2020 Volume 67 Issue 8 Pages 803-818
2020 Volume 67 Issue 8 Pages 803-818
This 4-year randomized, double-blind, multicenter trial (NCT01927861) investigated the long-term efficacy and safety of Norditropin® (NN-220; somatropin) in Japanese children with short stature due to Noonan syndrome. Pre-pubertal children with Noonan syndrome were randomized 1:1 to receive 0.033 mg/kg/day (n = 25, mean age 6.57 years) or 0.066 mg/kg/day (n = 26, mean age 6.06 years) GH. Height standard deviation score (SDS) change after 208 weeks from baseline was evaluated using an analysis of covariance model. Height SDS improved from –3.24 at baseline with a significantly greater increase (estimated mean [95% confidence interval]) with 0.066 vs. 0.033 mg/kg/day GH (1.84 [1.58; 2.10] vs. 0.85 [0.59; 1.12]; estimated mean difference 0.99 [0.62; 1.36]; p < 0.0001). The majority of treatment-emergent adverse events (TEAEs) were non-serious, mild and assessed as unlikely treatment-related. TEAE rates and frequencies of serious TEAEs were similar between groups. Three patients receiving 0.066 mg/kg/day were withdrawn; two due to TEAEs at days 1,041 and 1,289. Mean insulin-like growth factor-I SDS increased from –1.71 to –0.75 (0.033 mg/kg/day) and 0.57 (0.066 mg/kg/day) (statistically significant difference). In both groups, there were only minor glycosylated hemoglobin changes, similar oral glucose tolerance test insulin response increases and no clinically relevant changes in oral glucose tolerance test blood glucose, vital signs, electrocardiogram or transthoracic echocardiography. In conclusion, treatment with 0.033 and 0.066 mg/kg/day GH for 208 weeks improved height SDS in Japanese children with short stature due to Noonan syndrome with a significantly greater increase with 0.066 vs. 0.033 mg/kg/day GH and was well tolerated, with no new safety concerns.
NOONAN SYNDROME (NS) is a genetically heterogeneous disorder caused by up-regulated RAS MAPK signaling, typically inherited in an autosomal dominant manner, although it may also arise due to a de novo mutation [1-6]. NS occurs in all ethnic groups and both sexes are equally affected [7]. Characteristics of NS include facial dysmorphism (90%) [6, 7], short stature (up to 70%) [8], congenital heart defects such as pulmonic stenosis (50–60%), hypertrophic cardiomyopathy (20%) and atrial septal defects (6–10%) [4], short and webbed neck (55%), scoliosis (25%) [9], bleeding disorders (30–65%) and neurocognitive and behavioral issues [4, 6]. The incidence is estimated to range between 1 to 1,000 and 1 to 2,500 live births [4, 6]. The variable clinical phenotype of patients with NS can be partly explained by its genetic heterogeneity, which includes mutations in PTPN11, SOS1, KRAS, NRAS, RAF1, BRAF, RIT1, SHOC2 and RRAS2 genes, with PTPN11 accounting for 50–60% of identified mutations [1, 3, 6, 10-12]. However, in 20–30% of NS cases, causative mutations behind NS and NS-like disorders have not yet been identified [1].
Short stature affects up to 70% of children with NS [8, 13]. Birth weight and length are typically within a normal range, but a height loss of 1–1.5 standard deviation score (SDS) is often seen in NS patients within the first year [13, 14]. In childhood, growth is often below –2 SDS of the growth curve of normal children and puberty is often delayed [13, 14]. In untreated adult NS patients, height is reported to be below –2 SDS of the normal population [13, 14]. Mechanisms for short stature in NS are heterogeneous and include GH deficiency, neurosecretory dysfunction, and GH resistance [4, 15-17]. Disturbances in GH secretion have been reported for short children with NS, suggesting neurosecretory dysfunction [15]. Furthermore, it has been reported that NS patients with PTPN11 mutations have lower levels of insulin-like growth factor I (IGF-I) than those without the respective mutation due to a negative effect on GH receptor signaling mediated by phosphatase activities [18, 19].
Over the last two decades, a number of published studies have reported increased height SDS (HSDS) in response to GH treatment of short stature due to NS, with no significant adverse effects [20-24]. Results from an increasing number of long-term studies of GH treatment in NS demonstrate a significant improvement in adult height [25].
Norditropin® (NN-220; somatropin; Novo Nordisk A/S, Denmark) is a human GH (hGH) synthesized by genetic recombinant technology. It is approved in more than 100 countries, including Japan, for treatment of various growth disorders in children, e.g. GH deficiency (GHD), Turner syndrome, small for gestational age (SGA), and in adults with severe GHD [26]. Other indications include chronic renal insufficiency and Prader-Willi syndrome (USA and Switzerland), idiopathic short stature (USA and South Korea) and achondroplasia (Japan). Norditropin® was approved for treatment of ‘short stature associated with NS’ in the USA in 2007, Switzerland (2008), South Korea (2008), Israel (2011) and Brazil (2016). The regulatory submissions in these countries were based on two pivotal trials in Sweden, including a two-year, prospective, randomized, parallel-dose group trial (S/GHD/004/NOO) and a retrospective data collection and follow-up trial until adult height was reached (GHNOO-1658) [23].
Based on results from the 104-week pivotal phase of the present trial (GHLIQUID-4020) in which treatment with 0.033 mg/kg/day and 0.066 mg/kg/day GH was shown to improve HSDS in children with short stature due to NS without any safety issues [27], Norditropin® was approved in Japan in November 2017 for treatment of ‘short stature due to NS’.
This report presents results of the pivotal and extension phase of the trial evaluating the efficacy and safety of 208 weeks of treatment with two doses of GH in Japanese children with short stature due to NS.
This was a multicenter, randomized, parallel-group, double-blind trial (NCT01927861) investigating the long-term efficacy and safety of GH in Japanese children with short stature due to NS conducted at 26 sites in Japan between August 2013 and July 2018. The trial was conducted in accordance with the Declaration of Helsinki (2008) [28], International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Good Clinical Practice (GCP) (1996) [29], and the Ministry of Health and Welfare Ordinance on GCP (1997) [30]. The protocol was reviewed and approved according to local regulations by appropriate health authorities and institutional review boards. Written informed consent was obtained from all subjects’ parents or legally acceptable representative prior to any trial-related activities.
Children were treated for 104 weeks in the pivotal phase and for further 104 weeks in an extension phase, resulting in a total of 208 weeks of treatment. Treatment was extended to 234 weeks for subjects who agreed to continue treatment after completion of the extension phase. Subjects were randomized 1:1 to receive 0.033 mg/kg or 0.066 mg/kg GH using a trial-specific interactive voice/web response system. GH was administered as a once-daily subcutaneous injection, alternating between the upper arm, thigh, abdominal wall, or gluteal region, using 5 mg or 10 mg pre-filled pens (FlexPro® PenMate® injection supportive device), which were indistinguishable from one another.
Trial populationInclusion criteria were as follows: Japanese children (boys three to <eleven years of age; girls three to <ten years of age) with short stature due to NS, clinically diagnosed according to van der Burgt score list [31], where epiphyseal fusion had not yet taken place. Further inclusion criteria were a HSDS of –2 or below according to the Japanese national reference data for children [32], the availability of height records for the period between 40–64 weeks prior to screening, and pre-pubertal status as described previously [27, 33, 34]. All included patients were naïve with respect to GH treatment.
Key exclusion criteria were known or suspected hypersensitivity to hGH or related products, diabetic type diagnosed with the Japanese Diabetes Society Classification [35], a history or presence of active malignancy, and prior GH treatment. Further exclusion criteria were as described by Ozono et al. [27].
