2019 Volume 66 Issue 5 Pages 423-430
2019 Volume 66 Issue 5 Pages 423-430
T helper (Th) 17 cells and interleukin (IL)-17 play a significant role in the pathogenesis of autoimmune thyroid disease (AITD). However, it has recently become clear that Th17 cells are more heterogeneous and exhibit two different phenotypes, whereas IL-23 and IL-1β are crucial for the generation of pathogenic Th17 lymphocytes. We aimed to investigate the association between IL-17 and Th17-promoting cytokines in AITD by studying the immunoexpression patterns of IL-17, IL-23, and IL-1β in thyroid tissue. Following thyroidectomy, 29 patients with AITD (21 cases of Hashimoto’s thyroiditis (HT) and 8 cases of Graves’ disease (GD)) and 18 patients with colloid goiter, as controls, were enrolled in this study, and immunohistochemistry was performed. The expression level of IL-17 in thyrocytes was significantly higher in HT and GD patients than in colloid goiter patients. Immunopositivity for both IL-23 and IL-1β was significantly increased in HT patients compared to GD and colloid goiter patients. However, no difference was found between IL-23 or IL-1β expression in patients with GD and colloid goiter. A positive correlation between IL-17 and IL-23 as well as IL-17 and IL-1β expression was observed in HT patients (r = 0.574, p = 0.007 and r = 0.461, p = 0.036, respectively). In the GD group, IL-17 was positively correlated with IL-1β (r = 0.817, p = 0.013) but not with IL-23 expression. We found increased IL-23 and IL-1β expression in the HT group but not in the GD group. Furthermore, both interleukins were correlated with IL-17 immunopositivity in thyroid tissue, suggesting that pathogenic Th17-promoting cytokines may play a role in HT pathogenesis.
AUTOIMMUNE THYROID DISEASES (AITD), with their main clinically opposite manifestations as hypothyroidism in Hashimoto’s thyroiditis (HT) and hyperthyroidism in Graves’ disease (GD), are the most common autoimmune disorders [1]. Although both diseases belong to AITD, they are different in pathogenic mechanisms and the presentation of the dysregulated immune system. The absence of cytotoxicity and structural damage of the follicle and the prevalence of humoral autoimmune response against TSH receptor are the main features distinguishing GD from HT. In the case of HT, cytotoxic T lymphocytes induce programmed cell death in thyrocytes by the release of perforin and granzymes, subsequently leading to the development of hypothyroidism. Additionally, thyroid follicular cells can undergo caspase-mediated apoptosis by expressing Fas/Fas ligands [2]. Although the contribution of anti-thyroid peroxidase (TPO) antibodies to thyroid follicular destruction compared to T cell attack and cytotoxic effects is minor, antibodies have shown several pathogenic effects, such as complement activation and the inhibition of enzymatic activity [3]. Furthermore, higher oxidant parameters and lower antioxidant parameters have been found in euthyroid treated-naïve HT patients, indicating that the presence of TPO antibodies is the main independent risk factor for developing oxidative stress, irrespective of thyroid function [4].
Two classical thyroid autoimmunity phenotypes can be distinguished by a balance between T helper (Th) 1- and Th2-mediated immune activity. Thus, HT predominantly promotes the Th1 immune response, characterized by the upregulation of pro-inflammatory cytokines, such as interleukin (IL)-2, IL-1β, interferon (IFN)-γ, tumor necrosis factor (TNF)-α, leading to cell-mediated immunity and thyrocyte death in apoptotic pathways. In GD, there are increased levels of Th2-released cytokines, such as IL-4, IL-5, IL-6, IL-10, and IL-13; these cytokines promote the humoral response, stimulating the production of TSH receptor antibodies and enhancing the survival and growth of thyroid cells and apoptosis of infiltrating lymphocytes either by the downregulation of Fas/Fas ligand or the upregulation of the anti-apoptotic molecule Bcl-2 [5]. The fact that both thyroid disorders may coexist in the same individual is indicative of the presence of mixed Th1/Th2 and probably Th17 responses in both HT and GD patients.
