2018 Volume 65 Issue 2 Pages 203-211
2018 Volume 65 Issue 2 Pages 203-211
The clinical influence of macroprolactin (MPRL) is not clearly understood and the rate of patients potentially affected by MPRL is unknown. We investigated the influence of MPRL on the onset of galactorrhea and estimated the rate of patients with a proportion of MPRL fraction that may possibly affect galactorrhea. Data of patients with obstetric or gynecological symptoms who had undergone PRL fractionation testing were retrospectively analyzed. To evaluate factors influencing galactorrhea, a multivariate logistic regression analysis was performed and the adjusted odds ratios of MPRL for galactorrhea were calculated. Cutoff values for the total PRL level and the proportion of MPRL fractions for galactorrhea were determined by ROC analysis using a multivariate logistic model. The prevalence of patients with a proportion of MPRL fraction greater than or equal to the cutoff value for galactorrhea was estimated. The median proportion of MPRL fraction was 30.1% and increased as PRL level increased. Total PRL and MPRL had a significant influence on the onset of galactorrhea and the adjusted odds ratio was 1.09 in total PRL and 0.94 in MPRL. The rate of patients with a proportion of MPRL fraction that may possibly affect galactorrhea was estimated to be 33.5% of the study population, and thus found to be twelve times or more the number of macroprolactinemia patients. Future prospects for hyperprolactinemia may require diagnostic criteria using free prolactin levels and so MPRL fraction measurement is important for the diagnosis and treatment of patients with obstetric and gynecological symptoms.
HYPERPROLACTINEMIA is one of the most common hypothalamic pituitary endocrine disorders [1-3]. The causes of hyperprolactinemia include pregnancy, prolactinoma, intracranial tumors, medication, and hypothyroidism [1], and 9%–39% of hyperprolactinemia cases are classified as idiopathic [4, 5]. On the other hand, Sapin et al. reported that macroprolactin (MPRL) fraction accounts for 31.6% of total PRL in hyperprolactinemia patients with a MPRL fraction in the normal range [6]. In many cases, MPRL fraction may be affecting the prolactin level and the diagnosis of hyperprolactinemia. Human PRL is heterogeneous in molecular size: the major circulating form is little PRL (MW 23 kDa), the remainder consisting of big PRL (MW 50 kDa), and big-big PRL (MW greater than 150 kDa) [7, 8]. The monomeric isoform accounts for 80%–95% of PRL and is known to be both biologically and immunologically active in vivo [9]. Macroprolactinemia is a condition in which big-big PRL is substantially increased in the serum, and is usually diagnosed when the proportion of polyethylene glycol (PEG)-induced precipitable PRL exceeds 60% of the total PRL (recovery < 40%) [8, 10, 11]. The prevalence of macroprolactinemia is reportedly 10%–26% in patients with hyperprolactinemia [4, 11, 12-15] and 3.68% in the general population [16]. Although the prevalence of macroprolactinemia is low and its influence tends to be neglected, the MPRL fraction itself exists in most patients and may have a clinical influence even in patients who are not diagnosed as having macroprolactinemia. However, the influence of MPRL has not been elucidated and the rate of patients affected by MPRL is unknown.
In this study, we investigated the clinical influence of M-RPL on galactorrhea which is a typical symptom caused by PRL and estimated the rate of patients potentially affected by MPRL.
Data for patients with obstetric or gynecological symptoms who had undergone PRL fractionation testing at Hamada Hospital, Tokyo, from 2012 to 2014, were retrospectively analyzed. This study followed the ethical principles of the declaration of Helsinki and the ethical principles for medical research involving human subjects implemented by the Ministry of Health, Labour and Welfare in Japan. Informed consent was obtained from all patients, and the study protocol was approved by the ethics committee at Hamada Hospital.
