2021 Volume 68 Issue 1 Pages 63-68
2021 Volume 68 Issue 1 Pages 63-68
Anaplastic thyroid cancer (ATC) is a rarely occurring refractory disease. While recent clinical trials have demonstrated the efficacy of tyrosine kinase inhibitor (TKI) therapy for ATC, evidence is scarce in clinical practice. In this study, we reviewed our initial experiences with TKI treatment in ATC patients with the aim of revealing the efficacy and safety of the same in clinical practice. We retrospectively reviewed our experiences with TKI treatment use in ATC patients diagnosed at our institute from 2014 to 2019. Changes in the patients’ neutrophil-to-lymphocyte ratio (NLR) by TKI therapy introduction as well as their clinical factors to indicate the efficacy were examined. Seven patients showed no indication for TKI treatment, while 13 (65%) received treatment. The median duration of TKI treatment was 1.9 months. All patients died, and the overall survival period from diagnosis was 4.7 (95% confidence interval: 2.0–11.5) months. Adverse events ≥Grade 3 were observed commonly (92.3%), and resulted in the termination of TKI treatment in six cases (46.1%). Existence of multiple unfavorable characteristics (higher Prognostic Index) was associated with poor survival. The NLR decreased after the introduction of TKIs and increased again when treatment failed. The response rate to TKI among the ATC patients were approximately 30% in practice. Although the duration of the response was short, several patients demonstrated long survival durations when TKI treatment was provided after successful multidisciplinary treatment to control local disease. Decreases in high NLR values during treatment may suggest the continued effect of TKIs.
ANAPLASTIC THYROID CANCER (ATC) is a rarely occurring refractory disease that often shows resistance to conventional multidisciplinary therapies such as surgery, radiation and chemotherapy [1], which exert only a marginal effect on patient survival [2]. The median survival of such patients is only 4 months from disease diagnosis [2, 3]. Prognosis of the patients with advanced stage ATC was dismal [4]. Although several therapeutic approaches using cytotoxic chemotherapy showed limited efficacies [5, 6], the prognosis has not improved in the last 20 years [7]. The revised 2018 Japanese guideline for the treatment of thyroid tumors strongly recommends the use of molecular targeted therapy with tyrosine kinase inhibitors (TKIs) for ATC [8].
The efficacy of TKI has been clearly demonstrated in ATC management. There is basic rationale for attempting TKI in ATC management in some setting based on limited data. Sorafenib has shown a disease control effect [9], while lenvatinib demonstrated a significant response rate (RR) of 24% in a clinical trial [10]. More recently, dabrafenib with trametinib [11, 12] or combination treatment using immune check point inhibitors with them. [13] demonstrated a significant effect in ATC treatment. NCCN Clinical guidelines recommended these agents for systemic therapy for ATC [14]. At present, only lenvatinib has been approved for clinical use for ATC in Japan. Sorafenib inhibits the tyrosine kinase of the vascular endothelial growth factor receptor (VEGFR), RET gene, and the platelet-derived growth factor receptor (PDGFR) [15]. Lenvatinib strongly inhibits the tyrosine kinase of the VEGFR, RET gene and PDGFR too, and also inhibits the tyrosine kinase of the fibroblast growth factor receptor [16]. However, adverse events associated with TKI use have been frequently reported in Japanese patients, such as those with hypertension, proteinuria, hand-foot syndrome, fatigue, and appetite loss [17]. Sometimes, these adverse events may impair patients’ quality of life or cause life-threatening events that may lead to treatment termination. Although recent clinical trials have demonstrated the efficacy of TKI therapy for ATC, evidence is scarce in clinical practice. Two reports have shown the treatment results of TKI for ATC in clinical practice [13, 18]. Still, no insight has been demonstrated concerning the indicator to prospect the prognoses or monitor the efficacy.
