2018 Volume 65 Issue 3 Pages 345-357
2018 Volume 65 Issue 3 Pages 345-357
The lack of isolation ward throughout Japan has long been limiting the 131I radioactive iodine (RAI) ablation for differentiated thyroid cancer (DTC) cases. The 30 mCi RAI ablation was only recently permitted for outpatient basis. However, no patient selection tool nor response predictor has been proposed. This study evaluated factors to find response predictor and determinant for the suitable patients. The retrospective study reviewed 47 eligible non-metastatic papillary DTC patients whose had first 30 mCi RAI ablation after total thyroidectomy. Age, gender, clinical stage, risk category, and pre-ablation serum thyroglobulin (Tg) level were among covariates analyzed to determine the patient selection factors; while the thyroid bed uptake on initial whole body scan (WBS) was later also included in determining RAI ablation response. Thirteen (28%) patients had a low risk (T1-2) while 23 (49%) and 11 (23%) had an intermediate (T3) or high risk (T4), respectively. Twenty-five patients were responders, and 22 were non-responders. All factors were similar between responders and non-responders except pre-ablation serum Tg level (p < 0.001). In multivariate analysis, pre-ablation serum Tg level was the only significant factor for both patient selection (odd ratio (OR) = 1.52, 95% confidence interval (CI) = 1.13–2.06) and response predictor (OR = 1.48; 95% CI = 1.12–1.95). With the cut-off of 5.4 ng/mL, pre-ablation serum Tg level predicts RAI ablation response with 92% specificity and 73% sensitivity. Pre-ablation serum Tg level may help patient selection and predict the response to outpatient 30 mCi RAI ablation among post total thyroidectomy non-metastatic DTC patients.
THYROID CANCER contributes 1.9% cancer incident and 0.37% cancer death among all malignancies in 2015 worldwide. Despite the steady declined in mortality, the incidence from 2005 to 2015 was rapidly increasing and almost doubled [1]. In Japan, estimated incidence of thyroid cancer was 3,505 cases (0.8% of all malignancies) for male and 9,590 cases (3% of all malignancies) for female in 2009 only [2], with papillary thyroid carcinoma (PTC), —most of them were differentiated thyroid carcinoma (DTC), —dominated (>80%) cases in both sexes [3]. Even though DTC is naturally indolent with very high 10-years survival rate (93%), the 10-years recurrence rate was up to 32% and sometimes cases with certain characteristics may become life-threatening [4]. The completeness of near-total or total thyroidectomy is an important determinant of outcome, but thyroid tissue remains in about 90% of patients. Thus, radioactive iodine (RAI) ablation plays an adjunctive role to the surgery and has long remained the standard treatment to eradicate possible residual thyroid cancer tissue [5].
The recommended RAI dose is varied from 30–100 milliCuries (mCi) for low-risk patients and higher (100–200 mCi) for them with suspected micro-metastasis, distant metastasis or more aggressive subtypes [6]. The two largest European randomized controlled trial (RCT) to date showed that the low-dose (30 mCi) RAI ablation in low to intermediate risk DTC had similar efficacy to that of high-dose (100 mCi) [7, 8]. The largest Asian RCT (Korea) also recently revealed the same conclusion [9].
While there is a clear survival benefit of RAI ablation for high-risk patients, there is more controversy for patients with intermediate-risk disease, particularly the elderly with positive nodes, adverse histologies or tumor invasion [10]. A recent Korean study showed that 30 mCi was insufficient for intermediate-risk DTC cases [11]. The latest meta-analysis (17 RCTs, 3,737 patients) proved that higher RAI doses (>80 mCi) might be better than lower doses (<40 mCi) to achieve successful ablation [12]. On the contrary, in the previous meta-analysis (9 RCTs, 2,569 patients) involving low and intermediate risk cases, 30 mCi was sufficient, with similar quality of life, fewer adverse effects, and a shorter stay in isolation ward [13]. Not only the dose amount is debatable, the urgency of RAI for intermediate-risk patients is also questionable. Generally, a high-dose ablation would yield higher successful ablation rates up to >90%. However, successful ablation was not associated with a reduction in clinical recurrences. This approach also has to be weighed against its disadvantages: higher adverse events, increased risk of secondary malignancies, requirement for isolation room and also higher costs [14]. Recently, even the largest institution bodies taking care of the thyroid cancer therapeutic guidelines are equivocal regarding post-surgical RAI ablation [5, 15, 16].
Japan has several distinct socio-medical circumstances compared to other/Western countries, most importantly, radioisotope facilities are of limited availability [17], making the treatment plans for DTC different significantly [18]. Routine RAI ablation was not possible because of strict regulation of radioactive substances, and total thyroidectomy is also not routinely performed [19]. Instead, lobectomy without RAI ablation is the preferred treatment for PTC patients without high-risk features [20]; while RAI ablation tends to be restored to high-risk patients only, which usually required a higher RAI doses. However, the increasing RAI demand is not offset by the number of available isolation ward which on the contrary, decreased [21]. About 50% of RAI ablation centers admitted that their patients have to wait more than six months for RAI after surgery [22].