Endpoints and their assessmentThe primary endpoint was change in HSDS from baseline to 104 weeks of treatment based on Japanese national reference data for children as reported by Ozono et al. [27]. Change in HSDS from baseline to 208 weeks of treatment based on Japanese national reference data for children or on reference data from Japanese NS patients, respectively, were secondary efficacy endpoints. Furthermore, secondary efficacy endpoints included height velocity SDS and height velocity from 104 to 156 weeks, and from 156 to 208 weeks of treatment. To assess efficacy endpoints, specific characteristics, including height and body weight were recorded at each visit and HSDS values derived as described by Ozono et al. [27].
Safety endpoints included the incidence of treatment-emergent adverse events (TEAEs) during 208 and 234 weeks of treatment. TEAEs and serious TEAEs, defined as reported by Ozono et al. [27] and classified as mild (no or transient symptoms, no interference with the patient’s daily activities), moderate (marked symptoms, moderate interference with the patient’s daily activities) or severe (considerable interference with the patient’s daily activities, unacceptable), were recorded. Terms defining the relationship between each TEAE and the trial product were probable (good reasons and sufficient documentation to assume a causal relationship), possible (a causal relationship is conceivable and cannot be dismissed) or unlikely (the event is most likely related to etiology other than the trial product) as assessed by the investigator. In addition, changes from baseline to 208 weeks of treatment in IGF-I, glycosylated hemoglobin (HbA1c), clinical laboratory tests, glucose tolerance (area under the curve [AUC] of glucose and AUC of insulin based on the oral glucose tolerance test [OGTT]), bone age, bone age/chronological age, vital signs, urinalysis, blood coagulation tests, and electrocardiogram (ECG) were secondary safety endpoints. Clinical laboratory analyses, including hematology, biochemistry, blood coagulation tests, lipids, glucose metabolism, IGF-I, urinalysis, thyroid hormones and bone metabolism markers, were performed using standard procedures at a central laboratory. IGF-I SDS values were derived using reference data based on Japanese children.
Statistical analysisThe sample size was determined based on the pivotal phase of the trial and was calculated using a two-sided, two-sample t-test for μ1 = μ2, where μi is the population mean, i = 1, 2 represents 0.033 mg/kg/day and 0.066 mg/kg/day GH, respectively, with a 5% significance level and 80% power. A total of 21 subjects per dose group gave a power of 80%, with a mean difference between the two dose groups of 0.45 and SD of 0.5 in the primary endpoint. Considering 10% withdrawal rate, the sample size of 24 subjects per dose group and 48 subjects in total was set to ensure the power for the per protocol (PP) analysis set.
The full analysis set (FAS) included all randomized subjects. Analysis of all efficacy endpoints was performed based on the FAS. In the pivotal phase, the primary analysis of the primary endpoint was repeated on the PP analysis set [27]. The safety analysis set (SAS) included all subjects receiving at least one dose of GH (0.033 mg/kg/day or 0.066 mg/kg/day of GH). Safety endpoints were summarized using the safety analysis set. Missing values for endpoints other than OGTT, HbA1c and bone age were imputed using the last observation carried forward (LOCF) method. For OGTT, HbA1c and bone age, missing values were not imputed. Statistical analysis was performed at 104 weeks. A similar statistical analysis was performed at 208 weeks.
Results are shown as estimated mean treatment effects (LSMeans). Estimated mean treatment differences (or ratios) were presented together with two-sided 95% confidence intervals (CIs) and p-values for all endpoints analyzed statistically.
The primary objective of this trial was to evaluate the growth-promoting effect of GH from baseline to 104 weeks of treatment in patients with short stature due to NS. This was obtained by calculation of the change in HSDS. Change in HSDS from baseline to 208 weeks of treatment (based on Japanese national reference data for children) was a secondary efficacy endpoint and analyzed using an analysis of covariance (ANCOVA) model with treatment as a fixed effect and baseline HSDS as a covariate. Treatment difference at 208 weeks was estimated using this model. The HSDS was also calculated according to the Japanese NS reference data and change in HSDS (Japanese NS reference data) analyzed based on the ANCOVA model as described above.
A total of 51 children were randomized (Fig. 1). As previously reported by Ozono et al. [27], all 51 subjects completed the pivotal phase of 104 weeks exposure, and 48 subjects completed the pivotal and extension phase. Three subjects were withdrawn from the trial during the extension phase, all in the 0.066 mg/kg/day treatment arm. Two of the withdrawals were due to TEAEs (polymyositis at day 1,041 and scoliosis at day 1,289, respectively), both of which are possibly associated with NS and may be attributable to the causative gene mutation. One boy (seven years old at baseline) was withdrawn at week 156 because he had reached average height compared to the Japanese standard, as judged by the investigator.
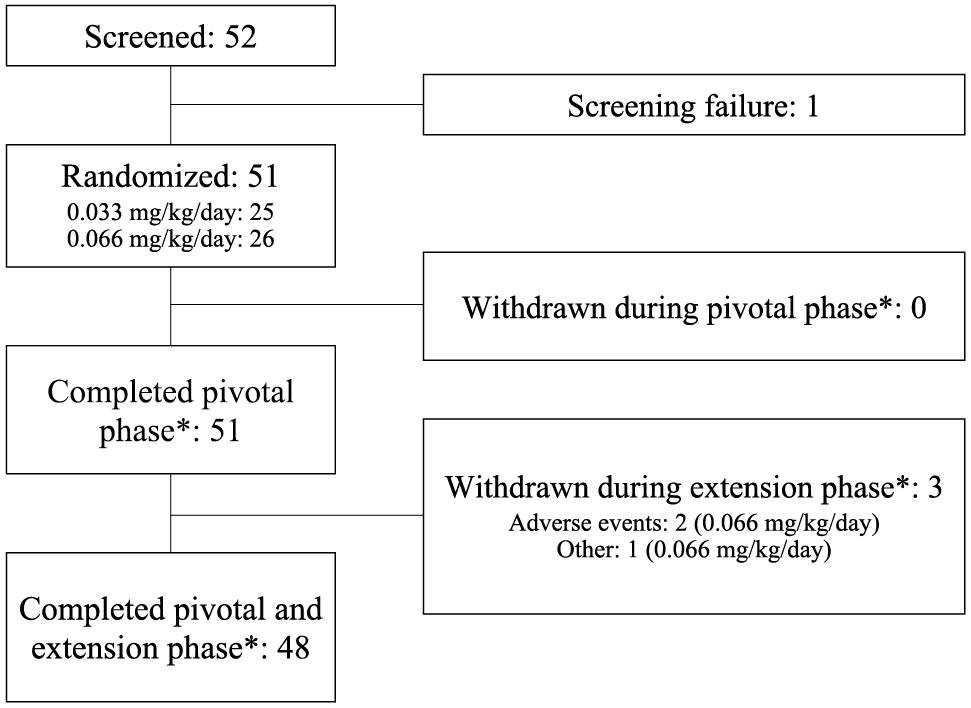
Patient flow
*Pivotal phase, 104 weeks of GH treatment; pivotal and extension phase, 208 weeks of GH treatment
The mean duration of exposure (including exposure data of patients who had agreed to extend treatment to 234 weeks) was 4.29 years in the 0.033 mg/kg/day dose group and 4.16 years in the 0.066 mg/kg/day dose group. There were no differences in total exposure between the two dose groups (0.033 mg/kg/day: 107.2 subject years; 0.066 mg/kg/day: 108.1 subject years).
