2018 Volume 65 Issue 9 Pages 963-967
2018 Volume 65 Issue 9 Pages 963-967
Intravenous (i.v.) glucocorticosteroids (GCs) constitute the first-line treatment for active and moderate-to-severe Graves’ orbitopathy (GO). In cases of persistent disease, rituximab, a monoclonal anti-CD20 antibody, may be used, although studies have yielded conflicting results. In case 1, a 50-year-old female heavy smoker presented with severe bilateral disfiguring eyelid edema of four months, bilateral exophthalmos and a clinical activity score (CAS) of 5/7. Laboratory investigation showed thyrotoxicosis and high thyroid-stimulating immunoglobulin (TSI) levels [32 IU/L (normal <1.75]. After minor improvement by i.v. methylprednisolone and standard retrobulbar radiotherapy (20 Gy), her visual acuity progressively declined to “hand motion”. Rituximab was administered (two pulses of 500 mg, two weeks apart), with significant response. At 3 1/2 years of follow-up, CAS is 0/7 and CD20+ lymphocytes remain at the lower normal range. In case 2, a 78-year-old non-smoker male was referred for management of severe active GO, one month after total thyroidectomy for Graves’ thyrotoxicosis (TSI: 6.74 IU/L). Over the preceding two-three months, severe GO manifested with chemosis, constant diplopia, loss of color vision and acuity of 1/10 bilaterally (CAS: 7/7). Following partial response to i.v. methylprednisolone and concomitant radiotherapy, rituximab (two pulses of 500 mg each, two weeks apart), was administered. Vision partially recovered and GO remains in remission one year later, even after 131I (100 mCi) administration for papillary thyroid carcinoma (TSI: 0.9 IU/L and CD20+ count at the lower normal range). In conclusion, rituximab may be an effective second-line therapy in GO patients, providing long-lasting remission.
INTRAVENOUS (I.V.) glucocorticosteroids (GCs), with or without combined radiotherapy are the first-line treatment for active and moderate-to-severe Graves’ orbitopathy (GO) [1]. GCs’ actions in GO, are directed against several aspects of the inflammatory cascade involving the extraocular muscles and soft orbital tissue and are effective in up to 80% of cases [1, 2]. However, in the rest of patients, response is unsatisfactory and, in 10–20%, GO relapses [1, 2]. In persistent or recurrent active GO, second-line options include a second course of i.v. GCs (if a cumulative dose of 8 g has not been exceeded), orbital radiotherapy and rituximab [1, 2].
Rituximab was the first chimeric monoclonal anti-CD20 antibody approved for the treatment of non-Hodgkin’s B-cell lymphoma, chronic lymphocytic leukemia and rheumatoid arthritis. However, it has also been used off-label in various autoimmune diseases, such as Wegener’s granulomatosis and multiple sclerosis, due to its direct B-cell-depleting action [3]. A number of case reports and uncontrolled open-label studies indicate rituximab as an effective treatment for GO, where GCs have failed to induce remission [1, 2, 4]. Two randomized controlled trials (RCTs) have yielded conflicting results [5, 6].
We describe two cases of very severe GO, treated successfully with rituximab to long-lasting remission.
A 50-year-old female heavy smoker presented with pronounced and worsening bilateral disfiguring eyelid edema, tearing, pruritus and intermittent vertical diplopia of several months. She had also bilateral exophthalmos (24/110/24) and inconstant diplopia only in extremes of gaze. Clinical activity score (CAS) was 5/7, but visual acuity and color perception were preserved. On physical examination, she presented resting tachycardia (110 bpm) and gross periorbital edema (Fig. 1A). Orbital computerized tomography (CT) showed enlargement of all extraocular muscles (Fig. 2A). Thyroid function tests showed: TSH: 0.005 μIU/L (normal 0.27–4.2), free-T4: 2.39 ng/dL (normal 0.7–2) and free-T3: 3.23 ng/mL (normal 0.8–2). Moreover, pretibial myxedema was detected on both shins. Thyroid-stimulating immunoglobulin (TSI) levels were 32 IU/L (normal <1.75) [7].