After The Ministry of Health, Labor and Welfare of Japan raised some restriction to overcome the situation, The Japanese Society of Nuclear Medicine verified the use of 30 mCi dose for RAI ablation in an outpatient basis, which included in the 2010 guidelines from The Japanese Society of Thyroid Surgery (JSTS)/Japan Association of Endocrine Surgeons (JAES) [19, 23]. A preliminary study in Tokyo (50 patients) showed that 30 mCi RAI ablation might also be useful for high-risk DTC, with ablation success rate of 82% [24]. Thus, the use of 30 mCi RAI ablation warrants a further clarification to be safely applied to the whole range of patients’ risk. In this regard, patient selection for 30 mCi dose in outpatient basis become critical.
As in many hospitals in Japan, our institution also has a limited capacity for hospitalization of RAI ablation patients. From 2012, the 30 mCi dose began to be administered on an outpatient basis. The aim of this study was to discover the possible factors, which may help to select the appropriate candidate of 30 mCi RAI ablation therapy and to predict the RAI ablation outcome in DTC patients.
After ethical committee approval, we retrospectively reviewed the electronic medical records of all consecutive patients with DTC, who had undergone total thyroidectomy and initial RAI ablation between March 2012 and October 2016. All patients gave written informed consent for future anonymous use of clinical data in clinical studies. Preoperative diagnoses of DTC (PTC) were primarily based on ultrasonography (US)-guided fine needle aspiration cytology. The preoperative workup consisted of an examination of thyroid hormones, serum TSH, serum Tg, and anti-Tg antibody levels and diagnostic imaging. A high-resolution neck US, CT, MRI or 18F-FDG PET were part of the preoperative workup, mainly to screen the distant metastasis. The final diagnosis of metastasis-free was made by surgeon responsible for the thyroidectomy.
During the above mentioned period, 259 patients received RAI ablation in our institution, and 74 of them received 30 mCi dose. Patients who had an invasive pathological subtype, including tall cell, insular, poorly differentiated, and diffuse sclerosing variants of anaplastic or medullary thyroid carcinoma; or had a history of other malignancies, or had received a 131I ablation dose of more than 30 mCi, were excluded. Patients older than 20 years, had undergone total thyroidectomy with curative intent and had preoperative biopsy evidence of papillary DTC, were included. Sixty-two patients satisfied inclusion and exclusion criteria and 15 among them were later excluded for having distant metastasis in their initial whole body scan (WBS) taken two days after RAI ablation. These distant metastases were not found during the preoperative diagnostic imaging.
Each patient was risk-stratified using the 7th edition of the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) staging system [25] and the 2015 American Thyroid Association (ATA) guidelines [5].
Preparation and procedure of RAI ablation, and WBSComplete surgical resection was defined as an extracapsular total thyroidectomy with therapeutic, compartment-oriented neck dissection for suspicious or biopsy-proven metastatic cervical lymphadenopathy. In the 47 patients, five patients underwent thyroid hormone replacement (THR) withdrawal while the remaining 42 received recombinant human thyroid stimulating hormone (rhTSH or thyrotropin alfa, Thyrogen®, Sanofi-Genzyme corp., Cambridge, MA) for their TSH level elevation. THR patients were given their levothyroxine withdrawn for four weeks and replaced with triiodothyronine for the first two weeks of this withdrawal interval. They were also instructed to take a low-iodine diet starting from two weeks before RAI ablation until 4 or 5 days after. Patients receiving rhTSH injection were continuously receiving THR before and after RAI ablation. Serum TSH levels were determined by radioimmunoassay (RIA). The minimum TSH level recommended for RAI ablation was 30 mU/L. No WBS was performed between the surgery and the RAI ablation.
RAI ablation was done using oral 30 mCi 131I dose. An initial WBS was obtained two days later to evaluate the thyroid remnant, metastasis detection and as a reference for therapeutic monitoring. A large field-of-view gamma camera (E.CAM, Toshiba Medical System, Tochigi, Japan) with medium-energy parallel-hole collimator was used. A 20% symmetric window was centered at 364 keV. The anterior images of the neck, chest, and abdomen were obtained, and a minimum of 100,000 counts was collected per image.
The thyroid bed uptake intensity was subjectively graded by two experienced nuclear medicine physicians (T.H. and D.D.B.) with reference to bilateral salivary glands; as 1) no/faint uptake (if no uptake or lower than the salivary glands’ uptake), 2) medium uptake (if similar to the salivary glands’s uptake), and 3) high uptake (if higher than salivary glands’ uptake, or there was a visible star effect). The grading was blinded from the RAI ablation response data. Representative thyroid bed uptake grade on WBS image was shown in Fig. 1.
