trelagliptin (weekly group). Randomization and allocation of participants were performed by a company independent from the medical institutions involved. Allocation factors comprised age at screening, total number of oral medications, work (yes or no), and which of the four medical institutions they attended. Physical examination data and medical history were collected via clinical examination.
The primary outcome of this study was the change in patients’ treatment satisfaction levels, as assessed by questionnaires. The DTSQ and DTR-QOL were performed simultaneously at each medical institution at the time of randomization (baseline) and at week 12 of the study. The patients filled out the questionnaires by themselves in private to avoid any influence of physicians or medical care providers. The changes in the scores on both questionnaires from baseline to week 12 were compared between the two groups. DTSQ and DTR-QOL are self-administered questionnaires assessing patient-reported outcomes. The DTSQ consists of eight items, six of which are designed to measure a person’s satisfaction (current treatment, convenience, flexibility, understanding, willingness to recommend, and willingness to continue), while the remaining two items relate to concern about hyperglycemia and hypoglycemia. The DTSQ scores for the first six items were summed and assessed via rating on a scale from +6 (“very satisfied”) to 0 (“very dissatisfied”). The total scores of the first six items of the DTSQ ranged from +36 to 0, with higher scores denoting greater treatment satisfaction. The perceived frequency of hyperglycemia and hypoglycemia were rated on a scale from +6 (“most of the time”) to 0 (“never”) [15]. Patients completed a Japanese version of the DTSQ in this study [16].
The DTR-QOL was established by Ishii et al. in 1995 to evaluate patient QOL [9]. The DTR-QOL includes 29 items, and responses are scored on an 8-point scale, from +7 to 0. The assessment covers four domains: domain 1, burden on social activities and daily activities; domain 2, anxiety and dissatisfaction with treatment; domain 3, hypoglycemia; and domain 4, satisfaction with treatment. Total DTR-QOL score and subscale scores were calculated as shown below and were converted to a score of 0 (lowest QOL) to 100 (highest QOL) as described previously [9]. Total DTR-QOL score = (overall points from questions 1 to 29 – 29) × (100/174). The scores for questions 26 to 29 were reversed so that a lower score indicated better QOL. Subscale scores were calculated in the same way: domain 1 = (total points from questions 1 to 13 − 13) × (100/78); domain 2 = (total points from questions 14 and 19 to 25 – 8) × (100/48); domain 3 = (total points from questions 15 to 18 − 4) × (100/24); and domain 4 = (total reversed points from questions 26 to 29 − 4) × (100/24). The secondary outcomes were changes in glycated hemoglobin (HbA1c), plasma glucose, and weight.
Drug adherence was also evaluated in this study. All participants were given a medication diary when they entered the study. When they took the medications, they filled in check marks against each prescribed medicine every day. Drug adherence was calculated and evaluated based on the numbers of check marks in the diaries.
Statistical analysis
Based on a previous study, which analyzed the change in DTSQ scores in Japanese patients with type 2 diabetes [17] a power calculation determined that a sample size of 22 individuals per group was required to have at least 80% power to detect a difference between treatments. Statistical significance was assumed at the 5% level. All tests were two-sided. Assuming a dropout rate of 10%, the sample size was set at 24 patients per group.
Results are expressed as means ± SD. Differences between the two groups were evaluated using unpaired t-tests and the Wilcoxon test. Correlation was evaluated by Spearman rank-order correlation analysis. p-values <0.05 were considered statistically significant. Data were analyzed using JMP 11 (SAS Institute Inc., Cary, NC, USA) and Microsoft Excel Statistics 2010 for Windows.
Ethics statement
The trial was registered with the University Hospital Medical Information Network (UMIN) Center under the identifier UMIN 000022858. This study was conducted with the approval of the Institutional Review Board of the Hokkaido University Hospital Clinical Research and Medical Innovation Center (701–7636) and conformed to the provisions of the Declaration of Helsinki. Signed informed consent was obtained from all participants.
