2019 Volume 66 Issue 8 Pages 683-689
2019 Volume 66 Issue 8 Pages 683-689
Primary hyperparathyroidism (PHPT) is a common endocrine disease. Although surgical treatment is curative in most cases, there are few alternative therapies for the hypercalcemia caused by PHPT. Cinacalcet is a positive allosteric modulator of the calcium sensing receptor and was conditionally approved in Japan in 2014 to treat PHPT cases. However, there have been few reports on the outcomes. In our present study, we investigated the efficacy and safety of cinacalcet in 61 PHPT patients who were treated with this agent at our hospital between January 2014 and March 2017. The corrected serum Ca and intact PTH levels were significantly reduced by this treatment, whereas the serum phosphorus levels significantly increased. There were no significant differences in the eGFR or urinary Ca to urinary creatinine ratio between baseline and the maintenance phase. In terms of bone mineral density, there were significant increases observed in the 16 cases for whom a baseline value was available, 11 of whom had been treated for osteoporosis. The most common adverse events from cinacalcet treatment were gastrointestinal symptom, such as nausea and appetite loss. Other adverse events included severe dehydration due to hypercalcemia, myalgia, hypocalcemia, and increased urinary calcium excretion. Seven patients were switched to surgical treatment, and the drug was discontinued in 9 other patients, due to adverse effects. Our present study findings demonstrate that cinacalcet is an effective therapeutic option for PHPT from the perspective of hypercalcemia improvement but that adverse gastrointestinal effects of this drug occur at a frequency of about 10%.
CINACALCET acts as an positive allosteric modulator of the calcium-sensing receptor (CaSR), resulting in a decreased serum calcium level via decreased parathyroid hormone secretion and increased renal Ca excretion [1]. Since cinacalcet was approved for secondary hyperparathyroidism (SHPT) on hemodialysis in 2007 in Japan, it has been widely used to treat this condition and therefore reduced the number of surgical therapies [2]. Cinacalcet was subsequently approved in 2014 in Japan for treating hypercalcemia in cases of parathyroid carcinoma, and in primary hyperparathyroidism (PHPT) patients who are unable to undergo a parathyroidectomy or who experience recurrent PHPT after surgery [3].
A parathyroidectomy is indicated by some guidelines for symptomatic PHPT cases or for asymptomatic PHPT cases who show increased levels of corrected serum calcium of greater than 1 mg/dL above the reference range according to the Payne formula (cCa), skeletal or renal manifestations, or who have been diagnosed at 50 years of age or younger [4, 5]. However, treatment for hypercalcemia is sometimes required for PHPT patients who are unable to undergo a parathyroidectomy, experience recurrences, or who do not wish to undergo surgery. It has been reported that hypercalcemia can be well controlled for 52 weeks with cinacalcet treatment [6] and has been alleviated with this drug across a wide spectrum of severity levels from asymptomatic PHPT that does not meet any of the indications for surgery to overt PHPT with a history of failed parathyroidectomy [7]. It has been further reported that a majority of PHPT patients achieve normocalcemia (sporadic 65%, familial 80%) with cinacalcet whilst only 6 in 100 patients withdraw from this treatment because of side effects [8]. To date, however, few data have become available on the outcomes of cinacalcet use for PHPT in Japan. In our current study, therefore, we retrospectively surveyed the efficacy and safety of cinacalcet to treat PHPT at our hospital.
All 202 patients who had been prescribed cinacalcet at our hospital between 1 January 2014 and 31 March 2017 were retrospectively evaluated and classified as PHPT (n = 62), SHPT (n = 132), or tertiary hyperparathyroidism (THPT; n = 7) cases, or as an undetermined case (n = 1) in which familial or acquired hypocalciuric hypercalcemia [9-11] could not be ruled out. Cinacalcet was prescribed for the PHPT patients who had shown not only moderate to severe hypercalcemia, but also mild hypercalcemia which might lead to the signs and symptoms of this disorder. Among our 62 PHPT cases, one patient was excluded as he underwent a heminephrectomy for papillary urothelial carcinoma just after an improvement in his hypercalcemia symptoms with cinacalcet treatment.