MethodsData of patients with obstetric or gynecological symptoms who had undergone PRL fractionation testing were retrospectively analyzed. As background factors, data on age, symptoms, PRL level determined by immunoassay, total and free PRL levels and precipitation rates (proportion of MPRL) determined by PEG precipitation method, and levels of LH, FSH, and estradiol (E2) were collected. For the analysis, correlations between PRL level determined by immunoassay and total and free PRL levels determined by PEG precipitation method were evaluated. The proportion of MPRL fraction by age and PRL level was also examined. For the extraction of factors influencing galactorrhea, univariate analysis was performed; age and levels of PRL, total PRL, LH, FSH, and E2 were compared between patients with and without galactorrhea. For the evaluation of factors influencing galactorrhea, a multivariate logistic regression analysis was performed and the adjusted odds ratios of these variables for galactorrhea were calculated. Cutoff values of total PRL and proportion of MPRL fractions for galactorrhea were determined using receiver operating characteristic (ROC) analysis of a multivariate logistic model. The prevalence of patients with a proportion of MPRL fraction greater than or equal to the cutoff value for galactorrhea was estimated based on the cumulative probability of the study population.
EndpointsPrimary endpoints were set to be the influence of MPRL on galactorrhea and the rate of patients potentially affected by MPRL.
Measurement of PRL and its sub-fractionsSerum PRL levels were determined using a chemiluminescence immunoassay system (Chemilumi ACS: Centaur, Siemens Healthcare Diagnostics, Inc., East Walpole, MA, USA) and a PRL level ≥20 ng/mL was defined as hyperprolactinemia. The total and free PRL levels and proportions of MPRL fraction were determined using the PEG precipitation method (PEG 6000, Wako Pure Chemical Industries, Osaka, Japan). Serum samples were mixed with the PEG solution and centrifuged. Free PRL concentration was measured in the supernatant after PEG precipitation. To determine total PRL concentration, serum samples were treated with water instead of PEG. The precipitation rate (MPRL fraction proportion) was calculated using the following formula: Proportion of MPRL fraction (%) = (total PRL – free PRL)/total PRL × 100
An MPRL fraction proportion ≥60% was defined as macroprolactinemia.
StatisticsDescriptive statistics were expressed as n (%) and median [interquartile range]. A Wilcoxon rank sum test was performed to compare continuous variables and a Chi-square test was performed to compare categorical variables. Correlation between total and free PRL levels (determined by PEG precipitation) and PRL levels (determined by immunoassay) were evaluated using Spearman’s rank correlation coefficient. Adjusted odds ratios for age, total PRL, and the proportion of MPRL for galactorrhea were calculated using multiple logistic regression analysis by the least-square method. Significant variables in comparison between patients with and without galactorrhea were used as independent variables for multiple logistic regression model. Statistics was performed using JMP, Version 12.0 software (SAS Institute Inc., Cary, NC, USA).
Data from a total of 1,506 patients were analyzed. Table 1 shows patient characteristics. The median age was 36 years old (y.o.) and the most common symptom was polycystic ovary syndrome (27.2%) followed by metrorrhagia (3.9%) and menopausal disorder (2.7%). Galactorrhea was found in 12 patients (0.8%). The median PRL level was 10.5 ng/mL and hyperprolactinemia was found in 186 patients (12.4%). The median proportion of MPRL fraction was 30.1% in all patients and 33.7% in patients with hyperprolactinemia. Macroprolactinemia was found in 43 of all patients (2.9%) and in 9 patients (4.8%) with hyperprolactinemia.