Baseline characteristics are summarized in Table 1. Nineteen girls and 32 boys were enrolled, with more boys than girls in the 0.066 mg/kg/day group. Medical history and concomitant illnesses among the trial population resembled the NS patient population. Overall, differences between the two dose groups observed at baseline were not clinically relevant. Mean age (SD) at baseline was 6.57 (2.42) years in the 0.033 mg/kg/day group and 6.06 (2.25) in the 0.066 mg/kg/day group, with an age range of 3.1 to 10.8 years for both groups. Mean HSDS at baseline was considerably below the reference data for Japanese children, with values being below –3 in both groups, as previously reported [27]. The NS genotype was reported for 36 (70.6%) subjects and included mutations in PTPN11, SOS1, KRAS, RAF1, BRAF, SHOC2, and RIT1 (Table 1).
| 0.033 mg/kg/day GH (N = 25) | 0.066 mg/kg/day GH (N = 26) | |
|---|---|---|
| Sex, Male/Female, n (%) | 14 (56.0)/11 (44.0) | 18 (69.2)/8 (30.8) |
| Chronological age (years) | 6.57 ± 2.42 | 6.06 ± 2.25 |
| Height (cm) | 100.27 ± 13.48 | 97.66 ± 12.55 |
| Height SDS (Japanese reference data) | –3.24 ± 0.76 | –3.25 ± 0.71 |
| Height SDS (Noonan reference data) | –0.73 ± 0.74 | –0.80 ± 0.72 |
| Height velocity SDS | –1.99 ± 1.17 | –1.70 ± 1.33 |
| Body weight (kg) | 15.73 ± 4.75 | 14.96 ± 3.50 |
| Body mass index (kg/m²) | 15.28 ± 1.38 | 15.63 ± 1.43 |
| Bone age (years) | 5.50 ± 2.15 | 5.03 ± 2.31 |
| IGF-I (ng/mL) | 70.2 ± 35.8 | 69.7 ± 35.2 |
| IGF-I SDS | –1.81 ± 0.88 | –1.61 ± 0.75 |
| Mutations detected, n (%) | ||
| PTPN11 | 16 (64.0) | 12 (46.2) |
| KRAS | 0 (0.0) | 1 (3.8) |
| SOS1 | 2 (8.0) | 0 (0.0) |
| RAF1 | 0 (0.0) | 2 (7.7) |
| BRAF | 0 (0.0) | 1 (3.8) |
| SHOC2 | 0 (0.0) | 1 (3.8) |
| RIT1 | 0 (0.0) | 1 (3.8) |
| None | 4 (16.0) | 6 (23.1) |
| Not analyzed | 3 (12.0) | 2 (7.7) |
| Concomitant illness*, n (%) | ||
| Pulmonary stenosis** | 5 (20.0) | 11 (42.3) |
| Atrial septal defect | 3 (12.0) | 7 (26.9) |
| Hypertrophic cardiomyopathy | 3 (12.0) | 5 (19.2) |
| Mitral valve incompetence | 3 (12.0) | 1 (3.8) |
| Aortic valve incompetence | 0 | 3 (11.5) |
| Aortic stenosis | 0 | 2 (7.7) |
| Arrhythmia | 1 (4.0) | 1 (3.8) |
| Cardiac hypertrophy | 1 (4.0) | 1 (3.8) |
| Cardiomyopathy (Noncompaction of myocardium)*** | 1 (4.0) | 0 |
| Supraventricular tachycardia | 1 (4.0) | 0 |
| Ventricular extrasystoles | 1 (4.0) | 0 |
| Bicuspid aortic valve | 1 (4.0) | 0 |
| Mitral valve stenosis | 0 | 1 (3.8) |
| Pulmonary valve incompetence | 0 | 1 (3.8) |
| Right ventricular hypertrophy | 0 | 1 (3.8) |
| Tricuspid valve incompetence | 0 | 1 (3.8) |
| Left ventricle outflow tract obstruction | 0 | 1 (3.8) |
Results are mean ± standard deviation unless otherwise stated.
* Only concomitant illnesses related to cardiac disorders are listed.
** Sum of patient numbers at Medical Dictionary for Regulatory Activities (MedDRA) Preferred Term (PT) of Pulmonary artery stenosis (0; 5), Pulmonary valve stenosis (3; 5), Pulmonary artery stenosis congenital (1; 1), Pulmonary valve stenosis congenital (1; 0). Numbers in brackets show numbers of patients in the 0.033 mg/kg/day group and in the 0.066 mg/kg/day group, respectively.
*** MedDRA PT is “Cardiomyopathy”, and reported term is “Noncompaction of myocardium”.
IGF-I, insulin-like growth factor I; N/n, number of patients; SDS, standard deviation score; %, percentage of patients
Based on the Japanese national reference data for children, there was an estimated mean gain in HSDS of 0.85 (95% CI: 0.59; 1.12) with 0.033 mg/kg/day and 1.84 (95% CI: 1.58; 2.10) with 0.066 mg/kg/day GH after 208 weeks of treatment (Fig. 2a). The gain in HSDS was significantly greater with 0.066 mg/kg/day compared to 0.033 mg/kg/day, the estimated mean difference between the two dose groups was 0.99 (95% CI: 0.62; 1.36), p < 0.0001, (Fig. 2a). The mean HSDS over time based on the Japanese national reference data for children had increased from –3.24 at baseline to –2.40 after 104 weeks [27] and to –2.39 after 208 weeks in the 0.033 mg/kg/day group and from –3.25 to –1.78 after 104 weeks [27] and to –1.41 after 208 weeks of treatment in the 0.066 mg/kg/day group (Fig. 3a). Eight subjects (32.0%) in the 0.033 mg/kg/day group and 20 subjects (76.9%) in the 0.066 mg/kg/day group exhibited a HSDS above –2.0 based on Japanese national reference data for children after 208 weeks of treatment compared to none at baseline.

Height SDS and change in height SDS at 208 weeks in the two treatment groups (0.033 mg/kg/day and 0.066 mg/kg/day GH) based on a) Japanese reference data for children; b) Japanese Noonan syndrome reference data.
Mean estimates are from an ANCOVA method with treatment as a fixed effect and baseline response as a covariate in full analysis set. Missing data is imputed using last observation carried forward. Solid line is the total average value at baseline (a: –3.24; b: –0.77). Mean ± standard error.
ANCOVA, analysis of covariance; CI, confidence interval; ETD, estimated treatment difference in height SDS for 0.066 mg/kg/day vs. 0.033 mg/kg/day GH; SDS, standard deviation score

Height SDS in the two treatment groups based on a) Japanese reference data for children; b) Japanese Noonan syndrome reference data and onset of puberty at each visit.
 0.033 mg/kg/day GH;
0.033 mg/kg/day GH;  0.066 mg/kg/day GH
0.066 mg/kg/day GH
Full analysis set, last observation carried forward imputed data. Mean ± standard error. Dashed line represents the lower end of the height SDS range for the Japanese national reference population (–2 SDS) (a) or the middle of the height SDS range for the Noonan syndrome population (b).