A growing body of evidence suggests that IL-17A, the prominent effector cytokine of Th17, can induce the release of different pro-inflammatory cytokines and chemokines responsible for the induction and development of chronic inflammatory responses in many autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, psoriasis, juvenile idiopathic arthritis, and inflammatory bowel disease [6-8]. Th17 cells also play an essential role in the pathogenesis of AITD.
Various cytokines and lineage-associated transcription factors are essential for the regulation of human Th0 lymphocyte differentiation into Th1, Th2, Th17, Th9, Th22, follicular T helper cells or regulatory T cell subsets. Thus, the development of the Th1 population is induced by IL-12 or IFN-γ, whereas IL-4 initiates the differentiation of the humoral response promoting Th2 cells [9]. Continuing studies have highlighted a spectrum of transcription factors and cytokines that can induce and maintain Th17 cell differentiation in both mouse models and humans. The combination of transforming growth factor (TGF)-β and pro-inflammatory cytokines, such as IL-1β, IL-6, IL-21, and IL-23, has been found to play a crucial role in humans. It has been shown that TGF-β in the presence of IL-6 and IL-21 promotes the initial differentiation of Th17 cells by the activation of RAR-related orphan receptor (ROR) C, while IL-23 and IL-1β are important for both the full maturation and stabilization of the Th17 phenotype [10, 11]. Interestingly, although IL-23 is not required for the induction of Th17 differentiation (naïve T cells do not express IL-23 receptor), IL-23-dependent signaling is crucial for the generation of highly pathogenic Th17 cells via signal transducer and activator of transcription (STAT) 4 [12]. It has recently been reported that IL-23 levels are enhanced in the peripheral blood [13] and thyroid tissues [14] of HT patients; however, it is still unknown whether IL-23 is involved in the pathogenesis of GD. IL-1β is another pro-inflammatory cytokine that participates in AITD [15] and is essential for the conversion of classical Th17 lymphocytes into the pathogenic Th17 subset [16]. Remarkably, IL-1β synergizes with both IL-23 and IL-6 to enhance the expression of interferon regulatory factor 4, which in combination with STAT3 further induces RORC during Th17 development [11].
Several studies have demonstrated a significant increase of Th17 lymphocytes and IL-17 in the peripheral blood and/or thyroid tissues of AITD patients, suggesting their contributions to thyroid autoimmunity. The overexpression of IL-17 within thyroid tissues from HT and GD patients compared to controls was also revealed in our previous study [17]. However, the role of interleukins in promoting the differentiation of Th17 cells has not been thoroughly evaluated in HT and GD pathogenesis. In the current study, we aimed to investigate the association between IL-17 and pathogenic Th17-promoting cytokines in AITD by studying the immunoexpression patterns of IL-17, IL-23, and IL-1β in thyroid tissue.
Twenty-nine adult patients with AITD (21 cases of HT and 8 of GD) who underwent thyroidectomy at Riga East Clinical University Hospital, Latvia, between January 2014 and December 2016 were enrolled in this study. Eighteen age- and gender-matched patients with colloid goiters displaying normal thyroid function served as the control group. The mean age of AITD patients and controls was 49.52 years (range, 28–68 years; 27 females) and 51.78 years (range, 33–72 years; 16 females), respectively. AITD was confirmed by the clinical manifestations of HT and GD, complemented by thyroid biochemical tests, ultrasound imaging, and histopathological examination. At the time of GD diagnosis, the patients had hyperthyroidism, positive anti-TSH receptor antibodies, and diffuse goiter on ultrasound examination. HT patients demonstrated positive antibodies to TPO and/or thyroglobulin, negativity for anti-TSH receptor antibodies, and features of chronic autoimmune thyroiditis on ultrasound examination. The eighteen patients with colloid goiters displaying normal thyroid hormone levels and an absence of thyroid antibodies were used as controls. The indications for surgery in HT patients were large thyroid nodules with or without obstructive symptoms and suspicion of malignancy. The main indications for thyroidectomy in the GD group were large goiters and the need for definitive treatment due to multiple relapses following medical treatment. Patients with colloid goiters underwent thyroidectomy due to large goiters and/or nodules, cosmetic reasons and suspicion of malignancy. Patients with thyroid malignancies confirmed on pathology were excluded. The current study was approved by the Riga Stradins University Ethics Committee, and written informed consent was obtained from all patients.