| n | 1,506 |
| Age, median [IQR] | 36 [32, 41] |
| PRL: immunoassay | |
| PRL level (ng/mL), median [IQR] | 10.5 [7.7, 15.1] |
| Patients with hyperprolactinemia, n (%) | 186 (12.4%) |
| PRL: PEG precipitation method | |
| Total PRL level (ng/mL), median [IQR] | 8.4 [6, 12.1] |
| Free PRL level (ng/mL), median [IQR] | 5.65 [4, 8] |
| Proportion of MPRL fraction (%), median [IQR] | 30.11 [24.09, 37.5] |
| Patients with hyperprolactinemia | 33.7 [23.9, 40.7] |
| Macroprolactinemia (Proportion of MPRL ≥60%), n (%) | 43 (2.9%) |
| Patients with hyperprolactinemia | 9 (4.8%) |
| LH level (mIU/mL), median [IQR] | 5.7 [3.7, 10.9] |
| FSH level (mIU/mL), median [IQR] | 7.1 [5.6, 9.6] |
| E2 level (pg/mL), median [IQR] | 52.3 [36, 83] |
| Symptoms, n (%) | |
| Polycystic ovary syndrome | 410 (27.2%) |
| Metrorrhagia | 59 (3.9%) |
| Menopausal disorder | 40 (2.7%) |
| Ovarian dysfunction | 37 (2.5%) |
| Infertility screening | 16 (1.1%) |
| Galactorrhea | 12 (0.8%) |
| Others | 4 (0.3%) |
IQR, interquartile range; PRL, prolactin; PRL level, prolactin measured by immunoassay; total PRL level, prolactin measured under a PEG-free condition; free PRL level, non-precipitated prolactin fraction measured by PEG precipitation method; MPRL, macroprolactin; Proportion of MPRL fraction, precipitated fraction determined by PEG precipitation method
There were significant correlations between the PRL level determined by immunoassay and the total and free PRL levels determined by PEG precipitation (Fig. 1).
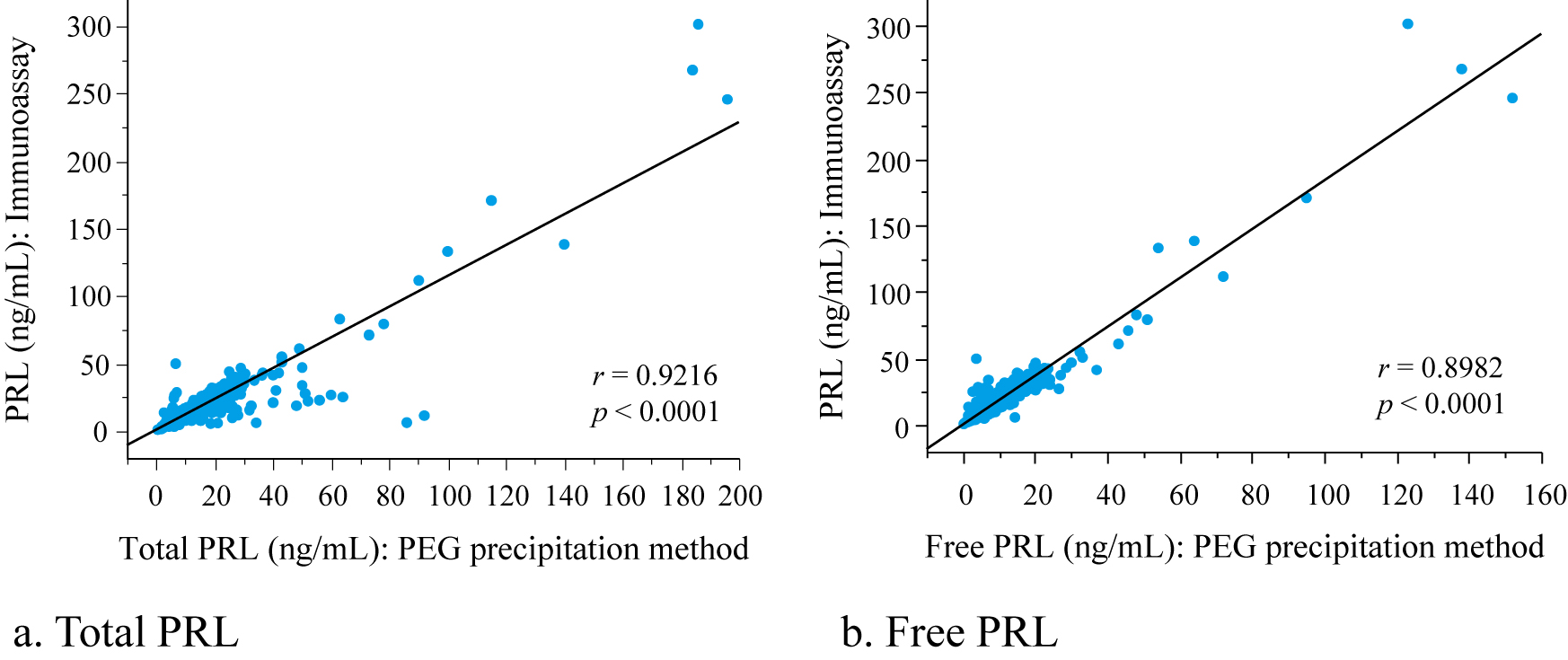
Correlation of PRL levels with immunoassay and PEG precipitation methods
r, Spearman’s rank correlation coefficient; Regression equation: a. PRL = 1.14 × total PRL + 0.93, b. PRL = 1.83 × free PRL + 0.35
PRL, prolactin measured by immunoassay; free PRL, non-precipitated prolactin fraction measured by PEG precipitation method; total PRL, prolactin measured under a PEG-free condition
Significant differences were found in the median proportion of MPRL fraction amongst age groups, PRL level groups, and total PRL level groups (Table 2). The proportion of MPRL fraction showed a decrease with age and increases with PRL and total PRL increased.
| n | Median [IQR] | p | |
|---|---|---|---|
| Total | 1,506 | 30.1% [24.0, 37.5] | |
| By age group | |||
| ≤30 y.o. | 321 | 31.4% [25.0, 38.1] | 0.0142 |
| 31–35 y.o. | 379 | 30.6% [25.0, 38.0] | |
| 36–40 y.o. | 383 | 30.0% [23.2, 37.1] | |
| >40 y.o. | 423 | 28.2% [23.0, 36.8] | |
| By PRL level group: immunoassay | |||
| 1st quartile: PRL ≤7.7 (ng/mL) | 381 | 27.7% [22.9, 35.0] | <0.0001 |
| 2nd quartile: PRL >7.7, ≤10.5 (ng/mL) | 379 | 29.6% [25.0, 37.0] | |
| 3rd quartile: PRL >10.5, ≤15.1 (ng/mL) | 370 | 30.9% [24.1, 37.9] | |