CI, confidence interval; ETD, estimated treatment difference in height SDS for 0.066 mg/kg/day vs. 0.033 mg/kg/day GH; SDS, standard deviation score
Whereas only a small number of subjects had entered puberty after 104 weeks of treatment in the pivotal phase of the trial (seven out of 51), the number steadily increased after this time point. At the end of treatment, approximately half of all subjects (26 out of 51) had entered puberty (Fig. 3a and b, lower panels). Generally, a similar number of subjects entered puberty at a certain time point with no differences between the two dose groups. At the end of the trial, the majority of girls (0.033 mg/kg/day: eight of total eleven girls; 0.066 mg/kg/day: four of total eight girls) had not entered puberty, while the majority of boys (0.033 mg/kg/day: ten of total 14 boys; 0.066 mg/kg/day: eleven of total 18 boys) had entered puberty (including patients who remained in the trial for 234 weeks). Four boys (two in each dose group) entered puberty already at five to seven years of age. In girls, the earliest onset of puberty was at nine to ten years of age (two in the 0.066 mg/kg/day group). No TEAEs of precocious puberty were recorded.
When analyzed based on the Japanese NS reference data, there was an estimated mean gain in HSDS of 0.96 (95% CI: 0.74; 1.18) with 0.033 mg/kg/day and 1.91 (95% CI: 1.70; 2.12) with 0.066 mg/kg/day after 208 weeks of treatment (Fig. 2b). The gain in HSDS was significantly greater with 0.066 mg/kg/day compared to 0.033 mg/kg/day, the estimated mean difference between the two dose groups was 0.95 (95% CI: 0.65; 1.25), p < 0.0001, (Fig. 2b). The mean HSDS over time based on the Japanese NS reference data had increased from –0.73 at baseline to 0.02 after 104 weeks [27] and to 0.22 after 208 weeks in the 0.033 mg/kg/day group and from –0.80 to 0.68 after 104 weeks [27] and to 1.12 after 208 weeks of treatment in the 0.066 mg/kg/day group (Fig. 3b).
An exploratory analysis showed that age at treatment initiation appeared to have an effect on HSDS increase based on Japanese NS reference data at 208 weeks, with a greater change seen when treatment was initiated at a younger age (Fig. 4), an observation that was also reported by Ozono et al. after 104 weeks of treatment [27]. Furthermore, there was a positive correlation between change in HSDS (Japanese NS reference data) after 208 weeks and change in IGF-I SDS, with a Pearson correlation coefficient of r = 0.7483, p < 0.0001 (Fig. 5).

Change in height SDS in the two treatment groups relative to Japanese Noonan syndrome reference data after 208 weeks of treatment vs. age at start of treatment.
 0.033 mg/kg/day GH;
0.033 mg/kg/day GH;  0.066 mg/kg/day GH
0.066 mg/kg/day GH
Full analysis set, last observation carried forward imputed data.
SDS, standard deviation score

Change in height SDS in the two treatment groups relative to Japanese Noonan syndrome reference data after 208 weeks of treatment vs. change in IGF-I SDS.
 0.033 mg/kg/day GH;
0.033 mg/kg/day GH;  0.066 mg/kg/day GH
0.066 mg/kg/day GH
Full analysis set, last observation carried forward imputed data. Pearson correlation coefficients: r = 0.7483, p < 0.0001.
IGF-I, insulin-like growth factor I; SDS, standard deviation score
During the first year of treatment, the height velocity SDS calculated based on the height velocity for Japanese children [36] increased from the lower border of the reference range at baseline to values above the upper border of the reference range (Fig. 6a). After the first year of increase, the mean height velocity SDS gradually decreased in both dose groups [27]. The mean height velocity SDS was maintained above 0 in both groups after two years of treatment (pivotal phase) [27] and also after three and four years of treatment in the 0.066 mg/kg/day group. Specifically, the mean height velocity SDS increased from baseline values of –1.99 (0.033 mg/kg/day) and –1.70 (0.066 mg/kg/day) to 0.58 and 2.65, respectively, after two years of treatment [27], to –0.39 and 1.44, respectively, after three years of treatment, and to –0.73 and 0.92, respectively, after four years of treatment (Fig. 6a). Similarly, the mean height velocity (cm/year) increased from baseline values of 4.74 (0.033 mg/kg/day) and 5.08 (0.066 mg/kg/day) to 6.20 and 7.99, respectively, after two years of treatment [27], to 5.77 and 7.01, respectively, after three years of treatment, and to 5.64 and 6.11, respectively, after four years of treatment (Fig. 6b). Although mean height velocity decreased gradually after one year of treatment, as was previously reported in Ozono et al. [27], it was still greater than at baseline after three and four years of treatment in both treatment groups (Fig. 6b). Throughout the trial period, both mean height velocity SDS and mean height velocity were greater with 0.066 mg/kg/day than with 0.033 mg/kg/day (Fig. 6).

a) Height velocity SDS over time in the two treatment groups; b) Height velocity (cm/year) over time in the two treatment groups.
 0.033 mg/kg/day GH;
0.033 mg/kg/day GH;  0.066 mg/kg/day GH
0.066 mg/kg/day GH
Full analysis set, last observation carried forward imputed data. Mean ± standard error. Baseline: Height velocity SDS (a) or height velocity (b) from 1 year prior to screening to week 0. Dashed line (a) represents the middle of the height velocity SDS range for the Japanese national reference population.
SDS, standard deviation score
An overview of TEAEs during 208 and 234 weeks of treatment is presented in Table 2A. The proportion of subjects with TEAEs and the event rates of TEAEs were similar between the 0.033 mg/kg/day group (96%; 507 events; 471 events/100 patient years) and the 0.066 mg/kg/day group (100%; 539 events; 496 events/100 patient years). Overall, differences in the distribution of TEAEs between dose groups were not clinically relevant. As previously reported by Ozono et al. [27], the most frequent TEAEs with an incidence ≥5% were signs and symptoms generally observed in children (i.e. general infections, respiratory tract infections, otitis media, eczema, fever) in both dose groups.
| 0.033 mg/kg/day GH (N = 25) | 0.066 mg/kg/day GH (N = 26) | |||||||
|---|---|---|---|---|---|---|---|---|
| N | % | E | Rate | N | % | E | Rate | |
| TEAEs | 24 | 96.0 | 507 | 471 | 26 | 100.0 | 539 | 496 |
| Serious TEAEs | 9 | 36.0 | 10 | 9 | 10 | 38.5 | 12 | 11 |
| Severity | ||||||||
| Severe | 2 | 8.0 | 2 | 2 | 3 | 11.5 | 4 | 4 |
| Moderate | 8 | 32.0 | 12 | 11 | 10 | 38.5 | 10 | 9 |
| Mild | 24 | 96.0 | 493 | 458 | 26 | 100.0 | 525 | 483 |
| Events leading to withdrawal | 0 | – | – | – | 2 | 7.7 | 2 | 2 |
| Related to trial product | ||||||||
| Probable | 1 | 4.0 | 1 | 1 | 4 | 15.4 | 4 | 4 |
| Possible | 8 | 32.0 | 13 | 12 | 7 | 26.9 | 8 | 7 |
| Unlikely | 24 | 96.0 | 493 | 458 | 26 | 100.0 | 527 | 485 |
| Outcome | ||||||||
| Recovered/resolved | 24 | 96.0 | 481 | 447 | 26 | 100.0 | 511 | 470 |
| Recovering/resolving | 4 | 16.0 | 4 | 4 | 3 | 11.5 | 3 | 3 |
| Not recovered/not resolved | 12 | 48.0 | 22 | 20 | 15 | 57.7 | 25 | 23 |
| Fatal | 0 | – | – | – | 0 | – | – | – |
E, number of events; N, number of patients; Rate, event rate per 100 exposure years; %, percentage of patients
The majority of TEAEs were non-serious, mild or moderate in severity and assessed as unlikely related to GH by the investigator (Table 2A). Adverse events possibly or probably related to GH are listed in Table 2B. No deaths were reported.