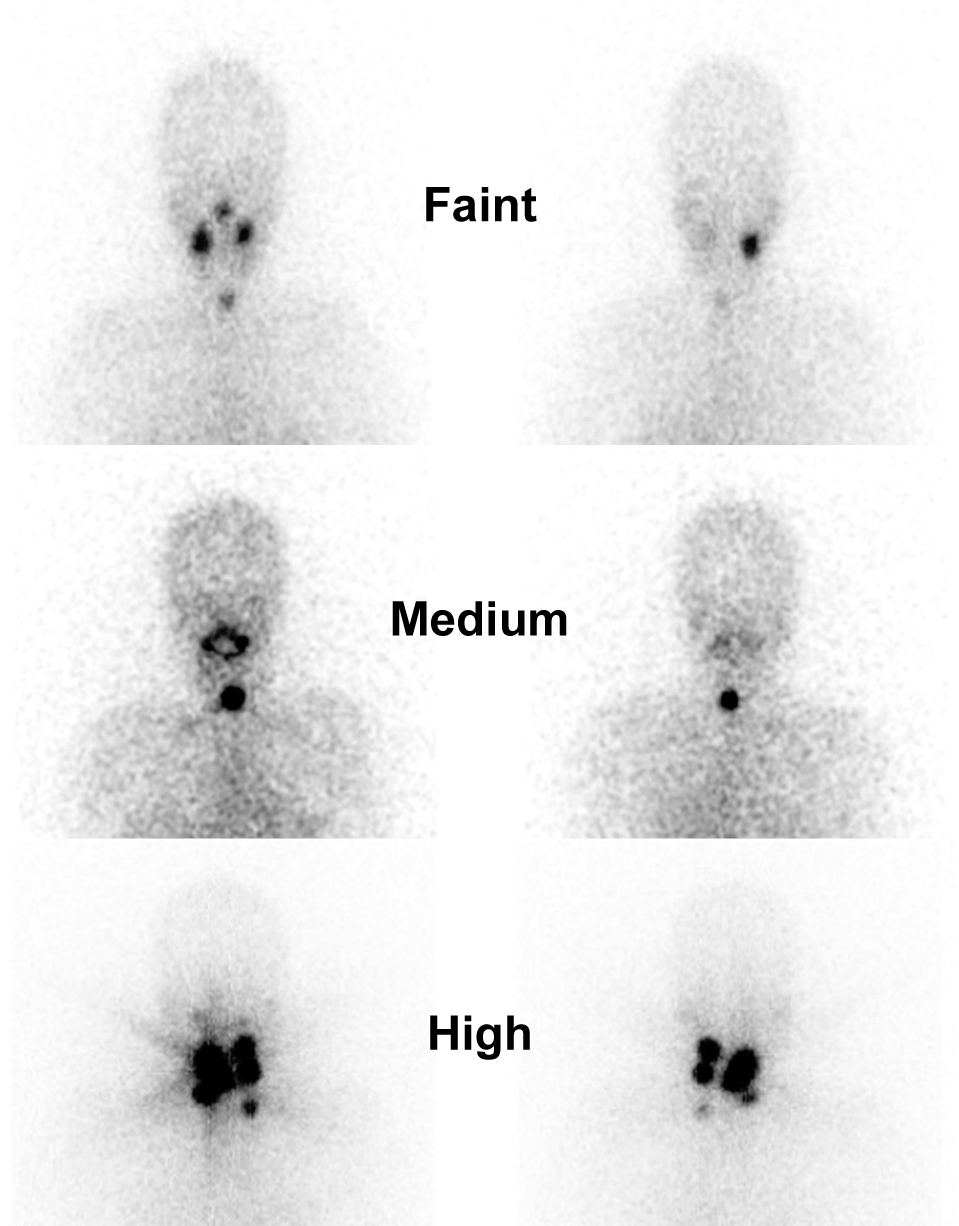
Representative neck region of initial WBS images for thyroid bed uptake grading. The initial WBS was obtained two days after oral ingestion of 30 mCi for RAI ablation. Bilateral salivary glands were the organ of reference.
After the initial WBS, THR therapy was restarted. Six months after RAI ablation, patients had at least one WBS (10 mCi 131I, oral dose), and their serum Tg level measurement was obtained either on rhTSH stimulation or THR withdrawal at our center.
Response criteria for RAI ablation therapyResponse to 30 mCi RAI ablation was evaluated based on our institutional criteria. Patients belonged to the responder group when their evaluation six months after RAI ablation showed both: 1) negative thyroid bed uptake on WBS image and 2) stimulated Tg level of ≤5 ng/mL. Patients with stimulated Tg level >5 ng/mL with any finding on WBS were belong to the non-responder group. The lowest detection limit of serum Tg level assay in our institution was 5 ng/mL throughout the study period.
The new 2015 ATA guidelines would categorize DTC patients with negative imaging on WBS as a biochemically incomplete response if their post-therapy stimulated Tg level is ≥10 ng/mL, but nowhere categorized those with post-therapy stimulated Tg level between 1 to 10 ng/mL [5]. In other parts of the world, the standard of post-ablation serum Tg as part of successful ablation criteria is widely dispersed (from <0.2 to ≤10 ng/mL); some center even solely rely on WBS negative finding as reported in a recent meta-analysis [12]. The Japanese clinical criteria does not strictly implement a certain cut-off value. Indeed a stimulated Tg level of 10 ng/mL was referred as having sensitivity and specificity of 100% and 93% to predict recurrence [26]. Based on above references, we also evaluated the possibility to obtain factors for patient selection tool or response predictor under a new response criteria: 1) negative thyroid bed uptake on WBS image and 2) stimulated Tg level of ≤10 ng/mL in evaluation six months after RAI ablation.