| 4th quartile: PRL >15.1 (ng/mL) | 376 | 32.3% [25.5, 40.0] | |
| By total PRL level group: PEG precipitation method | |||
| 1st quartile: total PRL ≤6.0 (ng/mL) | 389 | 27.7% [34.8, 22.9] | <0.0001 |
| 2nd quartile: total PRL >6.0, ≤8.4 (ng/mL) | 373 | 28.5% [35.5, 23.9] | |
| 3rd quartile: total PRL >8.4, ≤12.1 (ng/mL) | 375 | 30.6% [37.2, 23.9] | |
| 4th quartile: total PRL >12.1 (ng/mL) | 369 | 34.4% [42.9, 26.8] |
IQR, interquartile range; PRL, prolactin; PRL level, prolactin measured by immunoassay; total PRL level, prolactin measured under a PEG-free condition
Patient data by age and the presence or absence of galactorrhea are shown in Table 3. PRL levels, total PRL levels, and free PRL levels were significantly higher in patients with galactorrhea compared to non-galactorrhea patients. Although, no significant difference was found in the proportion of MPRL fraction between galactorrhea and non-galactorrhea patients, the proportion of MPRL fraction was significantly lower in patients with galactorrhea compared to non-galactorrhea patients aged ≥40 y.o. No significant differences were found in levels of LH, FSH, and E2 between groups. For the multivariate analytic evaluation of factors influencing galactorrhea, independent variables were chosen from the significant variables between with and without galactorrhea from the univariate analysis. Although there were significant differences amongst all PRL, total PRL, and free PRL levels in univariate analysis, total PRL was chosen due to consideration of multicollinearity. Since there was a significant difference between with and without galactorrhea in the proportion of MPRL in patients aged ≥40 y.o. and there was a significant difference in MPRL level among age groups (Table 2), age and MPRL were chosen. Therefore, age, total PRL, and MPRL were used as independent variables for multivariate analysis. Table 4 shows the results from multivariate logistic regression analysis of factors influencing galactorrhea. Both total PRL level and the proportion of MPRL fraction were significant influencing factors for galactorrhea. Total PRL was found to be a significant variable for an increase in galactorrhea with an odds ratio of 1.09 and MPRL was a significant variable for the reduction of galactorrhea with an odds ratio of 0.94. In this multivariate logistic model, cutoff values of total PRL and MPRL determined by ROC analysis for galactorrhea (AUC: 0.989) were 26.0 ng/mL and 34.6%, respectively. When converting the total PRL level determined by the PEG method to the PRL level determined by immunoassay using a regression equation (Fig. 1), the cutoff value for galactorrhea was 30.6 ng/mL of PRL.