This retrospective study was approved by the University of Tokyo Hospital Research Ethics Committee (Comprehensive approval of retrospective studies: authentication number 2879-(5)).
Measurements and extracted dataSerum calcium (Ca), albumin, phosphorus, and creatinine were measured in our hospital using LABOSPECT008® (Hitachi, Ltd. Tokyo, Japan) and the serum intact PTH was measured using the ECLIA method with a Cobas 6000® (Roche diagnostics K.K. Tokyo, Japan). The estimated glomerular filtration rate (eGFR) was calculated as follows: eGFR (mL/min/1.73 m2) = 194 × Cre–1.094 × Age–0.28 (female: ×0.739). Bone mineral density (BMD) was measured in the lumbar spine and/or femoral neck by dual-energy X-ray absorptiometry (DXA).
Data were collected from the electronic medical records of the study patients on the biochemical test results, BMD, symptoms, imaging studies, past histories, and medications. The timing of the biochemical tests was casual and was not limited to fasting or post-prandial evaluations. BMD was measured at the lumbar spine (L2–4) in most cases and at the femoral neck in 2 patients with a vertebral fracture. The cCa, serum phosphorus (iP), intact PTH, and eGFR values, the urinary calcium to urinary creatinine ratio (U-Ca/U-Cre), and the BMD values at the last visit prior to cinacalcet administration were adopted as the baseline data. All values at the second visit after the cinacalcet dosage had been settled other than the BMD were adopted as the maintenance phase data. In the case of the BMD values, the data at the latest measurement after introducing cinacalcet were adopted as the post-cinacalcet data.
Statistical analysisWe used a paired t test for the analysis of data differences with a normal distribution and a Wilcoxon signed-rank test for those with a non-normal distribution. All statistical analysis was performed using R 2.4.0 software (The R Foundation of Statistical Computing; www.r-project.org). A p value < 0.05 was considered statistically significant.
Sixty-one PHPT patients were analyzed (one patient who underwent a heminephrectomy was excluded). The mean age of this cohort was 67.8 years (range, 28–91 years), and the majority of these patients were women (51 cases). The mean baseline cCa and intact PTH values were above the reference range, and the mean iP value was in the low normal range (11.0 ± 0.8 mg/dL, 178 ± 181 pg/mL, and 2.7 ± 0.6 mg/dL, respectively) (Table 1). The 25-hydroxyvitamin D levels had not been routinely tested in our cohort as this had not been covered by medical insurance in Japan during the current study period. Thirty-two of our PHPT cases showed increased cCa levels of more than 1 mg/dL above the upper limit of the reference range, 40 of our cases had renal manifestations (eGFR <60 mL/min/1.73 m2, urolithiasis, and/or U-Ca/U-Cre (urine Ca to urine creatinine ratio) >400 mg/gCre), and 38 of our cases had skeletal manifestations (decreased BMD, compression fractures, and/or treatment for osteoporosis) (Fig. 1, Table 2). Twenty-six of our current study patients were treated for osteoporosis with bisphosphonates, denosumab, or selective estrogen receptor modulators (SERM) (Table 2). It was documented in the electronic medical records that 10 patients had received natural vitamin D (1,000 IU/day).