Forty-seven paraffin-embedded thyroid tissue blocks from 47 patients along with their histopathology reports were obtained from the Pathology Center of Riga East Clinical University Hospital and used for immunohistochemical study.
ImmunohistochemistryInitially, 4–5 μm thick sections were cut from formalin-fixed and paraffin-embedded thyroid tissue samples and mounted on slides; consecutive sections were used as negative controls for the immunohistochemical reactions. The sections were deparaffinized, endogenous peroxidase activity was blocked with 0.1% H2O2 in methanol and heat-induced antigen retrieval was accomplished in 10 mmol/L citrate buffer, pH 6.0. Thereafter, the sections were successively incubated at 4°C overnight with the following primary antibodies: polyclonal rabbit anti-IL-17A (Biorbyt, Cambridge, UK, 1:300); polyclonal rabbit anti-IL-23 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA, 1:100); and polyclonal rabbit anti-IL-1β (Santa Cruz Biotechnology, Inc., 1:50) antibodies. The results of the immunohistochemistry (IHC) reactions were visualized by HRP HiDef Polymer Detection™ System (CellMarque, Rocklin, CA, USA). For visualization of the antigen sites, 3,3'-diaminobenzidine tetrahydrochloride (DAB + Chromogen and DAB + Substrate buffer (CellMarque, Rocklin, CA, USA)) was used. The thyroid tissue sections were then counterstained with hematoxylin, washed, dehydrated and coverslipped.
IHC controls included substitution of the primary antibody with buffer solution. The assessment of immunostaining was performed by two independent observers blinded to the clinicopathological data. Tissue specimens were analyzed at both medium and high power magnification (×200 to ×400) using a DMRB light microscope (Leica, Wetzlar, Germany) equipped with a DFC 450C digital camera. The slides were scanned and digitized using a Glissando Slide Scanner (Objective Imaging Ltd., Cambridge, UK), and the images were captured using Aperio ImageScope software (Leica Biosystems, version 12.2.2.5015).
The cells labeled by the aforementioned antibodies displayed a brown cytoplasmic staining pattern and were considered immunopositive. The levels of immunopositivity for each antibody were expressed in a semiquantitative manner and graded as negative (0), weak (1), moderate (2) or strong (3), when thyroid follicular cells were positive at 0–5, 6–25, 26–50, or >50%, respectively.
Additionally, to provide clear visual details about the cellular distribution and localization of the cytokines, thyroid tissue specimens obtained from five HT patients during thyroidectomy were fixed in aldehyde and further processed for immunofluorescent labeling and confocal microscopy. Immunofluorescence (IF) staining of IL-1β, IL-17A, and IL-23 was performed using goat anti-mouse IgG-FITC: sc-2010 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, 1:300) as the secondary antibody; the samples were counterstained with 4',6-diamidino-2-phenylindole (DAPI) (Thermo Fisher Scientific, Invitrogen, UK, 1:3,000) and then embedded in Prolong Gold with DAPI (Thermo Fisher Scientific, Invitrogen, UK). Digital photos were taken using a confocal microscope Eclipse Ti-E (Nikon, Tokyo, Japan).
Statistical analysisThe results are shown as the median and interquartile range of the immunohistochemical findings. Nonparametric statistics were used, i.e., Mann-Whitney test, to assess differences between two independent groups and the Spearman rank correlation test was used to describe correlations. A significance level α = 0.05 was applied. Statistical analyses were performed by using IBM SPSS software, version 23.