| Galactorrhea | Non-galactorrhea | p | |
|---|---|---|---|
| n | 12 | 1,494 | |
| Age, median [IQR] | 36 [32.5, 41] | 36 [32, 41] | 0.8177 |
| <40 y.o., n (%) | 8 (66.7%) | 1,007 (67.4%) | 0.9568 |
| ≥40 y.o., n (%) | 4 (33.3%) | 487 (32.6%) | |
| PRL: immunoassay | |||
| PRL level (ng/mL), median [IQR] | 77 [43.6, 219] | 10.4 [7.7, 14.9] | <0.0001 |
| <40 y.o. | 63.1 [40.8, 235.2] | 11.1 [8, 15.8] | <0.0001 |
| ≥40 y.o. | 97.3 [52.6, 212.3] | 9.5 [7, 13.7] | 0.0006 |
| PRL: PEG precipitation method | |||
| Total PRL level (ng/mL), median [IQR] | 68 [29.3, 173] | 8.3 [6, 12] | <0.0001 |
| <40 y.o. | 58 [26.8, 173] | 8.7 [6.3, 12.6] | <0.0001 |
| ≥40 y.o. | 76.5 [38.3, 169.5] | 7.7 [5.4, 10.9] | 0.0007 |
| Free PRL level (ng/mL), median [IQR] | 46.9 [21.2, 110.3] | 5.6 [4, 8] | <0.0001 |
| <40 y.o. | 59.1 [20.1, 108.3] | 6 [4.2, 8.3] | <0.0001 |
| ≥40 y.o. | 60 [29.6, 132] | 5.2 [3.7, 7.2] | 0.0006 |
| Proportion of MPRL fraction (%), median [IQR] | 24.8 [21.9, 34.4] | 30.1 [24.1, 37.5] | 0.2824 |
| <40 y.o. | 32.5 [24.7, 36.7] | 30.9 [25, 37.9] | 0.9065 |
| ≥40 y.o. | 22.1 [20.4, 23.5] | 38.6 [23.3, 36.8] | 0.0391 |
| LH level (mIU/mL), median [IQR] | 7.2 [3.3, 11.1] | 5.7 [3.7, 10.9] | 0.7684 |
| <40 y.o. | 7.6 [4.6, 10.1] | 5.4 [3.6, 8.8] | 0.4209 |
| ≥40 y.o. | 6.3 [0.7, 22.4] | 7 [3.8, 23.4] | 0.5785 |
| FSH level (mIU/mL), median [IQR] | 7.3 [5.1, 9.2] | 7.1 [5.6, 9.6] | 0.9381 |
| <40 y.o. | 7.5 [4.4, 9] | 6.7 [5.4, 8] | 0.7848 |
| ≥40 y.o. | 7.3 [5.4, 68.5] | 9.9 [6.4, 37.2] | 0.8006 |
| E2 level (pg/mL), median [IQR] | 46.4 [26.7, 66.6] | 52.3 [36, 83.1] | 0.4071 |
| <40 y.o. | 37.4 [21.2, 66] | 51.4 [37, 74.5] | 0.2955 |
| ≥40 y.o. | 52.9 [39.9, 68.3] | 55.2 [31.3, 114.4] | 0.8631 |
IQR, interquartile range; PRL, prolactin; PRL level, prolactin measured by immunoassay; Total PRL level, prolactin measured under a PEG-free condition; free PRL level, non-precipitated prolactin fraction measured by PEG precipitation method; MPRL, macroprolactin; Proportion of MPRL fraction, precipitated fraction determined by PEG precipitation method
| Variable | Estimate | S.E. | p | Adjusted Odds (/unit) [95%CI] |
|---|---|---|---|---|
| Intercept | –3.355 | 2.00 | ||
| Age | –0.044 | 0.05 | 0.3888 | 0.95 [0.86, 1.05] |
| Total PRL | 0.086 | 0.01 | <0.0001 | 1.09 [1.06, 1.13] |
| MPRL | –0.064 | 0.03 | 0.0132 | 0.94 [0.87, 0.99] |