| n = 61 | Range | Mean ± SD | Reference range | |
|---|---|---|---|---|
| Age | (y.o.) | 28–91 | 67.8 ± 13.0 | |
| cCa | (mg/dL) | 9.8–13.4 | 11.0 ± 0.8 | 8.4–9.7 |
| iP | (mg/dL) | 1.1–5.0 | 2.7 ± 0.6 | 2.5–4.5 |
| eGFR | (mL/min/1.73 m2) | 7.6–149.8 | 70.3 ± 25.1 | ≥60 |
| Intact PTH | (pg/mL) | 49–1,130 | 178 ± 181 | 15–65 |
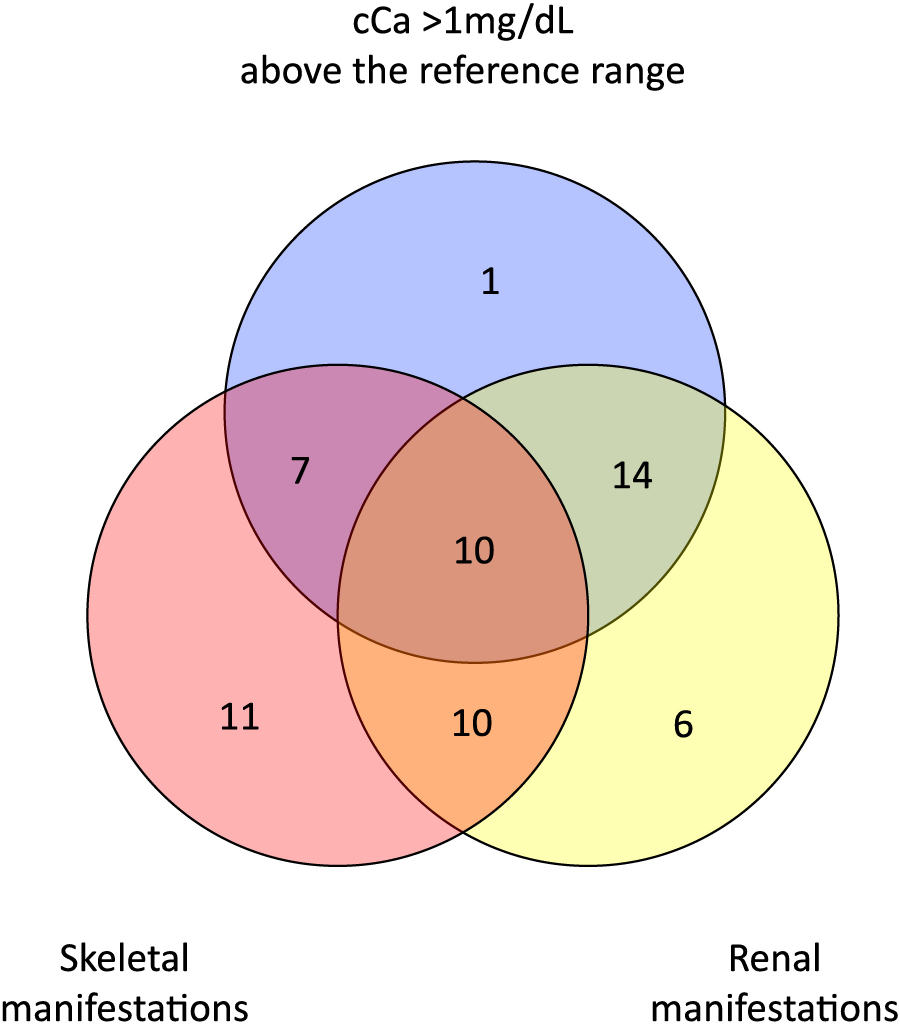
Clinical manifestations at baseline that meet surgical criteria
Surgical criteria comprised increased cCa levels of greater than 1 mg/dL above the upper limit of the reference range, renal manifestations (eGFR <60 mL/min/1.73 m2, urolithiasis, and/or U-Ca/U-Cre >400 mg/gCre), skeletal manifestations (T-score <–2.5 at any site, fragility fractures, and/or treatment for osteoporosis), and an age under 50. Since only 4 of the current study patients met the age criterion, a three-circle Venn diagram involving cCa (greater than 1 mg/dL above the reference range), renal manifestations, and skeletal manifestations is shown.
| Skeletal manifestations (n = 38) | n* | Renal manifestations (n = 40) | n* |
|---|---|---|---|
| BMD (T-score <–2.5) | 21 | eGFR <60 mL/min/1.73 m2 | 19 |
| Fragility fractures | 9 | urolithiasis | 27 |
| Treatment for osteoporosis | 26 | U-Ca/U-Cre >400 mg/gCre | 7 |
* The numbers are duplicated.
The cinacalcet doses were adjusted based on the cCa levels and on the symptoms caused by the hypercalcemia. The mean maintenance dose of cinacalcet was 43.4 mg (25 mg for 31 cases, 50 mg for 22 cases, 75 mg for 2 cases, and 100 mg for 6 cases), and most patients were maintained at 25 to 50 mg per day. The pretreatment cCa levels were significantly higher in the patients who were treated with 75–100 mg of cinacalcet than those receiving 25–50mg (median cCa level, 12.25 mg/dL and 10.60 mg/dL, respectively; p < 0.001, Mann-Whitney U test), indicating that a high dose of cinacalcet is needed to control moderate to severe hypercalcemia.