| 0.033 mg/kg/day GH (N = 25) | 0.066 mg/kg/day GH (N = 26) | |||||||
|---|---|---|---|---|---|---|---|---|
| N | % | E | Rate | N | % | E | Rate | |
| Total number | 8 | 32.0 | 14 | 13 | 11 | 42.3 | 12 | 11 |
| Metabolism and nutrition disorders | 0 | — | — | — | 4 | 15.4 | 4 | 4 |
| Hyperinsulinemia | 0 | — | — | — | 2 | 7.7 | 2 | 2 |
| Glucose tolerance impaired | 0 | — | — | — | 1 | 3.8 | 1 | 1 |
| Insulin resistance | 0 | — | — | — | 1 | 3.8 | 1 | 1 |
| Musculoskeletal and connective tissue disorders | 1 | 4.0 | 1 | 1 | 3 | 11.5 | 3 | 3 |
| Arthralgia | 0 | — | — | — | 1 | 3.8 | 1 | 1 |
| Polymyositis | 0 | — | — | — | 1 | 3.8 | 1 | 1 |
| Scoliosis | 0 | — | — | — | 1 | 3.8 | 1 | 1 |
| Sever’s disease | 1 | 4.0 | 1 | 1 | 0 | — | — | — |
| Respiratory, thoracic and mediastinal disorders | 3 | 12.0 | 3 | 3 | 1 | 3.8 | 1 | 1 |
| Asthma | 0 | — | — | — | 1 | 3.8 | 1 | 1 |
| Nasal polyps | 1 | 4.0 | 1 | 1 | 0 | — | — | — |
| Rhinorrhoea | 1 | 4.0 | 1 | 1 | 0 | — | — | — |
| Tonsillar hypertrophy | 1 | 4.0 | 1 | 1 | 0 | — | — | — |
| Skin and subcutaneous tissue disorders | 2 | 8.0 | 2 | 2 | 1 | 3.8 | 1 | 1 |
| Eczema | 0 | — | — | — | 1 | 3.8 | 1 | 1 |
| Hemorrhage subcutaneous | 1 | 4.0 | 1 | 1 | 0 | — | — | — |
| Urticaria | 1 | 4.0 | 1 | 1 | 0 | — | — | — |
| General disorders and administration site conditions | 1 | 4.0 | 2 | 2 | 1 | 3.8 | 1 | 1 |
| Administration site bruise | 0 | — | — | — | 1 | 3.8 | 1 | 1 |
| Chest pain | 1 | 4.0 | 1 | 1 | 0 | — | — | — |
| Oedema peripheral | 1 | 4.0 | 1 | 1 | 0 | — | — | — |
| Congenital, familial and genetic disorders | 1 | 4.0 | 1 | 1 | 0 | — | — | — |
| Congenital nevus | 1 | 4.0 | 1 | 1 | 0 | — | — | — |
| Endocrine disorders | 1 | 4.0 | 1 | 1 | 0 | — | — | — |
| Autoimmune thyroiditis | 1 | 4.0 | 1 | 1 | 0 | — | — | — |
| Infections and infestations | 1 | 4.0 | 2 | 2 | 0 | — | — | — |
| Skin infection | 1 | 4.0 | 2 | 2 | 0 | — | — | — |
| Nervous system disorders | 1 | 4.0 | 1 | 1 | 0 | — | — | — |
| Headache | 1 | 4.0 | 1 | 1 | 0 | — | — | — |
| Renal and urinary disorders | 0 | — | — | — | 1 | 3.8 | 1 | 1 |
| Proteinuria | 0 | — | — | — | 1 | 3.8 | 1 | 1 |
| Reproductive system and breast disorders | 0 | — | — | — | 1 | 3.8 | 1 | 1 |
| Breast enlargement | 0 | — | — | — | 1 | 3.8 | 1 | 1 |
| Vascular disorders | 1 | 4.0 | 1 | 1 | 0 | — | — | — |
| Superficial vein prominence | 1 | 4.0 | 1 | 1 | 0 | — | — | — |
E, number of events; N, number of patients; Rate, event rate per 100 exposure years; %, percentage of patients
A total of 22 serious TEAEs were reported in 19 children, with similar frequencies in the dose groups: Nine (36.0%) children had ten events in the 0.033 mg/kg/day group and ten (38.5%) children had twelve events in the 0.066 mg/kg/day group (Table 2A). In general, the serious TEAEs were single events with no apparent pattern of distribution between dose groups. All but one of the serious TEAEs were considered unlikely related to GH. The related serious TEAE (polymyositis) in the 0.066 mg/kg/day group was assessed as probably related to GH by the investigator and led to withdrawal of the patient from the trial. Of the 22 serious TEAEs, 18 subjects recovered or were recovering from 20 of the reported serious TEAEs by the end of the trial. Two subjects were reported as having ‘not recovered/not resolved’ serious TEAEs (‘dental caries’ in the 0.033 mg/kg/day group and ‘conductive deafness’ in the 0.066 mg/kg/day group), both of mild severity.
Two subjects in the 0.066 mg/kg/day group withdrew from the trial due to TEAEs. One three-year-old girl experienced ‘polymyositis’ at day 1,041 after onset of treatment. The TEAE was serious, severe in severity, assessed as probably related to GH by the investigator and had a duration of 132 days. The reported outcome was ‘recovering/resolving’. As the patient was treated with steroids and immunosuppressive therapy, the investigator decided to withdraw the child from the trial. One nine-year-old girl experienced ‘scoliosis’ at day 1,289 after onset of treatment. The TEAE was non-serious, moderate in severity and assessed as possibly related to GH by the investigator. The reported outcome was ‘not recovered/not resolved’.
Four children experienced nine cardiac disease TEAEs. All four children had congenital heart disorders. One child treated with 0.033 mg/kg/day reported six of the TEAEs, of which all were related to tachycardia, non-serious, of mild severity, judged as unlikely related to GH by the investigator and resolved. One child treated with 0.066 mg/kg/day experienced a severe serious TEAE (‘atrial fibrillation’), which was judged as unlikely related to GH by the investigator and reported as recovered. One child treated with 0.066 mg/kg/day experienced a non-serious and mild TEAE of ‘Wolff-Parkinson-White syndrome’, judged as unlikely related to GH by the investigator and did not recover. One child treated with 0.066 mg/kg experienced a non-serious and mild TEAE of ‘ventricular extrasystoles’, as previously reported by Ozono et al. [27], which was judged as unlikely related to GH by the investigator and was reported as recovered. No trends were seen regarding the incidence of cardiac TEAEs in relation to demographics and baseline characteristics.
One TEAE of ‘glucose tolerance impaired’ (reported in a subject who was evaluated as having impaired glucose tolerance at screening) and one TEAE of ‘insulin resistance’ (reported term ‘insulin resistance suspected’) were reported in the 0.066 mg/kg/day group. These TEAEs were captured in the pre-specified Medical Dictionary for Regulatory Activities search for hyperglycemia and new onset of diabetes mellitus.