Statistical methodsAll data were expressed as a mean ± standard deviation (SD). Parametric and non-parametric statistical tests were used accordingly (student’s t, chi-square, Kruskal-Wallis H, and Mann-Whitney U test). The binomial logistic regression analyses (univariate and multivariate analysis) were employed to predict the impact of several factors to the patients’ response to RAI ablation.
With our limited samples and events in mind, the number of covariates included in the regression was determined carefully to avoid overfitting. Recent studies showed that the duration between thyroidectomy and RAI ablation has no impact on the outcome [27], neither does the method of TSH level elevation (thyrotropin alpha (Thyrogen®) vs. withdrawal from thyroid hormone replacement) [7] nor the pre-ablation TSH level [28]. Thus, these three factors were precluded from the logistic regression analysis. Several factors that being evaluated were patient’s age (<45 and ≥45 y.o.), gender, AJCC/UICC clinical stage, ATA risk stratification, thyroid bed uptake grade on initial WBS and pre-ablation serum Tg level.
To avoid multicollinearity, we evaluated correlation coefficients between the independent variables in advance (Spearman rank correlation for categorical variables, Pearson correlation for continuous variables). If two variables had a corelation coefficient more than absolute value of 0.7 (<–0.7 or >0.7), simultaneous inclusion in the same multivariate logistic regression model was considered inappropriate. All factors satisfied this criteria (correlation coefficients ranged from –0.543 to 0.321). Finally, to fulfill the assumptions of logistic regression analysis, the Box-Tidwell test was used to check the assumption of a linear relationship between pre-ablation serum Tg level (the only continuous independent variable) and the logit transformation of the RAI ablation response (the dependent variable).
The logistic regression analysis was performed for two different purpose: 1) to find factors for patients selection tools, and 2) to find factors as a RAI response predictor. In the first analysis, the thyroid bed uptake on initial WBS was excluded since it was taken after RAI ablation. The thyroid bed uptake grade on initial WBS was included logistic regression analysis to determine the RAI ablation response.
The p-values of 0.05 were considered as the limit of significance level. A receiver operating characteristic (ROC) curve analysis would be performed once factor(s) significantly contribute to the logistic regression model was obtained. Statistical analysis was performed using IBM SPSS statistics software (Version 23; SPSS Inc., Chicago, IL).
Patient characteristics and categories based on RAI ablation responses are summarized in Table 1. In the 47 patients, 25 patients responded to RAI ablation while the remaining 22 did not, according to our hospital criteria; while based on the new response criteria, 28 were responders and 19 were non-responders. All the factors among both groups (with either response criteria) were similar, except pre-ablation serum Tg level (p < 0.001). As a note, only three patients (4%) have pre-ablation stimulated TSH level <30 mIU/L and one of them (TSH level = 23.57 mU/L) was a non-responder. Box-Tidwell tests before logistic regression showed that the assumption was satisfied. In the univariate analysis, Tg level was the only significant predictor for RAI ablation response based on either our hospital criteria or the new criteria (Table 2).
| Baseline characteristics | Based on our hospital’s RAI ablation response criteria* | Based on new RAI ablation response criteria** | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Responder | Non-responder | p values | Responder | Non-responder | p values | |||
| N | 47 | 25 | 22 | 28 | 19 | ||||
| Age | Mean ± SD | 55 ± 12 | 57.16 ± 11.3 | 52.77 ± 12.3 | 0.286 | 57.07 ± 11.40 | 52.21 ± 12.29 | 0.246 | |
| Median | 55.0 | ||||||||
| Range | 25–79 | ||||||||
| <45 years old | 30 | 8 | 0.184 | 24 | 14 | 0.304 | |||
| ≥45 years old | 7 | 2 | 4 | 5 | |||||
| Gender | |||||||||
| Female/Male | 34/13 | 72/28% | 20/5 | 14/8 | 0.211 | 21/7 | 13/6 | 0.621 | |
| AJCC/UICC clinical stage | |||||||||