Model, p < 0.0001; Total PRL, prolactin measured under a PEG-free condition; MPRL, precipitated macroprolactin fraction determined by PEG precipitation method
Fig. 2 shows patient distribution according to proportion of MPRL faction. The median proportion of MPRL fraction showed a wide distribution with minimum and maximum values of 1.96% and 97.7%, respectively. The rate of patients with MPRL fraction ≥34.6% (cutoff value for galactorrhea) calculated from the cumulative probability of patients was estimated to be 33.5% and the rate of patients with macroprolactinemia (MPRL ≥60.0%) was estimated to be 2.7%. Calculations show the rate of the number of patients with a proportion of MPRL fraction that may possibly affect galactorrhea was more than twelvefold (33.5%/2.7%) that of patients with macroprolactinemia.
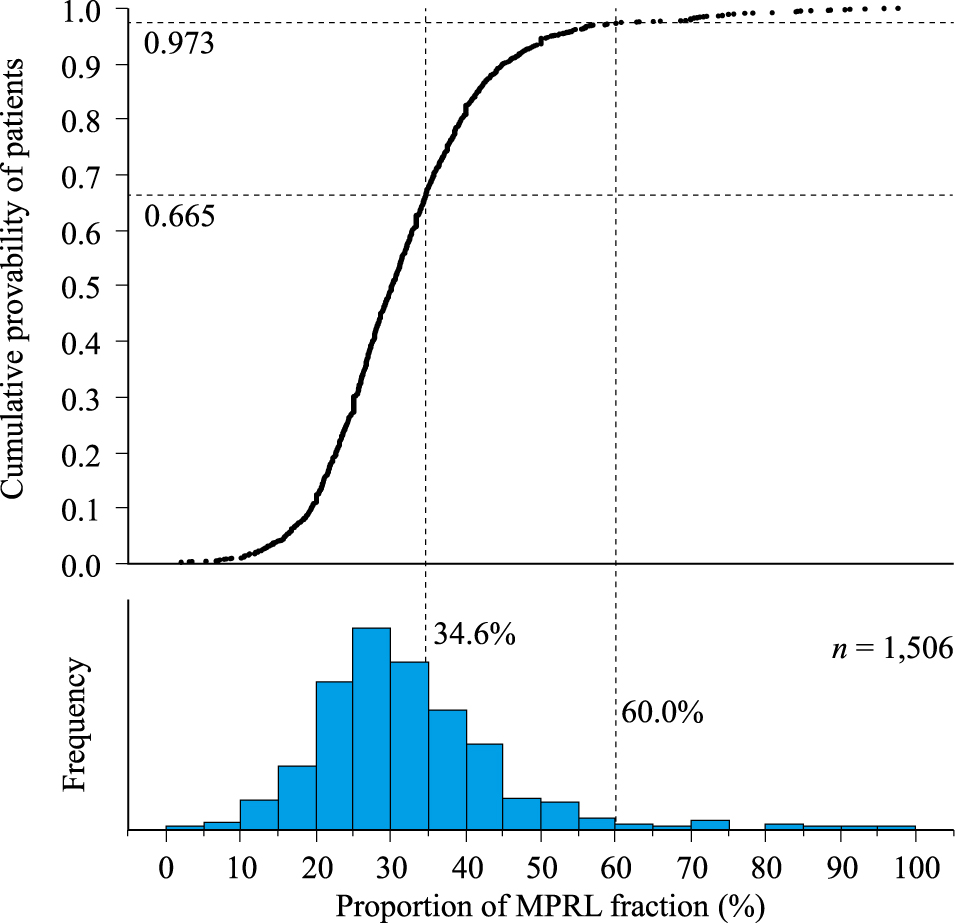
Patient distribution according to proportion of MPRL fraction
MPRL, macroprolactin (PEG precipitation rate); MPRL fraction ≥60.0%, cutoff value of macroprolactinemia; MPRL fraction ≥34.6%, cutoff value possibly affecting galactorrhea
We investigated the influence of MPRL on the onset of galactorrhea and the rate of patients with a proportion of MPRL that may possibly affect galactorrhea from the data of 1,506 patients with obstetric or gynecological symptoms. The median proportion of MPRL fraction was 30.1% and increased as the PRL level increased. MPRL was found to be a significant factor in the reduction of the onset of galactorrhea with an adjusted odds ratio of 0.94. The proportion of patients with MPRL ≥34.6%, which may possibly affect galactorrhea, was estimated to be 33.5% of the study population, more than twelvefold the rate for patients with macroprolactinemia.
The prevalence of macroprolactinemia was 2.9% in the total study population and 4.8% in patients with hyperprolactinemia. The prevalence of macroprolactinemia has been reported to be 10%–26% [4, 11-15], a deviation from our results, presumably due to the following. Of the abovementioned studies, Jamaluddin et al. [14] and Strachan et al. [15] both reported that the prevalence of macroprolactinemia was 21% in patients with PRL >700 mIU/L (33.0 ng/mL). Beda-Maluga et al. reported the prevalence of macroprolactinemia to be 8.6% in patients with a PRL level >30 ng/mL [11]. The present study targeted patients with obstetric or gynecological symptoms and