Other safety endpointsInitial steep increases in mean IGF-I and mean IGF-I SDS were observed in the first four weeks of treatment, with higher levels in the 0.066 mg/kg/day group compared to the 0.033 mg/kg/day group [27]. From week four and onwards, IGF-I increased further, whereas IGF-I SDS remained stable [27]. The estimated mean IGF-I increased from 69.9 ng/mL at baseline to 203.0 ng/mL in the 0.033 mg/kg/day group and to 287.5 ng/mL in the 0.066 mg/kg/day group after 208 weeks of treatment, corresponding to an estimated mean increase of 133.0 ng/mL and 217.6 ng/mL, respectively. This increase was significantly greater with 0.066 mg/kg/day compared to 0.033 mg/kg/day, with an estimated mean difference of 84.6 ng/mL (95% CI: 46.4; 122.7), p < 0.0001 (data not shown). The changes in IGF-I were reflected in the IGF-I SDS after 208 weeks of treatment. The estimated mean IGF-I SDS increased from –1.71 at baseline to –0.64 and –0.75 after 104 and 208 weeks, respectively, in the 0.033 mg/kg/day group and to 0.63 and 0.57 after 104 and 208 weeks, respectively, in the 0.066 mg/kg/day group (Fig. 7). This corresponded to an estimated mean increase in IGF-I SDS of 0.96 and 2.28, respectively, after 208 weeks of treatment. This increase was statistically significantly greater with 0.066 mg/kg/day compared to 0.033 mg/kg/day, with an estimated difference of 1.32 (95% CI: 0.82; 1.83), p < 0.0001. Overall, IGF-I SDS was >+2 (upper limit of normal) in 14 patients throughout the trial: for twelve patients in the 0.066 mg/kg/day group at 75 visits in total (ranging from 1 to 13 visits per patient), and for two patients in the 0.033 mg/kg/day group at six visits in total (ranging from 1 to 5 visits per patient). At the end of the trial, IGF-I SDS levels above +2 were recorded in one child in the 0.033 mg/kg/day group with a value of 2.6 and five children in the 0.066 mg/kg/day group with values of 2.1, 2.2, 2.3, 2.5, and 3.5. No malignancy events were reported throughout the trial period.
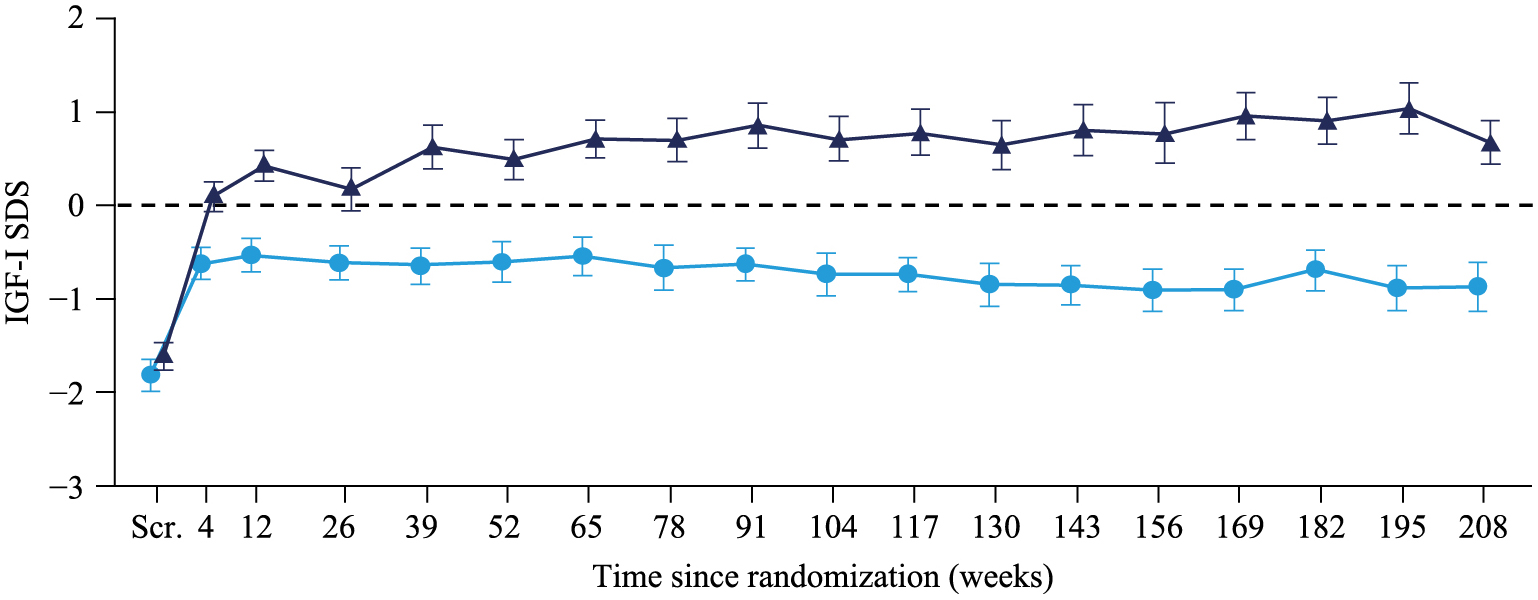
Mean IGF-I SDS in the two treatment groups over time.
 0.033 mg/kg/day GH;
0.033 mg/kg/day GH;  0.066 mg/kg/day GH
0.066 mg/kg/day GH
Safety analysis set, last observation carried forward imputed data. Mean ± standard error.
IGF-I, insulin-like growth factor I; SDS, standard deviation score; Scr., screening
There were only minor changes in HbA1c from baseline to 208 weeks of treatment and no apparent differences between dose groups were observed (0.033 mg/kg/day: +0.19%; 0.066 mg/kg/day: +0.20%) (not shown). The mean HbA1c levels were within the normal range throughout the 208 weeks of treatment. There were no clinically relevant changes compared to baseline in OGTT blood glucose after 208 weeks of treatment (Fig. 8a). There was no significant difference in the estimated treatment ratio of mean AUC of glucose between dose groups. At 208 weeks of treatment, the estimated treatment ratio for AUC of glucose (0.066 mg/kg/day / 0.033 mg/kg/day) was 1.06 (95% CI: 0.99; 1.14), p = 0.1081. The OGTT insulin response was increased after 208 weeks of treatment compared to baseline in both dose groups (Fig. 8b). After 208 weeks of treatment, the estimated mean AUC of insulin was 344.8 pmol*h/L in the 0.033 mg/kg/day group and 500.3 pmol*h/L in the 0.066 mg/kg/day group. This corresponded to an estimated treatment ratio (0.066 mg/kg/day / 0.033 mg/kg/day) of 1.45 (95% CI: 1.12; 1.87) which was statistically significant (p = 0.0053).

a) Blood glucose levels in the two treatment groups, based on OGTT; b) Insulin levels in the two treatment groups, based on OGTT.
0.033 mg/kg/day GH:  Screening,
Screening,  Week 52,
Week 52,  Week 104,
Week 104,  Week 156,
Week 156,  Week 208
Week 208
0.066 mg/kg/day GH::  Screening,
Screening,  Week 52,
Week 52,  Week 104,
Week 104,  Week 156,
Week 156,  Week 208
Week 208
Safety analysis set. Mean ± standard error.
OGTT, oral glucose tolerance test
At baseline, the mean bone age (SD) of children in both dose groups combined was 5.26 (2.22) years and thus approximately one year behind the chronological age of 6.31 (2.32) years (Table 1). The mean bone age increased steadily during the trial, and after 208 weeks, it had approached chronological age in both dose groups, with an estimated mean increase in bone age of 3.90 years with 0.033 mg/kg/day and 4.67 years with 0.066 mg/kg/day. The increase in bone age was significantly greater with 0.066 mg/kg/day compared to 0.033 mg/kg/day, with an estimated mean difference of 0.78 years (95% CI: 0.04; 1.51), p = 0.0398 between the two dose groups. The advanced bone age corresponded to the bone age/chronological age ratios, which was close to 1.0 after 208 weeks (0.88 in the 0.033 mg/kg/day group and 0.96 in the 0.066 mg/kg/day group) (Fig. 9). The advancement in bone age/chronological age ratio was significantly greater with 0.066 mg/kg/day compared to 0.033 mg/kg/day, with an estimated mean difference of 0.08 (95% CI: 0.02; 0.15), p = 0.0119, between the two dose groups (data not shown).