Results
Patient enrollment and baseline characteristics
Forty-nine patients (33 men and 16 women) were initially enrolled in the study. Each patient was randomly assigned to either the daily group or the weekly group, and 47 patients completed the study (daily group, 23 patients; weekly group, 24 patients). One patient in the daily group moved home and therefore withdrew from the study. One patient in the weekly group withdrew from the study because of an allergic reaction (Fig. 1). The final follow-up rate was 96%. The baseline clinical and metabolic characteristics of both groups are shown in Table 1. There were no statistically significant differences between the two groups in the baseline characteristics before randomization (age, sex, presence of work, body weight, plasma glucose level, HbA1c, total number of oral medications, oral administration daily frequency, type of daily DPP-4 inhibitors). Baseline DTSQ and DTR-QOL scores, including total and subscale scores, were also comparable between the two groups.

Table 1
Baseline characteristics of the participants
|
total |
Daily Group |
Weekly Group |
p |
| N |
49 |
24 |
25 |
|
| Age (years) |
62.0 ± 10.9 |
61.8 ± 11.0 |
62.2 ± 11.1 |
0.897 |
| Sex (male/female) |
33/16 |
17/7 |
16/9 |
0.762 |
| Work (yes/no) |
26/23 |
12/12 |
14/11 |
0.778 |
| Body weight (kg) |
63.8 ± 12.7 |
63.9 ± 11.9 |
63.8 ± 13.6 |
0.971 |
| Body Mass Index (kg/m2) |
23.8 ± 4.0 |
23.6 ± 4.1 |
23.9 ± 4.0 |
0.804 |
| Plasma glucose (mg/dL) |
140.0 ± 36.9 |
140.8 ± 39.4 |
138.8 ± 35.0 |
0.846 |
| HbA1c (%) |
6.7 ± 0.5 |
6.8 ± 0.5 |
6.7 ± 0.5 |
0.870 |
| eGFR (mL/min/1.73 m2) |
76.6 ± 17.4 |
75.4 ± 19.1 |
78.5 ± 16.2 |
0.586 |
| Number of concomitant medicine |
3.1 ± 1.3 |
3.3 ± 1.2 |
2.9 ± 1.5 |
0.335 |
| Oral administration times a day |
2.0 ± 0.7 |
2.0 ± 0.7 |
1.9 ± 0.7 |
0.696 |
| Kinds of daily DPP-4 inhibitors |
| Sitagliptin (n) |
23 |
10 |
13 |
|
| Vildagliptin (n) |
6 |
4 |
2 |
|
| Teneligliptin (n) |
7 |
2 |
5 |
|
| Others (n) |
13 |
8 |
5 |
|
| Antidiabetic medicine other than DPP-4 inhibitors |
| Biguanide (n) |
35 |
21 |
14 |
|
| Sulfonylurea (n) |
9 |
7 |
2 |
|
| SGLT-2 inhibitor (n) |
7 |
3 |
4 |
|
| Alpha-glucosidase inhibitor (n) |
4 |
2 |
2 |
|
| Thiazolidinedione (n) |
3 |
2 |
1 |
|
| Glinide (n) |
2 |
1 |
1 |
|
| Antihypertensive medicine |
| Angiotensin II receptor blocker (n) |
19 |
8 |
11 |
|
| Calcium channel blocker (n) |
14 |
7 |
7 |
|
| Diuretic medicine (n) |
2 |
2 |
0 |
|
| Selective aldosterone blocker (n) |
1 |
1 |
0 |
|
| Lipid lowering medicine |
| Statin (n) |
24 |
13 |
11 |
|
| Fibrate (n) |
3 |
2 |
1 |
|
| Ezetimibe (n) |
2 |
1 |
1 |
|
Number of medications refers to the mean number of medications other than DPP-4 inhibitors. Daily Group: the group that continued taking daily DPP-4 inhibitors. Weekly Group: the group that switched from daily DPP-4 inhibitors to trelagliptin.