Severe eyelid edema before (A) and after treatment with rituximab (B)
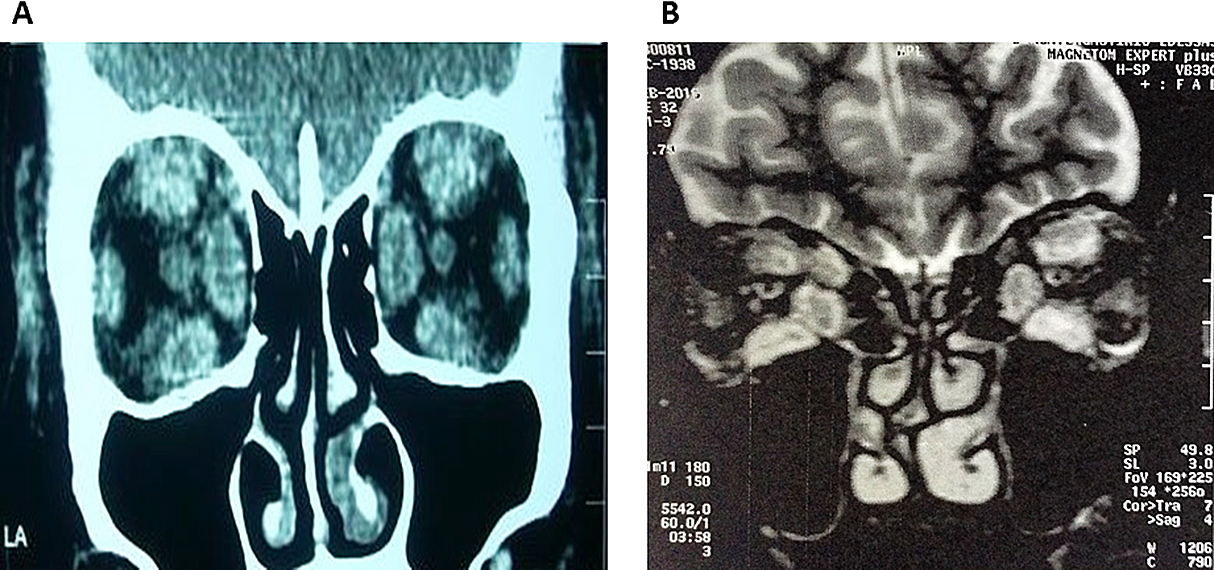
Coronal images of case 1 (CT) (A) and case 2 (MRI) (B), before rituximab therapy
The patient received i.v. methylprednisolone over three months (cumulative dose 6 g), with minor improvement [7]. Standard retrobulbar radiotherapy (20 Gy) followed, with little additional benefit. After ineffective treatment of thyrotoxicosis with methimazole, lithium was added and the patient underwent an uneventful thyroidectomy.
However, the patient’s visual acuity declined to “hand motion” three months after completion of the above treatments. The patient was deemed at high anesthetic risk for orbital decompression because of severe chronic obstructive airway disease and obesity, so “salvage therapy” with rituximab ensued. Two pulses of rituximab [375 mg/m2 (500 mg)], two weeks apart, were administered. During the first infusion, she developed diffuse erythroderma and bronchospasm, which abated with supportive therapy, while the infusion continued and was completed at a slower rate. CD20+ depletion was immediate (within the hour) and sustained at six months. The patient perceived an improvement in visual acuity within a month and when assessed at 3 months, acuity was 4/10 in the right eye and 2/10 in the left. At six months, visual acuity was 4/10 bilaterally.
At 3 1/2 years of follow-up, CD19+ and CD20+ lymphocytes are currently present in the peripheral blood, albeit at the lower range of normal. The vast majority of circulating B lymphocytes (96.7%) are naive and IgM memory cells are still very low (2.5%, normal 13.4–21.4). CAS is 0/7 (Fig. 1B) and the patient maintains an acuity of 2/10 (Fig. 1B). She continues to smoke (20–30 cigarettes/d). Of note, TSI remain at 14.2 IU/L and the patient’s pretibial myxedema has evolved to a remarkable bilateral multinodular dermopathy, compatible with nodular mucinosis.