Bone age/chronological age in the two treatment groups.
 0.033 mg/kg/day GH;
0.033 mg/kg/day GH;  0.066 mg/kg/day GH
0.066 mg/kg/day GH
Safety analysis set. Mean ± standard error. Dashed line indicates a ratio of 1. Centralized evaluation of bone age was done by RUS score method of Tanner-Whitehouse II method.
RUS, radius, ulna and short bones
During the first 104 weeks (pivotal phase), the mean body mass index (BMI) fluctuated around baseline levels in both dose groups. From 104 weeks and onwards, the mean BMI increased slightly in both dose groups with higher levels in the 0.066 mg/kg/day (increase of 0.88 kg/m²) compared to the 0.033 mg/kg/day (increase of 0.27 kg/m²) group at 208 weeks.
As previously reported for 104 weeks of GH treatment by Ozono et al. [27], there were no clinically relevant changes from baseline to 208 weeks or differences between dose groups in other clinical laboratory safety parameters (hematology, biochemistry, lipids, thyroid hormones, blood coagulation tests, and urinalysis), vital signs (blood pressure and pulse), ECG or transthoracic echocardiography.
In this trial, the growth-promoting effect and safety of GH for the treatment of Japanese children with short stature due to NS were investigated. Overall, the results showed that after 208 weeks of treatment with GH there was a gain in HSDS, and that this increase was significantly greater with a daily dose of 0.066 mg/kg compared with 0.033 mg/kg. This is in line with results of the pivotal phase of the trial at 104 weeks of treatment [27]. No new safety concerns were observed during the entire treatment period.
Of the 36 (70.6%) subjects clinically diagnosed with NS for whom genotyping data were available, 28 (77.8%) had a PTPN11 mutation, 2 (5.6%) each had a SOS1 or RAF1 mutation and 1 (2.8%) each had mutations of KRAS, BRAF, SHOC2 or RIT1. This rate of genetic mutations was generally similar to the frequency previously reported for patients with NS [6].
Both GH doses resulted in estimated mean gains in HSDS after 208 weeks of treatment, with a significantly greater increase in the 0.066 mg/kg/day group (1.84) than in the 0.033 mg/kg/day group (0.85) relative to Japanese national reference data for children. These results were reflected when analyzed relative to Japanese NS reference data. In addition, 76.9% of subjects in the 0.066 mg/kg/day group reached a HSDS >–2 after 208 weeks of treatment compared to 32.0% in the 0.033 mg/kg/day group (Japanese national reference data for children). The estimated mean HSDS for subjects treated with 0.033 mg/kg/day was –2.39 at 208 weeks, which was still below the normal range of –2 to +2, whereas the estimated mean HSDS was within the normal range for subjects treated with 0.066 mg/kg/day (–1.40).
Other studies of three to four years duration with GH treatment at doses of 0.047–0.059 mg/kg/day in children with NS have also shown an increase in HSDS. A study with 23 children with NS showed an increase in HSDS from –2.7 at baseline to –1.9 after three years of treatment with 0.33 mg/kg/week GH [37]. In an observational study assessing HSDS in NS patients for up to four years of treatment with 0.047–0.054 mg/kg/day GH, twelve of 17 patients (71%) achieved height normal for age and gender (HSDS >–2 SD), with mean HSDS increasing from –2.65 at baseline to –1.32 after four years of GH treatment [38]. Treatment with 0.05–0.075 mg/kg/day GH lead to an increase in HSDS from –2.64 at baseline to –1.54 after three years in a study with 15 pre-pubertal children with NS conducted in Korea [39]. These observations are in line with gains in HSDS reported for the current study.
Whereas the mean HSDS continued to increase over the 104-week treatment period (pivotal phase) in both dose groups, as was previously reported by Ozono et al. [27], this increase continued only in the 0.066 mg/kg/day group over 208 weeks of treatment (estimated mean HSDS increase of 1.47 at 104 weeks [27] and 1.84 at 208 weeks). In the 0.033 mg/kg/day group, the mean HSDS appeared to reach a plateau after 104 weeks (estimated mean HSDS increase of 0.84 at 104 weeks [27] and 0.85 at 208 weeks). Thus, mean HSDS was almost unchanged from 104 to 208 weeks of treatment in the 0.033 mg/kg/day group (–2.40 vs. –2.39), but had increased in the 0.066 mg/kg/day group in this time period (–1.78 at 104 weeks to –1.41 at 208 weeks) [27]. In contrast to values based on the Japanese national reference data for children, mean HSDS based on the Japanese NS reference data increased continuously over 208 weeks in both dose groups, although to a lesser extent in the 0.033 mg/kg/day group than in the 0.066 mg/kg/day group.
Previous studies have reported that the increase in mean HSDS was highest after one year of treatment with GH, but further increases after one year of treatment were significantly lower. The analysis of 402 NS patients enrolled in the KIGS database who were treated with GH showed a mean HSDS increase of 0.54 after one year of treatment, but significantly lower incremental increases after two and three years of GH therapy (0.13 each) [25]. In another clinical trial, HSDS was 0.5 after one year of treatment, but again the annual incremental increase was lower after two and three years of GH treatment (0.1 and 0.2, respectively) [37]. In contrast, an observational study with 17 NS patients treated with GH over a period of four years, mean HSDS changes increased with every year of treatment (year 1: 0.4; year 2: 0.75; year 3: 1.0; year 4: 1.07) [38]. This may be explained by the increase in mean GH dose from 0.047 mg/kg/day at the start of the study to 0.059 in the fourth year, and is in line with the observation made in this trial that only the higher dose of 0.066 mg/kg/day GH lead to a continuous increase of mean HSDS over 208 weeks of treatment.
In addition, height velocity SDS in both dose groups also increased in the first year of treatment, after which it gradually decreased, as was previously reported by Ozono et al. [27]. After four years of treatment, the mean height velocity SDS was maintained above 0 in the 0.066 mg/kg/day group, and was slightly below 0 in the 0.033 mg/kg/day group. This reflects an increase of height velocity SDS from the lower border of the reference range at baseline to values above the upper border of the reference range within the first treatment year, which then decrease to remain within the reference range.
As onset of puberty can be delayed by up to two years in NS patients, they may grow for a longer period of time and at an older age than individuals among the overall population, allowing for a longer growth period [14]. The steadily increasing number of subjects entering puberty after 104 weeks of treatment may partly explain why HSDS appeared to plateau or increase more slowly after this time point. However, the number of subjects entering puberty at certain time points after initiation of GH treatment was similar in the two dose groups. Generally, it has to be taken into account that adolescence is a period during which growth spurts are observed with sex hormones affecting increases in height. In the current study, approximately half of all subjects were in a pubertal stage during the trial period. It is therefore important to consider that the increase in height observed in this trial may also be affected by sex hormones associated with the onset of puberty when interpreting the data. HSDS increases during puberty have been reported previously [23] indicating that GH may improve height gain during puberty in addition to the height increase associated with the presence of sex hormones. Further investigations will need to be performed in order to explore the effect of GH treatment on adult height of Noonan syndrome.
In addition, the mean bone age advanced steadily in both dose groups during the trial, with a greater advancement seen in the 0.066 mg/kg/day group compared with the 0.033 mg/kg/day group. After 208 weeks, the bone age/chronological age approached 1.0 in both dose groups (0.033 mg/kg/day: 0.88; 0.066 mg/kg/day: 0.96) with a significant difference between dose groups in favor of 0.066 mg/kg/day.