| I | 9 | 19% | 2 | 7 | 0.121 | 3 | 6 | 0.096 | |
| III | 21 | 45% | 13 | 8 | 13 | 8 | |||
| IVa | 17 | 36% | 10 | 7 | 12 | 5 | |||
| ATA initial risk classification | |||||||||
| Low (T1–2) | 13 | 28% | 7 | 6 | 0.982 | 8 | 5 | 0.944 | |
| Intermediate (T3) | 23 | 49% | 12 | 11 | 13 | 10 | |||
| High (T4) | 11 | 23% | 6 | 5 | 7 | 4 | |||
| Pre-ablation serum Tg level (ng/mL) | |||||||||
| Mean ± SD | 11.6 ± 12.8 | 5.23 ± 0.99 | 18.82 ± 15.88 | < 0.001 | 5.89 ± 3.52 | 20.00 ± 16.50 | <0.001 | ||
| Range | 5.0–65.0 | (5–9.9) | (5–65) | (5–23.2) | (5–65) | ||||
| ≤5 | 23 | 6 | <0.001 | 24 | 5 | <0.001 | |||
| >5 | 2 | 16 | 4 | 14 | |||||
| Pre-ablation TSH level (mIU/L) | |||||||||
| Mean ± SD | 119.9 ± 56.0 | 121.32 ± 66.48 | 118.45 ± 42.07 | 0.864 | 121.26 ± 63.91 | 118.08 ± 43.41 | 0.761 | ||
| Range | 20.2–312.9 | (20.18–312.88) | (23.57–181.71) | (20.18–312.88) | (23.57–181.71) | ||||
| TSH elevation method | |||||||||
| rhTSH (Thyrogen) | 42 | 89% | 24 | 18 | 0.116 | 25 | 17 | 0.984 | |
| THR withdrawal | 5 | 11% | 1 | 4 | 3 | 2 | |||
| Thyroid bed uptake on initial WBS | |||||||||
| Faint uptake | 5 | 11% | 4 | 1 | 0.293 | 4 | 1 | 0.942 | |
| Medium uptake | 25 | 53% | 11 | 14 | 13 | 12 | |||
| Strong uptake | 17 | 36% | 10 | 7 | 11 | 6 | |||
| Duration between TT and RAI ablation (days) | |||||||||
| Mean ± SD | 195.6 ± 482.3 | 234 ± 575 | 152 ± 358 | 0.436 | 217 ± 544 | 164 ± 385 | 0.704 | ||
| Range | 39–2,709 | (39–2,709) | (41–1,745) | (39–2,709) | (43–1,745) | ||||
AJCC, American Joint Committee on Cancer; ATA, American Thyroid Association; rhTSH, recombinant human TSH; THR, thyroid hormone replacement; TT, total thyroidectomy; the UICC, Union for International Cancer Control; WBS, whole body scan. * Response to RAI ablation (evaluated 6-month post ablation) if: WBS (–) and serum Tg level ≤5 ng/mL. ** Response to RAI ablation (evaluated 6-month post ablation) if: WBS (–) and post-ablation serum Tg level ≤10 ng/mL
| Based on our hospital’s RAI ablation response criteria* |
Based on new RAI ablation response criteria** |
|||||
|---|---|---|---|---|---|---|
| p values | OR | 95% C.I. | p values | OR | 95% C.I. | |
| Age | ||||||
| <45 | 1.00 | 1.00 | ||||
| ≥45 | 0.194 | 0.36 | 0.08–1.68 | 0.310 | 0.47 | 0.10–2.03 |
| Gender (Female vs. Male) | 1.00 | |||||
| Female | 1.00 | |||||
| Male | 0.216 | 0.44 | 0.12–1.62 | 0.621 | 0.72 | 0.20–2.63 |
| AJCC/UICC clinical stage | ||||||
| Stage I | 0.151 | 1.00 | 0.200 | 1.00 | ||
| Stage III | 0.087 | 5.00 | 0.79–31.63 | 0.076 | 4.80 | 0.85–27.20 |
| Stage IV | 0.847 | 0.88 | 0.24–3.25 | 0.576 | 1.48 | 0.38–5.78 |
| ATA initial risk classification | ||||||
| Low risk | 0.990 | 1.00 | 0.912 | 1.00 | ||
| Intermediate risk | 0.973 | 1.03 | 0.20–5.15 | 0.916 | 1.09 | 0.21–5.76 |
| High risk | 0.897 | 1.10 | 0.26–4.65 | 0.694 | 1.35 | 0.31–5.91 |
| Pre-ablation serum Tg level (continuous value) | 0.036† | 1.53 | 1.03–2.27 | 0.002† | 1.26 | 1.08–1.46 |
| Post-ablation thyroid bed in WBS | ||||||
| Faint uptake | 0.315 | 1.00 | 0.457 | 1.00 | ||
| Medium uptake | 0.399 | 0.36 | 0.03–3.92 | 0.525 | 0.46 | 0.41–5.08 |
| Strong uptake | 0.348 | 1.82 | 0.52–6.33 | 0.416 | 1.69 | 0.48–6.01 |
* Response to RAI ablation if: WBS (–) and post-ablation serum Tg level ≤5 ng/mL
** Response to RAI ablation if: WBS (–) and post-ablation serum Tg level ≤10 ng/mL
† p < 0.05
The full model from logistic regression to obtain factor for patient selection was statistically significant χ2(7, n=47) = 30.38 (p < 0.00) and correctly classified 80.9% of cases based on our hospital criteria. Table 3 showed that the pre-ablation serum Tg level was the only factor that significantly contributes to the model (p < 0.05). Each serum Tg level increase was associated with the odds of RAI ablation failure increases by 1.74 times (95% CI = 1.07–2.83). Based on the new criteria, the full model was also significant, slightly better χ2(7, n=47) = 28.71 (p < 0.00) and also correctly classified 80.9% of cases. The pre-ablation serum Tg level was also the only factor that significantly contributes to the model (p < 0.01). Each serum Tg level increase was associated with the odds of RAI ablation failure increases by 1.52 times (95% CI = 1.13–2.06).