The semiquantitative data describing IHC expression of the antigens used in this study are summarized in Table 1. IL-17 immunopositivity was observed in the thyrocytes and inflammatory infiltrates of HT patients (Fig. 1A), and the level of expression was assessed mostly as moderate (13/21 patients showed staining grade ≥2). Additionally, the impaired integrity and destruction of follicular cells was frequently revealed in the IL-17-positive thyroid follicles of HT patients. In contrast to HT, patients with GD and colloid goiters showed predominantly weak and negative IL-17 expression, respectively. The expression level of IL-17 in the thyrocytes was significantly higher in HT and GD patients than in patients with colloid goiters (p < 0.001 for both) (Fig. 2A). Moreover, HT patients demonstrated higher IL-17 immunoreactivity than did GD patients, but the difference was not significant (p = 0.069).
| IL-17 | IL-23 | IL-1β | |
|---|---|---|---|
| Hashimoto’s thyroiditis | 2.2 (1.6; 2.8) | 0.29 (0.14; 0.57) | 0.71 (0.57; 1.42) |
| Graves’ disease | 1.3 (1; 2.3) | 0.14 (0.04; 0.25) | 0.22 (0.04; 0.29) |
| Colloid goiter | 0.6 (0.6; 1) | 0.14 (0.0; 0.14) | 0.07 (0.0; 0.14) |
Data are shown as median and interquartile range of the immunohistochemical findings. Interleukin (IL).

(A) HT patient evidencing IL-17-positive thyroid follicles and inflammatory cells (×400). (B) IL-23-positive cells within the inflammatory follicle in HT patients (×400). (C) IL-23 positivity demonstrated in the follicular epithelial cells of HT patients (×200). Confocal microscopy, representative image of IL-23 positive thyrocytes: green staining shows IL-23-specific staining, blue staining shows nuclei, insert (×1,000). (D) Strong cytoplasmic expression of IL-1β demonstrated in the thyrocytes of HT patients (×400). Confocal microscopy, representative image of IL-1β-positive thyrocytes: IL-1β-specific staining (green), nuclei (blue), insert (×1,000). (E) Weak IL-23 immunoreactivity in GD patients (×250). (F) Occasional IL-1β positivity revealed in GD patients (×400). (G) Colloid goiter showing almost nil IL-1β expression (×250). (H) Dendritic cells observed within inflammatory infiltrate in HT patients (×400). Graves’ disease (GD), Hashimoto’s thyroiditis (HT), interleukin (IL).
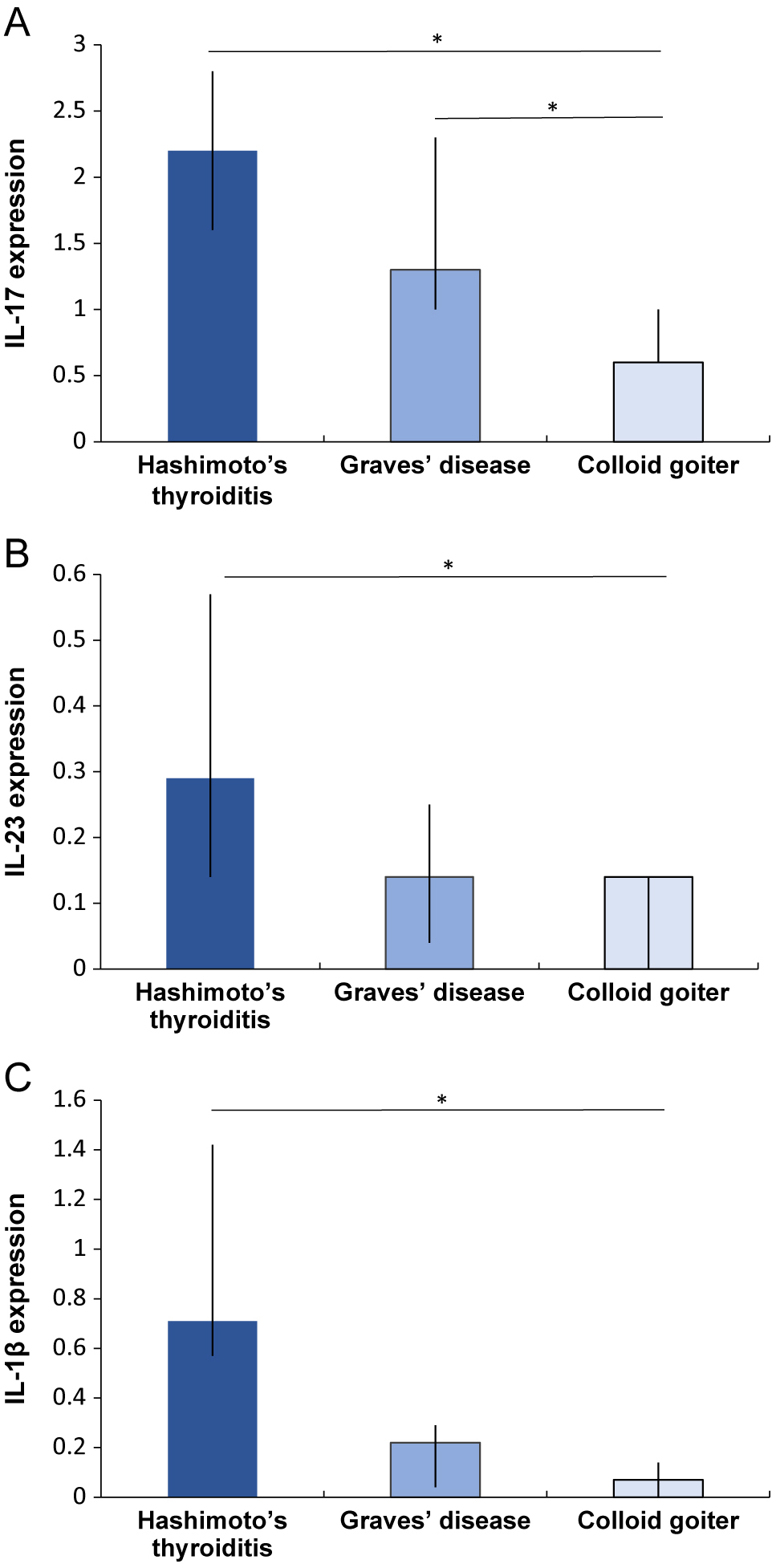