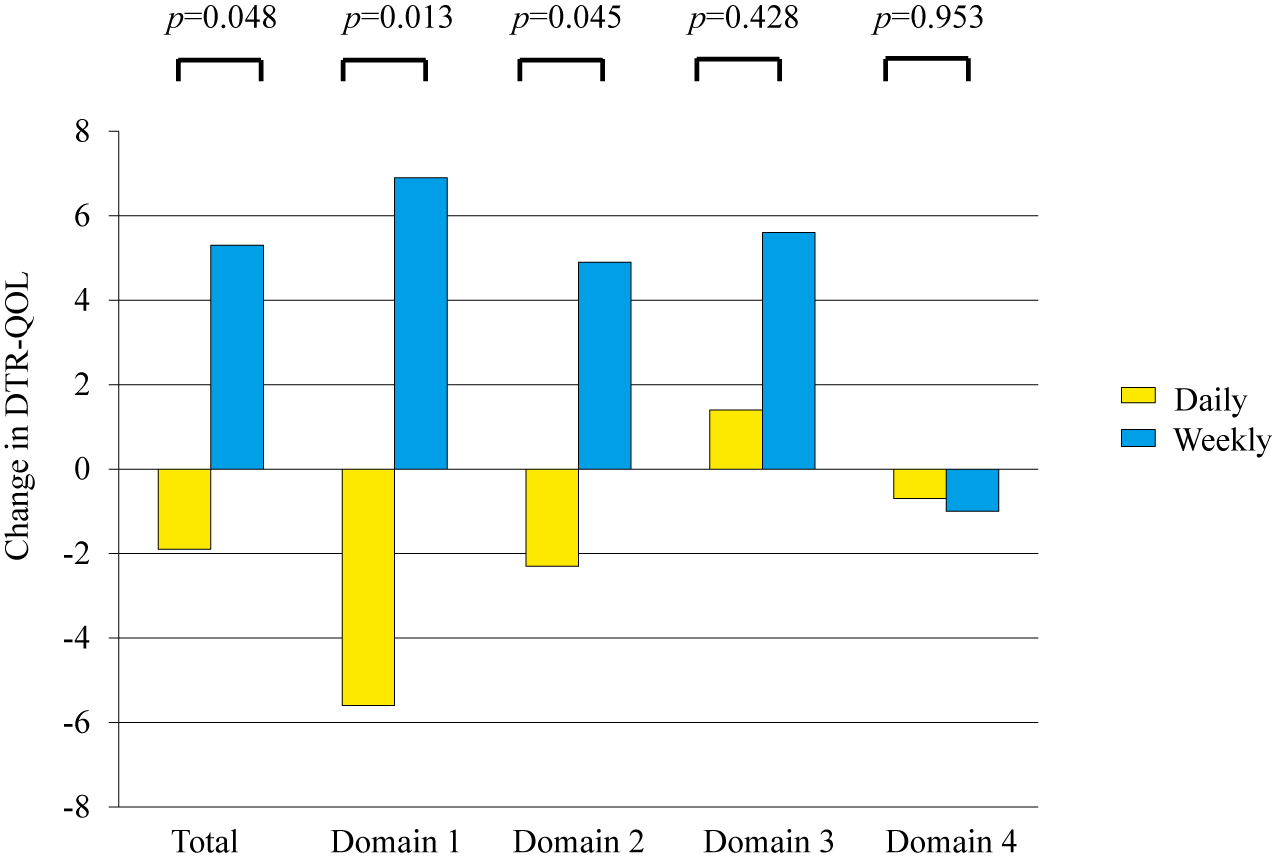
Patient-reported outcome measures were assessed in all 47 patients (23 and 24 patients in the daily and the weekly groups, respectively). At baseline, there was no significant difference in total DTSQ score (on items 1 and 4–8) between the two groups (25.6 ± 7.2 in the daily group vs. 22.9 ± 6.5 in the weekly group). The change in total DTSQ score from baseline to week 12 were not significant between the two groups (p = 0.655) (Table 2). However, the change in total DTR-QOL score from baseline to week 12 in the weekly group significantly improved compared with that in the daily group (total score, 75.4 ± 9.1 [baseline] to 73.5 ± 12.0 [week 12] in the daily group vs. 71.8 ± 13.1 [baseline] to 77.1 ± 11.4 [week 12] in the weekly group; p = 0.048). In the subscale analysis, there were significantly greater changes from baseline to week 12 in domain 1 (burden of social activities/personal activities) and domain 2 (anxiety and dissatisfaction with treatment) in the weekly group than in the daily group (p = 0.013 and 0.045, respectively). There was no significant difference in the changes in domains 3 and 4 between the groups (Table 2, Fig. 2). Further analysis of cost-effectiveness was performed because there were small changes in the cost to the patients of the medication when they switched to trelagliptin, even when the standard dose of DPP-4 inhibitors had been used in all the participants. Interestingly, total, domain 1 and domain 2 DTR-QOL scores showed significant improvement in the patients whose medication fee decreased after switching to trelagliptin (n = 7) compared with those whose payment remained unchanged (n = 17) (p = 0.034, 0.018, and 0.025, respectively).