| Based on our hospital’s RAI ablation response criteria* |
Based on new RAI ablation response criteria** |
|||||
|---|---|---|---|---|---|---|
| p values | OR | 95% C.I. | p values | OR | 95% C.I. | |
| Age | ||||||
| <45 | 1.00 | 1.00 | ||||
| ≥45 | 0.706 | 0.56 | 0.03–11.08 | 0.363 | 4.65 | 0.17–127.95 |
| Gender (Female vs. Male) | 1.00 | |||||
| Female | 1.00 | |||||
| Male | 1.000 | 1.00 | 0.11–8.94 | 0.320 | 3.59 | 0.29–44.55 |
| AJCC/UICC clinical stage | ||||||
| Stage I | 0.890 | 1.00 | 0.393 | 1.00 | ||
| Stage III | 0.768 | 0.47 | 0.00–68.30 | 0.945 | 0.87 | 0.01–53.19 |
| Stage IV | 0.782 | 1.30 | 0.20–8.29 | 0.181 | 4.13 | 0.52–33.06 |
| ATA initial risk classification | ||||||
| Low risk | 0.445 | 1.00 | 0.543 | 1.00 | ||
| Intermediate risk | 0.203 | 0.06 | 0.00–4.77 | 0.279 | 0.12 | 0.00–5.75 |
| High risk | 0.626 | 0.62 | 0.09–4.24 | 0.800 | 0.76 | 0.09–6.18 |
| Pre-ablation serum Tg level (continuous value) | 0.026† | 1.74 | 1.07–2.83 | 0.006† | 1.52 | 1.13–2.06 |
| Constant | 0.189 | 0.05 | 0.020 | 0.00 | ||
* Response to RAI ablation (evaluated 6-month post ablation) if: WBS (–) and post-ablation serum Tg level ≤5 ng/mL
** Response to RAI ablation (evaluated 6-month post ablation) if: WBS (–) and post-ablation serum Tg level ≤10 ng/mL
† p < 0.05
The full model from logistic regression to evaluate factors for RAI ablation response predictor was statistically significant χ2(9, n=47) = 32.84 (p < 0.001) and correctly classified 85.1 % of cases, based on our hospital criteria. The pre-ablation serum Tg level was the only factor that significantly contributes to the model (p < 0.05) as shown in Table 4. Each serum Tg level increase was associated with the odds of RAI ablation failure increases by 1.75 times (95% CI = 1.03–2.99). A ROC curve analysis was performed to explore further the cut-off value of pre-ablation serum Tg level to predict the RAI ablation response (Fig. 2). With the pre-ablation serum Tg cut-off of 10.5 ng/mL, the specificity to predict RAI ablation response was able to be optimized (100%), while the sensitivity was 64% (Table 5). Sensitivity may be kept reasonable (73%) with minimum compromise on the specificity (92%) when the pre-ablation serum Tg cut-off was set at 5.4 ng/mL.
| Based on our hospital’s RAI ablation response criteria* |
Based on new RAI ablation response criteria** |
|||||
|---|---|---|---|---|---|---|
| p values | OR | 95% C.I. | p values | OR | 95% C.I. | |
| Age | ||||||
| <45 | 1.00 | 1.00 | ||||
| ≥45 | 0.752 | 0.61 | 0.03–13.25 | 0.306 | 7.05 | 0.17–295.67 |
| Gender (Female vs. Male) | 1.00 | |||||
| Female | 1.00 | |||||
| Male | 0.638 | 1.79 | 0.16–20.30 | 0.242 | 4.76 | 0.35–65.09 |
| AJCC/UICC clinical stage | ||||||
| Stage I | 0.989 | 1.00 | 0.395 | 1.00 | ||
| Stage III | 0.963 | 1.13 | 0.01–174.44 | 0.703 | 2.35 | 0.03–188.77 |
| Stage IV | 0.882 | 1.16 | 0.16–8.46 | 0.174 | 4.66 | 0.51–42.80 |
| ATA initial risk classification | ||||||
| Low risk | 0.419 | 1.00 | 0.511 | 1.00 | ||
| Intermediate risk | 0.191 | 0.05 | 0.00–4.41 | 0.316 | 0.15 | 0.00–6.15 |
| High risk | 0.755 | 0.72 | 0.10–5.55 | 0.919 | 1.12 | 0.12–10.48 |
| Pre-ablation serum Tg level (continuous value) | 0.040† | 1.75 | 1.03–2.99 | 0.006† | 1.48 | 1.12–1.95 |
| Thyroid bed uptake in initial WBS | ||||||
| Faint uptake | 0.331 | 1.00 | 0.284 | 1.00 | ||
| Medium uptake | 0.819 | 1.46 | 0.06–36.40 | 0.441 | 3.97 | 0.12–133.02 |
| Strong uptake | 0.182 | 5.15 | 0.46–57.04 | 0.117 | 7.55 | 0.60–94.34 |
| Constant | 0.101 | 0.01 | 0.014 | 0.00 | ||
* Response to RAI ablation if: WBS (–) and post-ablation serum Tg level ≤5 ng/mL
** Response to RAI ablation if: WBS (–) and post-ablation serum Tg level ≤10 ng/mL
† p < 0.05
| Pre-ablation serum Tg value cut off |
Based on our hospital’s RAI ablation response criteria* |
Based on new RAI ablation response criteria** |
||
|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | |
| 5.40 | 0.73 | 0.92* | 0.74 | 0.86* |
| 5.95 | 0.68 | 0.96 | 0.68 | 0.89 |
| 10.50 | 0.64 | 1.00** | 0.68 | 0.96** |
| 16.25 | 0.55 | 1.00 | 0.58 | 0.96 |
* Response to RAI ablation if: WBS (–) and post-ablation serum Tg level ≤5 ng/mL
** Response to RAI ablation if: WBS (–) and post-ablation serum Tg level ≤10 ng/mL

ROC curves of the predictive value of pre-ablation serum Tg level to RAI ablation response; based on hospital’s criteria (blue line) and based on the new criteria (red line).