Comparison of IHC results of IL-17 (A), IL-23 (B) and IL-1β (C) in patients with autoimmune thyroid disease (Hashimoto’s thyroiditis, n = 21 and Graves’ disease, n = 8) and colloid goiter (n = 18). Comparisons were made by using the Mann–Whitney U test. Data are shown as the median (interquartile range), * = p < 0.05. Interleukin (IL).
Similarly, the expression of IL-23 (Fig. 1B, 1C) and IL-1β (Fig. 1D) was detected in the abundant inflammatory infiltrates characteristic of HT and thyrocytes, as confirmed by IHC and IF. Additionally, IL-23, IL-1β, and IL-17 were localized in the cytoplasm of thyroid follicular cells in HT patients by transmission electron microscopy after immunogold labeling (Fig. S1). In contrast, tissues obtained from GD patients demonstrated weak IL-23 (Fig. 1E) and IL-1β (Fig. 1F) thyrocytic immunostaining predominantly. Notably, a vast majority of patients with colloid goiters revealed the occasional or negative expression of IL-23 and IL-1β (Fig. 1G). The expression of IL-23 was significantly increased in HT patients when compared to both GD and colloid goiter patients (p = 0.043 and p < 0.001, respectively) (Fig. 2B). No difference was found between the expression level of IL-23 in patients with GD and colloid goiters (p = 0.324).
The highest immunopositivity of IL-1β was observed in patients with HT. Furthermore, the IL-1β immunopositivity was significantly higher than that in colloid goiter and GD patients (p < 0.001 for both) (Fig. 2C). Similar to IL-23, the expression level of IL-1β did not differ significantly between patients with GD and colloid goiters (p = 0.144).
Apart from thyrocytes and infiltrating lymphocytes, the expression of IL-23 and IL-1β was observed in dendritic cells demonstrating S100 positivity. The localization of S100 showed a brown granular immunostaining pattern in the cytoplasm and/or nuclei of dendritic cells (Fig. 1H).
When the association between IL-17 and Th17-promoting cytokines was analyzed, a moderate positive correlation between IL-17 and both IL-23 and IL-1β expression was found in HT patients (r = 0.574, p = 0.007 and r = 0.461, p = 0.036, respectively) (Fig. 3A, 3B). Furthermore, HT patients showed a positive correlation between IL-23 and IL-1β immunopositivity (r = 0.545, p = 0.011) (Fig. 3C). In the GD group, the expression of IL-17 was positively correlated with IL-1β immunopositivity (r = 0.817, p = 0.013). However, no significant relationship between IL-17 and IL-23 (r = 0.680, p = 0.063) or IL-23 and IL-1β immunoreactivity (r = 0.662, p = 0.074) was observed in this patient group.