After the introduction of cinacalcet, the mean cCa level was reduced to 9.6 ± 0.6 mg/dL, the mean intact PTH level was reduced to 131 ± 115 pg/mL, and the mean iP level was increased to 3.2 ± 0.5 mg/dL. In the maintenance phase, 74% (45/61 cases) of our study patients achieved a normal cCa level (cCa ≤9.8 mg/dL). The serum iP and intact PTH levels returned to within the reference range in 49 (80%) and 18 (30%) patients, respectively. Only 11 cases (18%) in our present series achieved a normal cCa, iP and intact PTH levels. Moreover, a significant decrease in the median cCa level (p < 0.001) and the median intact PTH level (p < 0.001) and a significant increase in the median iP level (p < 0.001), were observed after introducing cinacalcet. No difference was observed between the median U-Ca/U-Cre level or median eGFR level at baseline and in the maintenance phase (Fig. 2). In the 10 patients who had received natural vitamin D, the serum cCa levels significantly decreased without significant changes in the PTH levels or U-Ca/U-Cre values (data not shown).

Biochemical responses to cinacalcet treatment
The differences between the cCa, iP, intact PTH, eGFR, or U-Ca/U-Cre values at baseline (pre) and in the maintenance phase (post) of cinacalcet treatment were compared using a Wilcoxon signed-rank test. The box and whisker plots show the variations in the non-normal distribution of these values.
n.s.; not significant.
It was surprising that the PHPT spontaneously remitted in one of our current study patients. Her blood test results originally indicated PTH-dependent hypercalcemia (cCa 10.8 mg/dL, iP 3.1 mg/dL, intact PTH 75 pg/mL), and her biochemical status was improved after introducing a 25 mg dose of cinacalcet. About 2 months later, both her serum intact PTH level and cCa level dropped suddenly. Her serum cCa levels gradually improved to 8.9 mg/dL, accompanied by increased levels of intact PTH, at 13 months after commencing treatment which was subsequently ceased. After the discontinuation of cinacalcet, the serum cCa levels in this patient remained within the reference range, with slightly elevated intact PTH levels (Fig. 3).
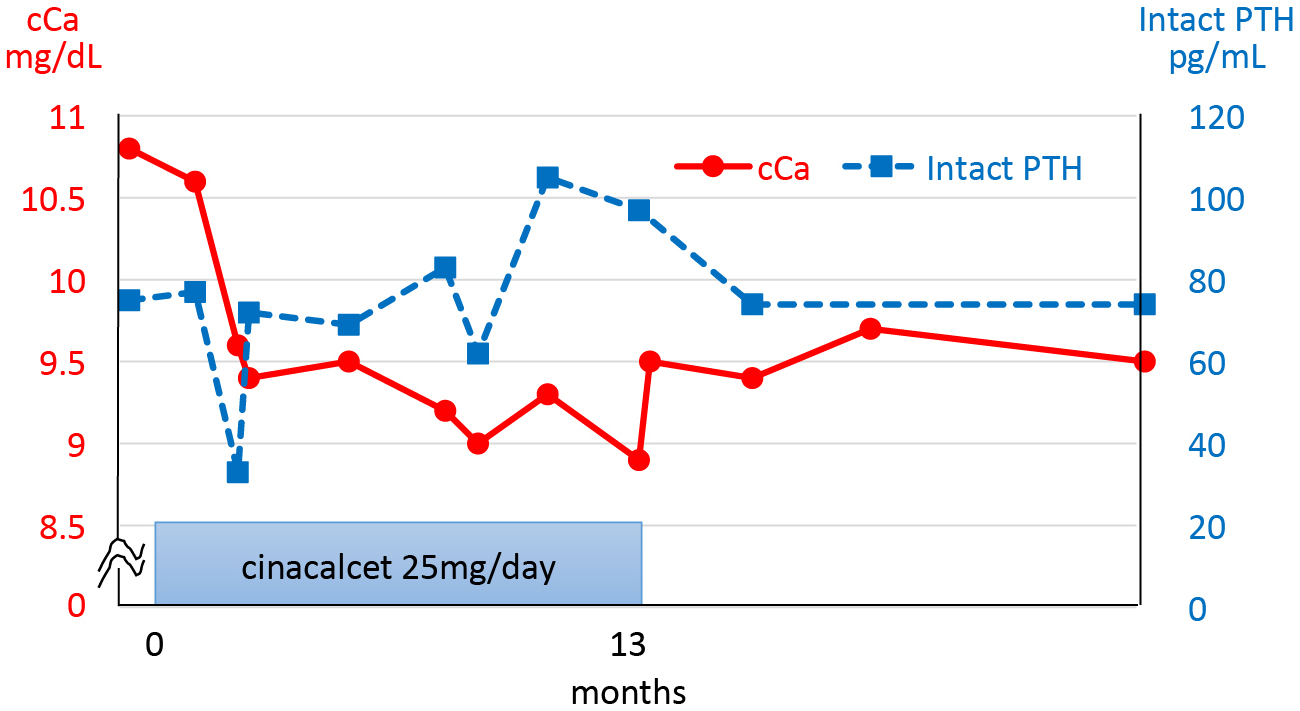
Clinical course in a case of spontaneous PHPT remission during cinacalcet treatment