Table 2
Changes in DTR-QOL scores and DTR-QOL scores from baseline to week 12
|
Daily Group |
|
Weekly Group |
p |
| Previous score |
Changes |
Previous score |
Changes |
| DTSQ |
|
|
|
|
|
|
| Total score (No. 1, 4~8) |
25.6 ± 7.2 |
0.7 |
|
22.9 ± 6.6 |
–0.2 |
0.655 |
| Subscale score |
|
|
|
|
|
|
| No. 1 Treatment satisfaction |
4.7 ± 1.1 |
–0.1 |
|
4.2 ± 1.3 |
–0.2 |
0.910 |
| No. 2 Frequency of hyperglycemia |
1.9 ± 1.7 |
0.4 |
|
1.7 ± 1.5 |
0.1 |
0.425 |
| No. 3 Frequency of hypoglycemia |
1.1 ± 1.3 |
0.2 |
|
0.4 ± 0.6 |
0.3 |
0.767 |
| No. 4 Convenience |
4.3 ± 1.5 |
0.04 |
|
3.5 ± 1.5 |
–0.04 |
0.855 |
| No. 5 Flexibility |
4.0 ± 1.7 |
0.4 |
|
3.6 ± 1.5 |
0.3 |
0.758 |
| No. 6 Understanding |
4.0 ± 1.4 |
0.1 |
|
3.9 ± 1.4 |
0.04 |
0.786 |
| No. 7 Recommend |
4.2 ± 1.6 |
0.3 |
|
3.9 ± 1.2 |
–0.3 |
0.208 |
| No. 8 Continue |
4.3 ± 1.5 |
–0.2 |
|
3.8 ± 1.2 |
–0.2 |
0.988 |
| DTR-QOL |
|
|
|
|
|
|
| Total score |
75.4 ± 9.1 |
–1.9 |
|
71.8 ± 13.1 |
5.3 |
0.048 |
| Subscale score |
|
|
|
|
|
|
| Domain 1 |
83.8 ± 12.1 |
–5.6 |
|
76.9 ± 16.7 |
6.9 |
0.013 |
| Domain 2 |
69.0 ± 14.2 |
–2.3 |
|
65.8 ± 15.6 |
4.9 |
0.045 |
| Domain 3 |
79.0 ± 19.7 |
1.4 |
|
81.9 ± 22.6 |
5.6 |
0.428 |
| Domain 4 |
59.4 ± 19.8 |
–0.7 |
|
57.1 ± 18.0 |
–1.0 |
0.953 |
The changes in values from baseline to week 12 are expressed as mean ± standard deviation.
As shown in Table 3, there was no significant difference in the mean change in HbA1c levels from baseline to week 12 between the daily group and the weekly group (+0.1 ± 0.3% vs. +0.2 ± 0.4%, respectively; p = 0.274). Similarly, there were no significant differences in the changes in plasma glucose level or body weight between the groups. Interestingly, the change in drug adherence during the study significantly improved in the weekly group compared with that in the daily group (–3.3 ± 3.3% vs. +5.7 ± 11.5%, respectively; p = 0.048) despite the comparable glycemic control.