Relationship between IHC expression levels of cytokines. In the HT group, IL-17A was positively correlated with IL-23 (A) and IL-1β (B) immunoreactivity. Furthermore, IL-23 was positively correlated with IL-1β immunopositivity (C). Correlations were determined by the Spearman rank correlation test. IHC, immunohistochemistry; IL, interleukin; HT, Hashimoto’s thyroiditis.
IL-17A/F, IL-21, IL-22 and granulocyte macrophage colony-stimulating factor (GM-CSF) represent the cytokine profile produced by a CD4+ T cell subset known as Th17. Recently, Th17 cells have been recognized as important contributors to the pathogenesis of Th1-dependent autoimmune diseases [18], and HT is now considered to be both a Th1- and Th17-mediated disease, while the role of Th17 cells in GD development and progress has not yet been clearly established.
Although Th17 cells have become associated with autoimmune inflammation leading to disease over the past decade, the understanding that Th17 cells are heterogeneous and exhibit under certain conditions different Th17 cell phenotypes—pathogenic and non-pathogenic has only recently been proposed [11]. These pathogenic lymphocytes can secrete both common and pathogenic molecules, such as IL-17A/F, IL-21, IL-22, IL-26, IFN-γ, TNF-α, and GM-CSF, thereby driving autoimmune inflammation.
IL-17 promotes neutrophil recruitment and activation, targeting immune and nonimmune cells, such as fibroblasts, epithelial and endothelial cells, thus triggering the release of cytokines, chemokines, and inflammatory mediators [19]. Previous studies have provided supportive evidence of Th17 and IL-17 participation in the pathogenesis of HT [20, 21], whereas their contributions to GD development remain poorly understood. Thus, an increased number of CD4+IL-17+ lymphocytes in the peripheral blood of pediatric patients with untreated HT but not in GD patients was demonstrated by Bossowski et al. [22], whereas Qin et al. [23] did not find a significant difference in IL-17 levels in the peripheral blood and thyroid tissues of GD patients compared to controls. In our study, the expression level of IL-17 in thyrocytes was higher in both HT and GD patients than in patients with colloid goiters, which may support a role for Th17 in AITD.
In contrast to the presence of convincing data regarding the cytokine combinations required for Th17 differentiation in mice, several studies have been performed recently to establish the cytokine profile, which can generate human Th17 cells expressing RORC. IL-23 has been shown to play an essential role in maintaining Th17 cell differentiation, suppressing IL-10 secretion and facilitating IL-22 and GM-CSF production [24]. Moreover, IL-23 was also found to be an important survival factor for Th17 lymphocytes [25]. Th0 cells upon exposure to IL-23, IL-1β, and IL-6 can generate highly pathogenic Th17 lymphocytes [10, 26]. IL-23, synthesized by macrophages and dendritic cells, is a member of the IL-12 family implicated in various autoimmune and inflammatory diseases.