there were no inclusion or exclusion criteria concerning PRL level and resulted in a median PRL level of 10.5 ng/mL. Furthermore, our study showed that the MPRL fraction increased as the PRL level increased. Thus, the difference in prevalence in macroprolactinemia is presumed to reflect the PRL level of the study population. We consider our results to be an indication of the frequency of macroprolactinemia in the general obstetrics and gynecology patient population.
The median proportion of MPRL fraction was 30.1% in all patients studied and 33.7% in patients with hyperprolactinemia. Even though the proportions of PRL sub-fraction were influenced by population and testing method [17] and the proportion of MPRL fraction have not been sufficiently elucidated, Sapin et al. reported that the mean proportion of MPRL was 31.6% in non-macroprolactinemia patients [6]. Our results also showed that around 30% of the PRL level was caused by the MPRL fraction, indicating that MPRL may possibly affect various symptoms associated with PRL in patients with obstetric or gynecological symptoms.
We found MPRL to be a significant factor influencing galactorrhea. Although some reports have shown an association between PRL and galactorrhea [18-20], an association between MPRL and galactorrhea has not been sufficiently elucidated. Thirunavakkarasu et al. [21] and Isik et al. [22] have reported that the prevalence of galactorrhea was significantly lower in patients with macroprolactinemia compared to patients with hyperprolactinemia. On the other hand, Can et al. [5] and McCudden et al. [23] reported, as a result of univariate analyses, there was no difference in the prevalence of galactorrhea relative to the proportion of MPRL fraction. Our study showed relationships between the proportion of MPRL, age and PRL level; thus, adjustments for these factors are necessary for the evaluation. Indeed, although no significant difference was found in the proportion of MPRL between the presence and absence of galactorrhea from univariate analysis, MPRL was found to be a significant variable for galactorrhea from multivariate analysis using age and total PRL as independent variables. Adjusted odds ratios for galactorrhea were 1.09 per 1 ng/mL increment of total PRL and 0.94 per 1% increment of MPRL, indicating that MPRL has sufficient influence to offset the effect of PRL.