Table 3
Changes in secondary outcomes from baseline to week 12
|
Daily Group |
|
Weekly Group |
p |
| Previous |
Changes |
Previous |
Changes |
| HbA1c (%) |
6.7 ± 0.4 |
0.1 |
|
6.8 ± 0.5 |
0.2 |
0.274 |
| Plasma glucose (mg/dL) |
139.0 ± 39.3 |
–6.3 |
|
134.8 ± 29.3 |
15.8 |
0.138 |
| Body weight (kg) |
63.7 ± 12.1 |
0.6 |
|
64.5 ± 13.5 |
0.3 |
0.322 |
| Drug adherence (%) |
94.6 ± 10.7 |
–3.3 |
|
93.9 ± 11.5 |
5.7 |
0.048 |
The changes in values from baseline to week 12 are expressed as mean ± standard deviation, and were analyzed using unpaired t-tests and the Wilcoxon test, respectively.
To reveal factors associated with patient satisfaction of the weekly group, we examined the relationships between treatment satisfaction and the clinical characteristics of the participants (Table 4). No correlations were found between treatment satisfaction scores (either DTSQ or DTR-QOL) and mean changes in HbA1c levels or body weight, or the number of concomitant medicines (Table 4). Among the baseline characteristics, a significant inverse correlation was observed between the change in treatment satisfaction score and patient age (ρ = –0.423, p = 0.040) (Table 4, Fig. 3). Another significant inverse correlation was observed between the change in treatment satisfaction score and the change in medication fee per day (ρ = –0.401, p = 0.049) (Table 4).
Table 4
Relationship between changes in DTR-QOL total score of the weekly group and various items
|
ρ |
p |
| Change in HbA1c (%) |
0.349 |
0.095 |
| Change in Body weight (kg) |
0.143 |
0.506 |
| Age (years) |
–0.423 |
0.040 |
| Number of concomitant medicine (n) |
–0.050 |
0.816 |
| Oral administration times a day (n) |
0.150 |
0.485 |
| Change in medication fee per day (yen) |
–0.401 |
0.049 |
Discussion
We examined the difference in patient satisfaction between daily and weekly DPP-4 inhibitors, using the DTSQ and DTR-QOL. This is the first study comparing daily and weekly DPP-4 inhibitors focusing on treatment satisfaction in patients with type 2 diabetes, There two evaluation methods were used for the first time in this study as well. Although there was no significant difference in DTSQ scores between the groups, a significantly greater improvement in DTR-QOL scores was detected in patients treated with a weekly DPP-4 inhibitor, trelagliptin, compared with those treated with conventional daily DPP-4 inhibitors.
A number of studies have evaluated patient satisfaction using the DTSQ [18]. However, in almost of those reports, treatment satisfaction was assessed in patients treated with injected therapies including insulin or glucagon-like peptide 1 receptor agonists [19-22]. We recently reported that a combined injection of basal insulin and glucagon-like peptide 1 receptor agonist improved DTSQ scores to a greater extent than multiple daily insulin injections in patients with type 2 diabetes [23]. From these reports, it appears that for injection therapies, lower injection frequency, lower HbA1c, and lower body weight were closely related to better treatment satisfaction among patients [23-25]. However, in this study, the participants did not receive an injected therapy, and switching from a daily to a weekly DPP-4 inhibitor did not alter HbA1c or body weight. Furthermore, we excluded patients who were not taking any medicine other than a daily DPP-4 inhibitor, meaning that participants had to take at least one medicine daily even after switching from a daily to a weekly DPP-4 inhibitor. These factors and the patients’ profiles likely made it difficult for the DTSQ score to reflect any difference resulting from a switch from a daily to a weekly DPP-4 inhibitor.