Based on the new response criteria, the full model from logistic regression was also statistically significant and slightly improved χ2(9, n=47) = 31.67 (p < 0.001) and maintained its accuracy by correctly classified 85.1% of cases. Table 4 showed that the pre-ablation serum Tg level remained the only predictive factor significantly contributed to the model (p < 0.001); and each increase of the pre-ablation serum Tg level increasing the non-response chance of RAI ablation by 1.48 times (95% CI = 1.12–1.96). A ROC analysis showed that by the use of pre-ablation serum Tg level cut-off of 10.5 ng/mL, specificity to predict RAI ablation response was 96%, and the sensitivity was slightly increased to 68%; while the use of cut-off of 5.4 ng/mL yielded 74% sensitivity and 86% specificity (Fig. 2, Table 5).
The current finding represented our 4.5-years experience regarding the use of 30 mCi dose RAI ablation for post total thyroidectomy DTC patients. To the best of our knowledge, this was the first study in Japan evaluating the patient/disease-related factors for patient selection tool for 30 mCi RAI ablation dose in the whole ATA risk classification-range since the release of Japanese clinical guidelines for Treatment of Thyroid Tumor in 2010. The use of low dose RAI ablation in Japan is frequently also being considered for high-risk DTC patients due to the lack of isolation wards [24]. However, no strong predictive factor nor patient selection tool has been proposed for such a low dose approach until recently. Consequently, a speculative clinical decision for RAI ablation sometimes inevitable, for example in the case of high-risk but non-metastatic elderly DTC patients.
The participants in this retrospective study represent most of the entire risk range according to ATA risk classification, despite the exclusion of metastatic cases. However, it is important to note that the definition of RAI ablation failure in this study was both the positive imaging on WBS and stimulated serum Tg level of ≥5 or ≥10 ng/mL; as evaluated six months after RAI ablation. As previously mentioned, this serum Tg level was much higher than that endorsed in 2015 ATA guidelines (<1 ng/mL) and elsewhere to categorize a patient as having an excellent response [5, 15]. Nonetheless, these successful ablation criteria are yet more stringent than that employed in several large-scale randomized controlled studies evaluating the efficacy of low dose RAI ablation, which some relied on negative imaging only [12].
Detectable serum Tg after total thyroidectomy may indicate incomplete surgery, which implies a high risk of recurrence. It also has been well recognized that detectable serum Tg frequently warns the existence of metastatic lesions. However, persistent disease (Tg detectable after total thyroidectomy) was reported to be a common finding in Japan, and most of them survived without RAI ablation if their Tg level was stable [29].
The multi-factor analyses in our study suggested that the pre-ablation serum Tg level post total thyroidectomy may be useful as a patient selection tool and response predictor for 30 mCi RAI ablation. Non-metastatic DTC patients with pre-ablation serum Tg level at least below 5.4 ng/mL may be the right candidate for this outpatient RAI ablation, and they likely achieve successful ablation, disregarding their age, gender, AJCC/UICC clinical stage, and their ATA risk classification grade. Patients with serum Tg level between 5.4 to 10.5 ng/mL might also be recommended in certain circumstances, such as low risk patients. On the other hand, those with serum Tg level of 10.5 ng/mL or higher would likely require RAI ablation with doses higher than 30 mCi. Our finding resonate the result from a recent study which focused only in intermediate-high risk patients in which Tg levels is among the most important factors for RAI ablation response. It was suggested that Tg levels greater than 10 ng/mL were significantly associated with RAI ablation failure [30]. Similar to that study, our finding also suggested that the thyroid bed uptake on initial WBS (two days after RIA ablation) failed to predict the RAI ablation response six months later.
Recently, pre-ablation serum Tg has been established as a predictive/prognostic factor for DTC, especially in long-term follow-up of 30 mCi RAI ablation prepared with withdrawal from thyroid hormone replacement [31]. In a large meta-analysis (n = 3,947), Webb et al. confirmed the previous findings that DTC patients with pre-ablation Tg level below 10.0 ng/mL have only a 6% likelihood to have a recurrent or persistent disease [32]. However, no studies from Japan were included in that meta-analysis, and more importantly, the RAI ablation dose was not limited to 30 mCi only. In the other hand, studies specifically evaluated the 30 mCi RAI ablation mostly treated those with low to intermediate risk. A Canadian study (n = 193, low to intermediate risk patients) revealed that pre-ablation Tg level cut-off of 6 ng/mL was predictive of 30 mCi RAI ablation response [33], while a Brazilian study (n = 237, low to intermediate risk patients) reported 10 ng/mL [34] which later included in their national consensus [35]. A Korean study (n = 176, low to intermediate risk patients), also indicated that 10 ng/mL cut-off was useful to predict response to 30 mCi RAI ablation [36], which was of particular interest since both Korean and Japanese are similar in their iodine-rich the dietary status [37].