Patients with a proportion of MPRL fraction of ≥34.6%, which may possibly affect galactorrhea, was calculated to be 33.5% of the study population. In general, macroprolactinemia is defined as having a MPRL fraction of ≥60%; thus, the prevalence of macroprolactinemia is low and its clinical influence tends to be underestimated. However, we found the number of patients with a MPRL level that may possibly affect galactorrhea to be more than twelvefold the number of patients with macroprolactinemia. Some studies on the MPRL biological activity as prolactin-like have been undertaken [24-27]. Although a few studies have revealed MPRL activity to be similar to monomeric prolactin in vitro [24, 25], they have not it to be apparent in vivo [26, 27]. The reason for this is presumed to be due to its high molecular weight [28, 29]. Regarding hyperprolactinemia treatment, a dopamine agonist (DA) has been confirmed to be effective against prolactinoma and is recommended in treatment guidelines [30, 31]. However, as far as we know, the clinical efficacy of a DA against macroprolactinemia has not been established. Alfonso et al. reported that only 28% of patients with macroprolactinemia had improved PRL levels after administration of a DA [32]. Lu et al. also reported that, compared to patients with true hyperprolactinemia, patients with macroprolactinemia were found to have no significant changes in clinical features or PRL levels after 1 year of cabergoline therapy [33]. Thus, it should be considered that administration of DA against hyperprolactinemia be judged after measurement of the MPRL fraction.
On the other hand, for the differential diagnosis in the guideline for gynecological practice in Japan for hyperparathyroidism, MRI examination is indicated if the PRL level is 100 ng/mL or more [30]. However, in this study, the median PRL level in 12 patients with galactorrhea was 77 ng/mL and the PRL levels in 7 patients (58%) were less than 100 ng/mL. Therefore, in the differential diagnosis of hyperprolactinemia accompanied by galactorrhea, there are cases for which it is necessary to decide whether or not image diagnosis should be performed. In these cases, measurement of MPRL fraction may provide information that will aid in this decision-making and there is the possibility that unnecessary examination will be avoided.
Our study showed that more than 30% of the study population patients showed a MPRL ≥34.6%, which may possibly affect galactorrhea. However, this cutoff value for MPRL was calculated for patients with obstetric or gynecological symptoms in our institution, and further consideration is needed on the external validity of this cutoff value. This indicates that, in the future, it may be necessary to have diagnostic criteria for free prolactin levels concerning PRL-related diseases including galactorrhea. Currently, screening is necessary to exclude, at the least, macroprolactinemia and it may help prevent unnecessary diagnostic testing and medication and lead to better decision-making for treatment for patients with obstetric and gynecological symptoms.
This study has the following limitations. This is a retrospective analysis and thus impossible to eliminate all bias and confounding factors. Disease and medications that may have an effect on PRL level were not included in the analysis. Our results need to be interpreted with consideration of the above.
MPRL was found to be a significant factor in reducing the onset of galactorrhea. The rate of patients with a proportion of MPRL fraction that may possibly affect galactorrhea was estimated to be 33.5% of the study population and was twelve or more times the number of macroprolactinemia patients. Future diagnostic criteria for hyperprolactinemia may possibly be free prolactin level. Currently, MPRL fraction measurement is necessary to exclude, at the least, macroprolactinemia in the diagnosis of patients with symptoms associated with prolactin.
The authors would like to express great appreciation to Professor Naoki Hattori, Ritsumeikan University, School of Pharmaceutical Sciences, for providing support to our study.
None of the authors have any potential conflicts of interest associated with this research.