The reliability and validity of the DTR-QOL as a method for evaluating patient QOL was demonstrated in 2012 [9]. To date, no studies have investigated QOL using both DTSQ and DTR-QOL. Therefore, with no previous studies for comparison, the cause of the differences in the results of the DTSQ and DTR-QOL in our study is not clear. However, the DTR-QOL contains 29 questionnaire items, of which 25 relate to the patient’s level of satisfaction with their diabetes treatment, whereas the DTSQ contains only eight items, and only six of these relate to patient satisfaction [26]. Therefore, the evaluation of patient satisfaction by the DTR-QOL may be more detailed and provide less of an underestimation compared with evaluation by the DTSQ. This may be the major reason why DTR-QOL score significantly improved in the weekly group, despite the comparable DTSQ scores between the groups. Among the four domains of the DTR-QOL, significant improvements were observed for domain 1 (burden on social activities/personal activities) and domain 2 (anxiety and dissatisfaction with treatment) (Table 2, Fig. 2), despite the comparable effects of treatment on glycemic control and body weight between the groups (Table 3). Although several studies have reported close correlations between changes in DTR-QOL score and HbA1c or body weight [27-29], we did not observe any such significant correlations (Table 4).
To clarify which subgroups of patients experienced improved QOL upon switching from a daily to a weekly DPP-4 inhibitor, we evaluated the association between total questionnaire scores or each questionnaire item and patient baseline characteristics or variables that changed during the study. Among the baseline characteristics and the variables that changed over the course of the study, patient age and change in medication fee per day exhibited significant inverse correlation with total DTR-QOL score. Switching to a weekly DPP-4 inhibitor could therefore have more of a positive effect on QOL in younger patients with type 2 diabetes than in older patients, and in patients using more expensive DPP-4 inhibitors (Table 4, Fig. 3). Saundankar et al. has recently experienced problems with low medication adherence and suspension of regular clinic visits, especially among young patients with type 2 diabetes [30]. Similarly, a recent study using an online survey of Japanese patients with type 2 diabetes found that younger patients (under 65 years old) tended to prefer once-weekly oral administration compared with older patients, primarily because the younger patients found it less burdensome not to have to take pills every day, and felt that they would be less likely to forget to take a weekly medication [31]. For such patients, a medication taken weekly may be appreciated more than we might have expected.
A joint statement issued by the American Diabetes Association (ADA) and the European Diabetes Association (EASD) stated that treatments should respect the needs, values, and preferences of individual patients, who should be involved in treatment decisions, which would result in better adherence to treatment [32]. In our study, glycemic control, body weight change, and adverse events were comparable between the groups after 12 weeks. Patient satisfaction level may vary independent of drug efficacy and safety, as shown in this trial. It is important for us to inform all patients of the existence of weekly DPP-4 inhibitors and discuss their use.
Potential limitations of our study were the small sample size, the short study duration and the lack of double blinding. To resolve these potential issues, our findings need to be validated in a larger population over a longer period of time.
In conclusion, a weekly DPP-4 inhibitor, trelagliptin, could partially improve patients’ treatment satisfaction compared with daily DPP-4 inhibitors, especially as regards the burden of the treatment on social/personal activities and anxiety/dissatisfaction with treatment, independent of changes in glycemic control or body weight in patients with type 2 diabetes.
Acknowledgments
We thank the participating patients at the Diabetes Outpatient Departments of the Hokkaido University Hospital, Manda Memorial Hospital, Kurihara Clinic and Aoki Clinic for their valuable contributions.
Disclosure Statement
H.M. has received honoraria for lectures from Astellas Pharma Inc., AstraZeneca, Dainippon Sumitomo Pharma Co., Ltd., Eli Lilly, Kissei, Mitsubishi Tanabe Pharma Co., MSD, Novartis Pharma, Novo Nordisk Pharma and Sanofi; and received basic research funding from Astellas Pharma Inc., AstraZeneca, Daiichi Sankyo, Eli Lilly, Mitsubishi Tanabe Pharma Co., MSD, Novo Nordisk Pharma, Sanofi, Takeda Pharmaceutical Co., Ltd. and Taisho Toyama Pharmaceutical Co., Ltd. A.N. has received honoraria for lectures from Sanofi. T.A. has received honoraria for lectures from Mitsubishi Tanabe Pharma Co., Chugai Pharmaceutical Co., Ltd., Astellas Pharma Inc., Takeda Pharmaceutical Co., Ltd., Pfizer Inc. and AbbVie Inc.; and received basic research funding from Astellas Pharma Inc., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Co., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo, and Otsuka Pharmaceutical Co., Ltd. The other authors have no potential conflicts of interest to declare in relation to the publication of this article.