The 2015 ATA guidelines allow the postsurgical Tg level as a tool to aid in adjuvant RAI ablation decision-making [5], including a suggestion to omit RAI ablation in low-risk patients. A recent 5-year median follow-up study in Brazil also confirmed that DTC patients with the postsurgical (non-stimulated) Tg level <0.3 ng/mL did not require RAI ablation [38]. However, their Western counterpart (European) strongly disagreed [16] and suggested that the postoperative serum Tg value may be more helpful in identifying patients that benefit from RAI ablation rather than in the identification of those who do not require ablation [39]. Apparently, in the European population (n = 1,298), the low dose (≤54 mCi) in the first RAI ablation yielded a worse long-term outcome, even for low-risk DTC patients [40].
In Japan, the concern is not only between RAI ablation or no ablation but also how to optimize the decreasing amount of isolation ward facilities with the raising demand of RAI ablation [18, 21]. In this critical situation, outpatient 30 mCi RAI ablation become a valuable option for high-risk patients. A Japanese study showed that 30 mCi might be enough for high-risk DTC patients [24]. However, successful ablation criteria in that study were only the negative finding on post-ablation WBS (mean, 7 months), which warrant careful interpretation, despite the excellent long-term outcome of thyroid cancer patients in this country. On the contrary, a more recent Japanese study showed that even 100 mCi dose may not be enough for intermediate-high risk DTC patients, if the pre-ablation Tg levels are more than 10.5 ng/mL [30].
The main considerations of using the post-surgical pre-ablative serum Tg to suspect a residual thyroid tissue are 1) serum Tg levels are dependent on circulating thyrotropin (TSH) concentrations, and 2) measurement techniques for serum Tg are not reliable in the presence of anti-Tg antibodies. However, TSH levels of responder and non-responder according to either our hospital-based or the new RAI ablation criteria were similar in this study. Also, even though only 62% (n = 29) of our patients had their anti-Tg antibody measurement result, 90% (n = 26) among them had observed as negative (<100 IU/mL).
As concluded in many studies, age was not a predictive factor for 30 mCi RAI ablation [33, 36]. However, American and European consensus determine 45 years old as the risk limit in a long-term outcome [5, 15]. In the Japanese population, the risk limit is apparently much higher. PTC patients with age >60 years old are prone to locoregional recurrence, distant recurrence and cancer-related death about 1.7, 2.9 and 5.9 times more likely than younger patients, respectively. Uniquely, young PTC patients (<30 years old) also have 1.6 times higher risk of locoregional recurrence, but not distant metastasis and death [41]. Therefore, such age consideration should also apply when 30 mCi RAI ablation become an available option.
This study was limited by the retrospective nature and a small sample size obtained from a single facility despite the 4.5-year period (the outpatient 30 mCi dose therapy was started in March 2012). Our institution has treatment beds for maximum six patients per month. Our situation might not be suitable for generalization for the most healthcare institution in Japan; however, the lack of isolation ward facility is real, and 30 mCi RAI ablation provided an emergency exit and temporary solution for DTC patients.
The limited sample size and outcome also confined the number of variables in the logistic regression model (only six variables at most with 25 outcome events) [42]. Careful exclusion of variables has been performed based on valid references to avoid overfitting due to the excessive complexity of the model. However, a larger study might allow evaluation of variables excluded in this study. The limited number of patients receiving rhTSH also prevented further statistical analysis of the possible role of TSH elevation methods.
Another major limitation of this study was the short period of evaluation. Most of the participant in this study had their ablation response evaluated within six months after ablation therapy. Some of the patients were referred from other centers without isolation facility for RAI ablation; they were referred back after their first evaluation. Long-term follow-up, particularly in high-risk patients, would be more appropriate to describe the actual response to 30 mCi RAI ablation. Particularly, in the Japanese population, the serum Tg-doubling time after total thyroidectomy has been reported to be a very strong prognostic factor for survival [29], which warrant a further study for this particular dose.
Pre-ablation serum Tg may help patient selection and predict response to outpatient 30 mCi RAI ablation dose among non-metastatic papillary DTC patients received total thyroidectomy. A larger-scale or multi-center study with longer follow-up period is necessary to confirm our finding.
For retrospective studies, written consent is deemed unnecessary according to national regulations. However, Institutional Review Board approval (from Gunma University/Gunma University Hospital) was obtained for this retrospective analysis. Patient consent was waived.
Consent for publicationNot applicable.
Availability of data and materialAll the data is contained within the manuscript.
Competing interestsNot applicable.
FundingNot applicable.
Authors’ contributionsTH designed the study, collect patients’ data, and edit and review the manuscript, AA analyzed the data and wrote the manuscript, DDB assisted in patients’ data collection, AB assisted in data analysis and edited the manuscript, YT edits and reviews the final manuscript. All authors contributed to manuscript preparation, and read and approved the final manuscript.
AcknowledgementsNot applicable.