BMD values measured by DXA were available in 31 of our study cases at baseline alone and in 16 patients both at baseline and after cinacalcet introduction (follow-up period range, 9–40 months; mean, 20.9 months). Eleven of these 16 cases were being treated for osteoporosis and the remaining 5 had received no medication for this condition. The mean BMD value significantly increased after introducing cinacalcet as determined using a paired t test. However, a BMD increase was evident only in the patients receiving medication for osteoporosis (Fig. 4).

BMD responses to cinacalcet treatment
The whole study group (16 cases) consisted of all study patients for whom BMD values were available both before and after the administration of cinacalcet. BMD measurements were performed at the lumbar spine (L2–4) in most patients and at the femoral neck in 2 patients with a vertebral fracture. The medication group comprised patients who had been receiving medication for osteoporosis, including bisphosphonatpthes (4 cases), denosumab (6 cases), or SERM (1 case). The non-medication group contained patients who received no medication for osteoporosis (5 cases). The differences between the mean BMD values at baseline and post-cinacalcet treatment were compared using a paired t test.
n.s.; not significant.
Seven of the PHPT patients in our current series developed nausea and the treatment was discontinued in 6 of these cases. One patient was admitted repeatedly for severe dehydration caused by hypercalcemia. Other side effects of the drug included myalgia, general malaise, increased urinary calcium excretion, and hypocalcemia. Treatment was discontinued in 16 patients for the following reasons: 7 patients were switched to a parathyroidectomy, 6 patients developed nausea and/or appetite loss, one patient developed general malaise, one patient showed increased levels of U-Ca/U-Cre, and one patient developed hypocalcemia.
We have retrospectively analyzed patients with mild to moderate hypercalcemia caused by PHPT who were treated with cinacalcet at our hospital. This therapy brought about biochemical improvements in the cCa, iP and intact PTH levels. Normal cCa levels and iP levels were achieved with maintenance doses of cinacalcet in most patients. The intact PTH levels showed a significant decrease with cinacalcet but still remained greater than the upper limit of the reference range in most patients. As a result, only 11 cases (18%) in our present series achieved normal cCa, iP, and intact PTH levels. Gastrointestinal symptoms, as the most common adverse events, are the main cause of the discontinuation of cinacalcet. In this regard, evocalcet, which is the next-generation oral calcimimetic, may be a promising alternative for PHPT patients as well as for SHPT cases [9].