These efficacy results indicate that doses of 0.033 mg/kg/day or 0.066 mg/kg/day can both be recommended in the treatment of short stature due to NS. This gives clinicians the option to individualize GH dosing based on the specific patient situation and is of great benefit when taking into account that other factors, such as age at treatment initiation, duration of treatment, age at onset of puberty or GH sensitivity may play a role with respect to the treatment effect [21, 23, 40]. The fact that a greater change in HSDS was seen when treatment was initiated at a younger age shows the importance of starting GH treatment at an early age.
No safety concerns were identified during 208 and 234 weeks of GH treatment. The proportion of subjects with TEAEs and the event rates of TEAEs were similar, and there were no clinically relevant differences in the distribution of TEAEs between dose groups. Of the 22 serious TEAEs reported during the trial (seven serious TEAEs and no withdrawals had been reported within the pivotal phase after 104 weeks of treatment) [27], all but one were considered unlikely related to GH. The related serious TEAE (‘polymyositis’) was reported in a patient treated with 0.066 mg/kg/day and led to withdrawal from the trial because the child was treated with steroids and immunosuppressive therapy. Polymyositis is an idiopathic inflammatory myopathy characterized by symmetrical, proximal muscle weakness, elevated skeletal muscle enzyme levels and characteristic electromyography and muscle biopsy findings. To date, polymyositis has not been reported as a treatment-related AE in association with GH, and GH treatment in general is not known to be myotoxic. Furthermore, drug-induced myopathies usually resolve within weeks after discontinuation of treatment with the causative agent, which was not the case with this patient. Therefore, the event is likely related to the patient’s underlying NS. The other TEAE leading to withdrawal was ‘scoliosis’ reported as possibly related to GH treatment. Skeletal abnormalities are a common feature in NS patients, and scoliosis was seen in 16 of 60 patients in a clinical review [9], again suggesting that the event is likely related to the patient’s NS and not to the treatment itself.
There was no evidence of a negative effect of GH on cardiac function, and all nine events occurred in four subjects with existing congenital heart disorders. All but one cardiac disease TEAEs were reported as transient, one non-serious and mild TEAE of ‘Wolff-Parkinson-White syndrome’ judged as unlikely related to GH was reported in a child treated with 0.066 mg/kg/day who did not recover by the end of the trial. After 104 weeks of treatment (pivotal phase), two cardiac disorders in two subjects had been reported [27]. In previous studies, cardiac side effects associated with GH treatment, including features of hypertrophic cardiomyopathy, were also absent [21, 37, 39]. No new safety findings were identified from the evaluations of clinical safety laboratory parameters.
Increased IGF-I levels (IGF-I SDS above +2) are generally a concern during long-term GH treatment in children, therefore IGF-I was monitored in order to ensure that there was no constant increase in IGF-I SDS levels above +2 SDS. Although health risks associated with such an increase are difficult to assess, it is preferable to maintain IGF-I levels below +2 SDS. Subjects in the trial generally had low IGF-I levels at baseline (0.033 mg/kg/day: 70.2 ng/mL; 0.066 mg/kg/day: 69.7 ng/mL). In the first four weeks of treatment, steep increases in IGF-I and IGF-I SDS were observed. This reflected a normalization of estimated mean IGF-I SDS from being just above the lower border of the reference range at baseline (–1.71) to estimated mean values within the normal range of –2 to +2 (0.033 mg/kg/day: –0.75; 0.066 mg/kg/day: 0.57) at 208 weeks of treatment. These values after the initial increase are comparable with the estimated mean IGF-I SDS levels reported after 104 weeks of treatment in the pivotal phase of the trial (0.033 mg/kg/day: –0.64; 0.066 mg/kg/days: 0.63) [27]. The initial steep increases in mean IGF-I and mean IGF-I SDS are therefore as expected with GH treatment. After four weeks and until 208 weeks, the mean IGF-I SDS remained stable (below or approximately +1 for the 0.066 mg/kg/day group and below 0 for the 0.033 mg/kg/day group). Overall, two children in the 0.033 mg/kg/day group and twelve children in the 0.066 mg/kg/day group had an IGF-I SDS above +2 at some of the visits within the 208 or 234 week treatment period. There was a positive correlation between change in HSDS (Japanese NS reference data) and change in IGF-I SDS at 208 weeks which was similarly shown at 104 weeks based on Japanese national reference data for children [27]. In some subjects, large increases in HSDS were seen, whose increases in IGF-I SDS was <2 SDS, and three subjects with an increase in HSDS showed IGF-I SDS changes <0 SDS, suggesting a possible direct effect of GH on bone.
There were only minor changes in HbA1c levels and no clinically relevant changes in OGTT blood glucose after 208 weeks of treatment and without apparent differences between dose groups. The OGTT insulin response was increased in both dose groups after 208 weeks of treatment. All changes in glucose metabolism were within the expected range when administering GH, which is known to induce a mild form of insulin resistance [41].
The strengths of this trial include the double-blind design, the comparison between two GH dose groups, and that the trial population, with 51 children, was relatively large. The use of reference values from Japanese patients with NS, in addition to the Japanese national reference data for children among the general population, to calculate HSDS values, was also a strength of this trial. The trial, however, has the limitation that no adult height data for the children enrolled in the trial are available.
In conclusion, GH at doses of 0.033 mg/kg/day and 0.066 mg/kg/day improved height in Japanese children with short stature due to NS. After 208 weeks of treatment, the increase in HSDS was significantly greater with 0.066 mg/kg/day compared to 0.033 mg/kg/day. Treatment with GH was well tolerated with a majority of TEAEs being non-serious, mild and assessed as unlikely related to treatment. TEAE rates and patterns were similar between dose groups. No new safety concerns were identified during the 208 weeks of treatment.
The authors would like to thank the patients who participated in the trial and their families.
The authors would like to acknowledge the contribution of the following investigators who were involved in the trial (in alphabetical sequence): Masanori Adachi, Hirokazu Arakawa, Ikuma Fujiwara, Tomonobu Hasegawa, Yukihiro Hasegawa, Shinobu Ida, Kenji Ihara, Tsuyoshi Isojima, Kenichi Kashimada, Tatsuya Kawano, Toru Kikuchi, Sachiko Kitanaka, Kanako Kojima-Ishii, Hideki Kumagai, Haruo Mizuno, Hiroshi Mochizuki, Jun Mori, Keisuke Nagasaki, Hisakazu Nakajima, Toshio Nakanishi, Yoshiaki Ohtsu, Shinji Saitoh, Hirotake Sawada, Shun Soneda, Hisashi Sugiyama, Yusuke Tanahashi, Hidefumi Tonoki, Maiko Utoyama, Shuichi Yatsuga, Ichiro Yokota and Tohru Yorifuji.
The study was funded by Novo Nordisk Pharma Ltd.
Medical writing support was provided by Physicians World Europe GmbH, Mannheim, Germany, and was financially supported by Novo Nordisk.
Yoichi Matsubara and Susumu Yokoya have nothing to declare. Takaaki Endo, Keiji Nishijima and Yoshihisa Ogawa are employees of Novo Nordisk Pharma Ltd. Reiko Horikawa, Tsutomu Ogata and Keiichi Ozono have received lecture fees and research grants from Novo Nordisk Pharma Ltd. Keiichi Ozono has further received lecture fees from Kyowa-Kirin and Alexion and research grants from Ribomic. Tsutomu Ogata and Keiichi Ozono have received scholarship donations from Novo Nordisk Pharma Ltd.