Interestingly, Ruggeri et al. described increased levels of serum IL-23 in untreated and euthyroid HT patients compared to controls, thus representing early pathogenetic events characteristic of HT [13]. Furthermore, the authors described IL-37 upregulation in HT. IL-37 exerts a protective role against oxidative stress and inflammation by inhibiting effects of Th1/Th17-mediated response [27]. Recently, another research group found the enhanced expression of IL-23 in the thyroid tissue of patients with HT when compared to controls [14]. Additionally, these authors demonstrated the accumulation of reactive oxygen species and the inhibition of autophagy in thyrocytes induced by increased IL-23. In the present study, we found that the immunoexpression of IL-23 in the thyroid follicular cells of HT patients was significantly higher than that in both GD and colloid goiter patients, which may further result in the increased generation of Th17 lymphocytes and production of IL-17. Furthermore, a positive correlation between IL-17 and IL-23 as well as IL-17 and IL-1β expression was observed in HT patients, supporting a role for Th17 cells in HT development. To our knowledge, the expression of IL-23 in the thyroid tissues of GD patients has not been studied before. There are only two studies in which the levels of IL-23 were measured in the serum of GD patients [20, 28]. Increased serum levels of IL-23 have been reported in the study conducted by Jia et al. [28], whereas Figueroa-Vega et al. did not observe any significant differences in the IL-23 levels in GD patients compared to controls [20]. In our study, no difference between IL-23 immunopositivity within thyroid tissues in GD and colloid goiter patients was found, suggesting that pathogenic Th17-promoting cytokines might be less involved in GD pathogenesis. However, further studies based on a larger number of thyroid tissue samples and different methods to confirm these data are needed. Although no significant relationship between IL-17 and IL-23 expression was observed in GD patients, IL-23 tended to correlate with IL-17 immunopositivity (p = 0.063). Therefore, these results as well as conclusions regarding the role of Th17 cells in GD pathogenesis must be interpreted with caution because the absolute number of patients with GD was only 8. Interestingly, Zheng et al. showed an increase in IL-17 and RORγt mRNA expression in IL-23-stimulated cultured cells from GD patients when compared to those from controls and cultures without IL-23 [14]. Similar results were demonstrated in euthyroid GD patients, indicating an independent effect of thyroid hormone levels [29]. Increased tissue levels of IL-23 have not yet been reported in GD, and very few studies have focused on the role of IL-23 receptor in these patients. Furthermore, IL-23 receptor gene polymorphisms have been strongly associated with GD, especially with Graves’ ophthalmopathy [30].
IL-1β, produced primarily by macrophages and dendritic cells, exhibits different immunomodulatory and inflammatory activities. In AITD, IL-1β impairs the barrier function of thyroid follicular cells, altering tight junction proteins by stimulating IL-6 production [15]. IL-1β has been shown to induce Fas expression, promoting thyroid cell death as well [31]. In the current study, we found enhanced levels of IL-1β expression in the thyroid tissue of patients with HT compared to patients with colloid goiter and GD. Similar to IL-23, IL-1β expression did not differ significantly between patients with GD and colloid goiter; however, the expression of IL-17 was upregulated in the GD group. Taking into account that IL-17 is a common Th17 molecule secreted by both cell phenotypes and that IL-23/IL-1β stimulates a shift towards a pathogenic Th17 phenotype [11], our findings indicate that pathogenic Th17 cells may play a lesser role in the development of GD than in HT. In this context, supportive evidence has been provided in a recent study performed by Vitales-Noyola et al., demonstrating the elevation of Th17 pathogenic cells in the peripheral blood of GD (especially in those with Graves’ ophthalmopathy) but not in HT patients; however, when analyzing the absolute number of pathogenic cells, no significant differences between HT, GD, and controls were found [32].
This study has some methodological limitations associated with a retrospective nature of the study and the assessment of archived paraffin-embedded thyroid tissues. We included and studied a small number of thyroid tissue specimens obtained from patients with GD because of the limited availability of these samples. Therefore, the results of GD group analyses must be concluded and interpreted discreetly because of a small sample size in this group. Furthermore, when considering recent data on the role of Th17 in the development and progression of Graves’ ophthalmopathy, more prospective studies analyzing this patient group are needed. Finally, although we did not aim to analyze the expression levels of other interleukins, it would be important to further investigate the role of different phenotypes of Th17 and their cytokine profiles in thyroid autoimmunity.
In conclusion, we found that both HT and GD patients had higher expression levels of IL-17, which simultaneously correlated with IL-23 and IL-1β immunopositivity within thyroid tissue in the HT group. Furthermore, increased expression of IL-23 and IL-1β, cytokines that to promote pathogenic Th17 cell differentiation, was observed in the HT but not in the GD group. Our data suggest that pathogenic Th17-promoting cytokines may play a role in HT pathogenesis. Further experimental studies to investigate the role and regulatory effects of Th17-related cytokines in the pathogenesis of AITD are required.
This study was supported in part by the Latvian National Research Program “BIOMEDICINE” No. 5.2.4.
None of the authors have any potential conflicts of interest associated with this research.