References
- 1 U.K. Prospective Diabetes Study Group (1995) U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes 44: 1249–1258.
- 2 Biderman A, Noff E, Harris SB, Friedman N, Levy A (2009) Treatment satisfaction of diabetic patients: what are the contributing factors? Fam Pract 26: 102–108.
- 3 Barbosa CD, Balp MM, Kulich K, Germain N, Rofail D (2012) A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence 6: 39–48.
- 4 Pladevall M, Williams LK, Potts LA, Divine G, Xi H, et al. (2004) Clinical outcomes and adherence to medications measured by claims data in patients with diabetes. Diabetes Care 27: 2800–2805.
- 5 Indelicato L, Mariano V, Galasso S, Boscari F, Cipponeri E, et al. (2017) Influence of health locus of control and fear of hypoglycaemia on glycaemic control and treatment satisfaction in people with Type 1 diabetes on insulin pump therapy. Diabet Med 34: 691–697.
- 6 Schectman JM, Nadkarni MM, Voss JD (2002) The association between diabetes metabolic control and drug adherence in an indigent population. Diabetes Care 25: 1015–1021.
- 7 Rozenfeld Y, Hunt JS, Plauschinat C, Wong KS (2008) Oral antidiabetic medication adherence and glycemic control in managed care. Am J Manag Care 14: 71–75.
- 8 Best JH, Boye KS, Rubint RR, Cao D, Kim TH, et al. (2009) Improved treatment satisfaction and weight-related quality of life with exenatide once weekly or twice daily. Diabet Med 26: 722–728.
- 9 Ishii H (2012) Development and psychometric validation of the diabetes therapy related QOL (DTR-QOL) questionnaire. J Med Econ 15: 556–563.
- 10 Galasso S, Facchinetti A, Bonora BM, Mariano V, Boscari F, et al. (2016) Switching from twice-daily glargine or detemir to once-daily degludec improves glucose control in type 1 diabetes. An observational study. Nutr Metab Cardiovasc Dis 26: 1112–1119.
- 11 Grandy S, Sternhufvud C, Ryden A, Sugg J, Rohwedder K (2016) Patient-reported outcomes among patients with type 2 diabetes mellitus treated with dapagliflozin in a triple-therapy regimen for 52 weeks. Diabetes Obes Metab 18: 306–309.
- 12 Kim HM, Lim JS, Lee BW, Kang ES, Lee HC, et al. (2015) Optimal candidates for the switch from glimepiride to sitagliptin to reduce hypoglycemia in patients with type 2 diabetes mellitus. Endocrinol Metab (Seoul) 30: 84–91.
- 13 Seino Y, Kuwata H, Yabe D (2016) Incretin-based drugs for type 2 diabetes: focus on East Asian perspectives. J Diabetes Investig 7: 102–109.
- 14 Inagaki N, Onouchi H, Maezawa H, Kuroda S, Kaku K (2015) Once-weekly trelagliptin versus daily alogliptin in Japanese patients with type 2 diabetes: a randomized, double-blind, phase 3, non-inferiority study. Lancet Diabetes Endocrinol 3: 191–197.
- 15 Bradley C (1994) The diabetes treatment satisfaction questionnaire: DTSQ. In: Bradley C (ed) Handbook of Psychology and Diabetes: A Guide to Psychological Measurement in Diabetes Research and Practice. Harwood Academic Publishers, Chur, Switzerland. 111–132.
- 16 Ishii H, Bradley C, Riazi A, Barendse S, Yamamoto T (2000) The Japanese version of the Diabetes Treatment Satisfaction Questionnaire (DTSQ): translation and clinical evaluation. J Clin Exp Med 192: 809–814 (In Japanese).
- 17 Nakadaira I, Gounai M, Kurasaki K, Hamano K (2014) A Study of Effect of Liraglutide on Workers with Irregular Working Times. JJOMT 62: 167–172 (In Japanese).