We observed one case of spontaneous remission of PHPT after introducing cinacalcet. A previously reported case of spontaneous PHPT remission was found to be due to infarction or hemorrhage of a parathyroid adenoma [10]. To our knowledge however, we have observed the first case of spontaneous PHPT remission during cinacalcet treatment (Fig. 3). Although no study to date has reported a parathyroid volume reduction caused by cinacalcet in PHPT patients, some reports have speculated that cinacalcet may reduce swollen parathyroid volumes in SHPT [11, 12] and demonstrated that it may induce apoptosis in parathyroid cells [13, 14]. Based on these earlier findings, we speculate that cinacalcet might induce apoptosis or infarction in limited cases of parathyroid adenoma.
Skeletal manifestations are among the most significant clinical impacts of PHPT because the fracture risk is increased at both the non-vertebral and vertebral sites in these patients [5, 15, 16]. Munro, et al. reported that treatment of PHPT patients with cinacalcet for up to 5.5 years had no significant effect on the BMD [17]. In contrast, it has been reported that denosumab or bisphosphonate therapy can increase the BMD of osteoporotic patients with PHPT [18-22]. In another observational study in PHPT patients however, bisphosphonate therapy showed an association with an increased fracture risk as opposed to parathyroidectomy, which was found to be associated with a decreased fracture risk [23]. With regard to the outcomes of a bisphosphonate and cinacalcet combination therapy in PHPT patients, only limited data are available. In our current study, the effects of cinacalcet on BMD appeared to be neutral as shown in Fig. 2, suggesting that it might not interfere with the positive effects of anti-osteoporotic agents on BMD. In terms of fracture risk, there were no reported fractures in our present cohort although a longer follow-up duration would be needed to draw a more definitive conclusion in this regard. The evidence thus far indicates that bisphosphonates or denosumab combined with cinacalcet may be a viable treatment approach for osteoporosis associated with PHPT.
Renal manifestations such as kidney stones and calcifications are another of the most significant clinical impacts of PHPT. Neither the 24 hour nor casual U-Ca/U-Cre ratios showed any significant improvement in our study subjects after introducing cinacalcet. Both decreased and increased urinary calcium excretion levels were observed in our PHPT cohort after introducing cinacalcet. Cinacalcet decreases the calcium load into the renal tubules as a result of its suppression of the serum cCa levels. However, it also affects calcium-sensing receptors expressed in the basolateral site of the thick ascending loop of Henle, resulting in an inhibition of the paracellular calcium shunt and elevated urinary calcium excretion. A combination of these effects may thus underlie the lack of any significant difference in the U-Ca/U-Cre ratio as a whole. At the very least, cinacalcet may not be harmful in patients with renal stones and calcifications.
A vitamin D deficiency or insufficiency is common in PHPT cases [24] and vitamin D supplementation has been reported to significantly reduce serum PTH levels and increase the BMD without causing hypercalcemia or hypercalciuria [25]. A recent cohort study from the United States [26] has reported that a vitamin D deficiency is no longer common in PHPT because of the nationwide vitamin D supplementation. Analysis of that cohort revealed lower PTH levels and higher BMD without significant changes in the serum or urinary calcium levels. In our present study series, at least 10 patients had received a daily natural vitamin D supplementation of 1,000 IU in combination with cinacalcet. Concerning these patients, the serum cCa levels also significantly decreased despite the lack of significant changes in the PTH levels. Since a vitamin D insufficiency is frequent [27] and vitamin D fortification has not been common in Japan, vitamin D supplementation might be beneficial in Japanese PHPT patients.
This study had several limitations of note including its retrospective design, single-center cohort, and small number of subjects (n = 61). However, cinacalcet has been approved for the treatment of PHPT on the basis of a phase 3 study in Japan which included only 7 patients [3] and phase 2 study in the Europe and the United States (n = 46). We believe that our present findings usefully support the effectiveness and safety of cinacalcet for PHPT interventions.
In summary, cinacalcet is a viable and effective option for treating PHPT from the perspective of hypercalcemia alleviation although adverse gastrointestinal effects of this drug occur at a frequency of 10% and cause its discontinuation. Evocalcet, as the next-generation oral calcimimetic [9], might prove to be a better option for PHPT patients.
This work was supported by a grant-in-aid for scientific research from the Ministry of Education, Science, Sports, and Culture, Japan (16K09797).
Conflicts of interestThe authors declare no competing interests in relation to this study.