A 78-year-old non-smoker male was referred to our combined thyroid-eye clinic for management of severe, active GO, one month after a total thyroidectomy for Graves’ thyrotoxicosis. An incidental papillary thyroid carcinoma of insular variant (1.05 cm) was detected in the right lobe. TSI levels were 6.74 IU/L (normal <1.75). His eye manifestations had evolved precipitously over the preceding two-three months with chemosis, constant diplopia, fixed globe, loss of color vision, acuity of 1/10 bilaterally and significant orbital pain. CAS was 7/7 (Fig. 3A). Orbital magnetic resonance imaging (MRI) showed enlargement of all extraocular muscles (Fig. 2B). His past history was remarkable for ischemic heart disease, aortic stenosis, arterial hypertension and dyslipidemia.
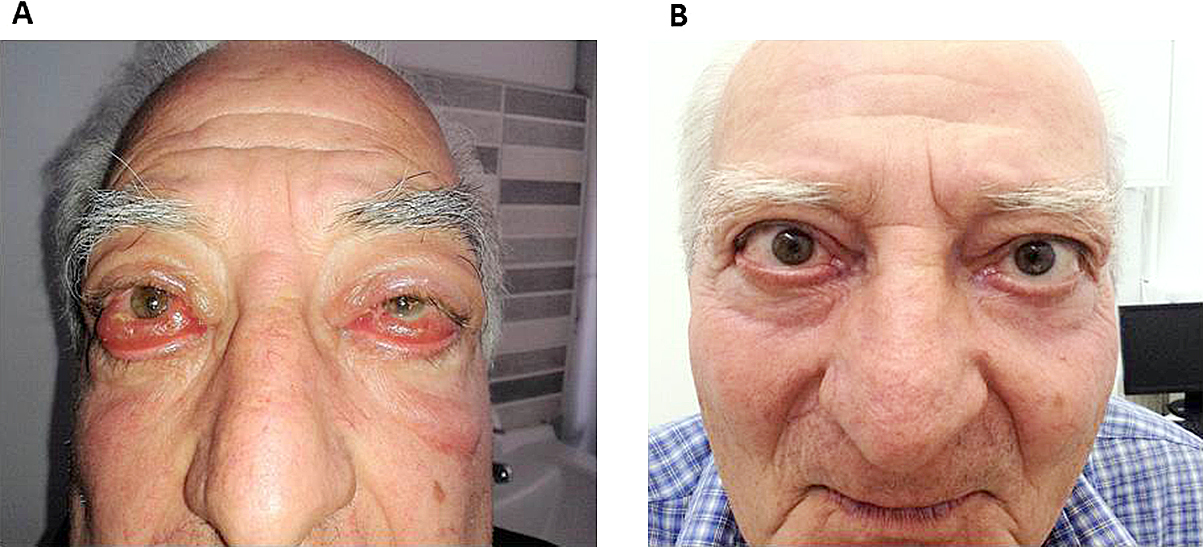
Severe sight threatening GO before (A) and after treatment with rituximab (B)
The patient was urgently initiated on i.v. methylprednisolone pulses of 1 g/d on two consecutive days a week every two weeks, to a cumulative dose of 8 g, which he tolerated well. Concomitant retrobulbar radiotherapy of 20 Gy was delivered, which resulted in partial remission of inflammation, chemosis and pain. However, acuity worsened to light perception on the right with a relative afferent pupillary defect on the left and frozen globes. Orbital decompression was deemed inappropriate due to high risk of globe rupture. Rituximab at two pulses of 500 mg each, two weeks apart, was administered without complications. Complete B-lymphocyte depletion was noted after the second infusion and persisted two months later. One month following completion of therapy, the patient reported improved vision in the right eye (2.5/10) and perception of moving objects on the left. He proceeded to cataract surgery on the left, with improvement to finger counting on this side. The vision gained, allowing the patient to return to his activities of daily living independently. His GO remains in remission one year later (Fig. 3B).