- 18 Bener A, Keskin FE, Kurtulus EM, Guzel M, Çekirdekçi EI, et al. (2017) Essential parameters and risk factors of the patients for diabetes care and treatment. Diabetes Metab Syndr Mar 6 [Epub ahead of print].
- 19 Galasso S, Facchinetti A, Bonora BM, Mariano V, Boscari F, et al. (2016) Switching form twice-daily glargine or detemir to once-daily degludec improves glucose control in type 1 diabetes. An observational study. Nutr Metab Cardiovasc Dis 26: 1112–1119.
- 20 Polonsky W, Traylor L, Wei W, Shi R, Ameer B, et al. (2014) More satisfied, but why? A pooled patient-level analysis of treatment satisfaction following the initiation of insulin glargine vs. comparators in insulin-naïve patients with type 2 diabetes mellitus. Diabetes Obes Metab 16: 255–261.
- 21 Wysham C, Blevins T, Arakaki R, Colon G, Garcia P, et al. (2014) Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care 37: 2159–2167.
- 22 Umpierrez G, Tofe Povedano S, Perez Manghi F, Shurzinske L, Pechtner V, et al. (2014) Efficacy and safety of dulaglutide monotherapy versus metformin in type 2diabetes in a randomized controlled trial (AWARD-3). Diabetes Care 37: 2168–2176.
- 23 Miya A, Nakamura A, Miyoshi H, Cho KY, Nagai S, et al. (2017) Satisfaction of switching to combination therapy with lixisenatide and basal insulin in patients with type 2 diabetes receiving multiple daily insulin injection therapy: a randomized controlled trial. J Diabetes Investig [Epub ahead of print].
- 24 Ishii H, Anderson JH Jr, Yamamura A, Takeuchi M, ikeda I, et al. (2008) Improvement of glycemic control and quality-of-life by insulin lispro therapy: assessing benefits by ITR-QOL questionnaires. Diabetes Res Clin Pract 81: 169–178.
- 25 Bode BW, Testa MA, Magwire M, Hale PM, Hammer M, et al. (2010) Patient-reported outcomes following treatment with the human GLP-1 analogue liraglutide or glimepiride in monotherapy: results from a randomized controlled trial in patients with type 2 diabetes. Diabetes Obes Metab 12: 604–612.
- 26 Bradley C, Gamsu DS (1994) Guidelines for encouraging psychological well-being: report of a Working Group of the World Health Organization Regional Office for Europe and International Diabetes Federation European Region St Vincent Declaration Action Programme for Diabetes. Diabet Med 11: 510–516.
- 27 Ishii H, Niiya T, Ono Y, Inaba N, Jinnouchi H, et al. (2017) Improvement of quality of life through glycemic control by liraglutide, a GLP-1 analog, in insulin-naive patients with type 2 diabetes mellitus: the PAGE1 study. Diabetol Metab Syndr Jan 7 [Epub ahead of print].
- 28 Okada M, Okada M, Nishigami J, Yamaaki N, Furukawa K, et al. (2015) Effect of switching basal insulin regimen to degludec on quality of life in Japanese patients with type 1 and type 2 diabetes mellitus. J Pharm Health Care Sci 1: 26.
- 29 Mashitani T, Hayashino Y, Okamura S, Kitatani M, Furuya M, et al. (2015) Diabetes treatment-related quality of life is associated with levels of self-care activities in insulin injection among Japanese patients with type 2 diabetes: Diabetes Distress and Care Registry at Tenri (DDCRT 8). Acta Diabetol 52: 639–647.
- 30 Saundankar V, Peng X, Fu H, Ascher-Svanum H, Rodriguez A, et al. (2016) Predictors of change in adherence status from 1 year to the next among patients with type 2 diabetes mellitus on oral antidiabetes drugs. J Manaq Care Spec Pharm 22: 467–482.
- 31 Sen R, Shields AL, Atsuda K (2016) Patient preference for once-weekly dosing in type 2 diabetes mellitus in Japan. JHEOR 4: 55–66.
- 32 Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, et al. (2012) Management of hyperglycemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35: 1364–1379.