131I (100 mCi) was administered two months after rituximab, without GO relapse. He is still in remission one year later, with negative TSI (0.9 IU/L) and anti-thyroglobulin antibodies, undetectable thyroglobulin (<0.1 ng/mL), with TSH: 1.57 μIU/mL and CD20+ count at the lower normal range.
Moderate-to severe and sight-threatening GO have a low prevalence in Europe, with the latter representing 2% of all GO [8]. However, it can negatively impact many aspects of the patient’s quality of life [1]. Regarding rituximab, two RCTs have been conducted so far. The first showed superiority (n = 15) over GCs (n = 15) (100% versus 69% with inactive disease, respectively) [5]. The second failed to show any difference between rituximab (n = 13) and placebo (n = 12) [6]. Longer disease duration before rituximab may be the main factor compromising response. Trials of rituximab combined with oral or i.v. GCs have not been conducted in GO.
The 2016 EUGOGO guidelines recommend caution with the use of rituximab in DON, as some cases of progression of GO to DON after treatment have been observed [1]. However, there are also several reports of sight threatening GO improvement with rituximab [9] and it is considered part of routine clinical care for unresponsive disease by some experts [10].
Several dosing schemes have been used, the classic scheme being 1,000 mg twice, two weeks apart, but it seems that lower than standard dosing is effective and may bring about equivalent clinical results in GO (a single 100 mg infusion or a single 500 mg infusion) [1, 5, 11]. Based on the available evidence, we chose to treat our patients with an intermediate dose regimen of 500 mg twice, two weeks apart.
Our patients have had a long-term remission of disease activity and dysthyroid optic neuropathy (DON) was offset, despite late partial B-cell recovery and persistent TSI in the first case. No further treatment for GO was required for our patients, except for lubricants. Relapse of inflammation can reach 30% in GC-treated patients [5], while no relapses have been reported with rituximab even after five years [5, 12]. It is unlikely that the steady improvement to restoration of inflammation and DON in our patients was a late beneficial effect of preceding treatments [13], as in both cases rituximab was administered >3 months after completion of conventional therapies. During this time inflammation was relentless and DON, which is irreversible if untreated, developed in the course. Visual field testing or visual evoked potentials were not tested for diagnosing DON in our patients. Visual loss is considered a late sign of DON, while color desaturation is an early sign [14]. Prior to rituximab, the first case developed visual decline and loss of color vision, while the second case had visual loss and a relative afferent papillary defect, indicating DON. B-cell depletion of long duration (≥6 months) has been reported in GO [4], following even a single pulse of a small dose [11]. While the prognostic significance of peripheral cell reconstitution in GO has not been studied, disease remission appears to be independent of B-cell recovery and persistent TSI titers [2]. Progression of GO to DON following rituximab treatment has rarely been reported, while most studies have reported improvement of DON [1, 2, 5, 6].
Infusion related adverse events are common, mostly mild, and include myalgias, skin reactions and gastrointestinal upset. Serious side effects are uncommon but may include a “cytokine release syndrome” [5, 15]. Pre-infusion prophylaxis with diphenhydramine and paracetamol may be recommended [9].
Due to lack of solid evidence of superiority and unfavorable adverse risk profile compared to GCs, rituximab use is reserved for steroid-resistant GO [1, 2]. Nevertheless, similarly to GCs, rituximab does not seem to improve proptosis, although it may act as a disease-modifying agent in some cases [2]. However, the evidence from two RCTs [5, 6] and several case series, points to a valuable role of rituximab in patients with persistent disease activity and progression on severity (such as development of DON) after first-line treatment with glucocorticoids and radiotherapy, when surgery is contraindicated or unacceptable.
In conclusion, rituximab is an effective second-line therapy for persistent and severe GO, can resuscitate vision and induce durable remission of inflammation, significantly restoring quality of life. Whether earlier timing of rituximab therapy in selected cases could reverse disease progression or not, remains to be explored.
The authors have no conflict of interest to disclose.