The prognostic index (PI) was designed to evaluate the prognoses of ATC patients [19] and has been confirmed reliable prospectively [20]. PI was, thus, listed in Japanese guidelines as a useful tool in determining appropriate therapeutic strategies [8]. This index was devised based on the number of four unfavorable characteristics the patient possessed which are as follows: 1) acute symptoms presenting within 1 month, 2) leukocytosis with a leukocyte level of 10,000/mm3 or greater, 3) tumor size greater than 5 cm, and 4) existence of distant metastasis. A PI of ≥2 indicates poor prognoses [19]. The neutrophil-to-lymphocyte ratio (NLR) is among the simple tools used for monitoring patient’s condition and evaluating treatment efficacy. NLR is calculated using the results of standardized blood tests; i.e. determined by dividing the neutrophil count by the lymphocyte count [21]. A high NLR indicates deterioration in the patient’s condition and/or the aggressiveness of the disease those resulted in poor response to the treatment and unfavorable prognoses [21].
In the present study, we reviewed our initial experiences with TKI treatment in ATC patients by two VEGF-inhibitory multi-kinase inhibitors; i.e. sorafenib and lenvatinib, with the aim of revealing the efficacy and safety of the same in clinical practice. We additionally investigated the possible indicators to prospect the efficacy of the treatment.
Data of ATC patients who received TKI therapy were retrospectively reviewed. A total of 20 patients were diagnosed with ATC at our institute from 2014 to 2019. Seven patients had no indication for anticancer treatment due to extreme disease progression and were referred for palliative care. Thirteen patients were treated with TKIs, after providing informed consent. Data on the patients’ clinical characteristics and treatment course were obtained from their records, including PI and NLR. We measured the NLR at shortly before induction of treatment, the lowest during treatment and the end of treatment. The survival period was defined as the period from the diagnosis of ATC to death of any cause. The effect on the tumor was evaluated using the RECIST (ver 1.1) criteria [22] while the adverse events (AE) was evaluated using the CTCAE (ver 4.0) criteria [23]. The institutional review board of our institute approved the present study (#4380).
Statistical analysis was performed using EZR [24]. Kaplan-Meier curves were created, and differences in the overall survival (OS) were examined by a log-rank test, with p < 0.05 denoting statistical significance.
Table 1 demonstrates the demography of the 13 ATC patients treated with TKIs. The patients comprised four men and nine women, with a median age of 74 (40 to 82) years. Seven patients had unresectable ATC, two had ATC recurrence after initial ATC treatment, and four showed ATC recurrence following initial treatment for differentiated thyroid cancer (DTC). Sorafenib and lenvatinib were administered in three and 10 patients, respectively. In two patients, sorafenib was provided as part of a clinical trial. These cases have already reported results in the trial [6]. Only a single regimen was provided to all patients, and no salvage therapy with other TKIs was administered.
| # | Age (y)/Sex | Indication1) | T | N | M | Stage2) | PI3) | Initial NLR4) | Treatment before TKI5) use | TKI | Maximal response | Treatment after TKI use | Cause of death | OS (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 82/M | Rec. ATC | X | 0 | 1 | IVA | 1 | 3.6 | Surgery (R0), EBRT6), CT7) | Sorafenib | PD8) | Surgery (lung) | Accident | 17.4 |
| 2 | 79/F | Primary | 3b | 1b | 0 | IVB | 1 | 2.1 | None | Sorafenib | SD9) | None | Suffocation | 3.2 |
| 3 | 79/F | Rec. DTC | X | X | 1 | IVC | 4 | 14.3 | None | Sorafenib | PD | None | Cachexia | 2.9 |
| 4 | 66/F | Rec. ATC | 3b | 0 | 1 | IVC | 2 | 4.7 | CT, Surgery (R0), EBRT, CT | Lenvatinib | PR10) | None | Cachexia | 22.0 |
| 5 | 67/F | Primary | 3b | 1b | 1 | IVC | 2 | 9.0 | Surgery (R2), EBRT, CT | Lenvatinib | PD | None | Cachexia | 11.5 |
| 6 | 77/F | Primary | 4a | X | 1 | IVC | 4 | 5.3 | EBRT, CT | Lenvatinib | SD | None | Cachexia | 4.7 |
| 7 | 74/F | Primary | 4a | 1b | 0 | IVB | 2 | 3.8 | None | Lenvatinib | SD | None | Suffocation | 1.8 |
| 8 | 76/F | Primary | 4a | 1b | 0 | IVB | 2 | 3.9 | None | Lenvatinib | PD | None | Suffocation | 1.6 |
| 9 | 77/F | Primary | 3b | 1b | 0 | IVB | 2 | 3.4 | None | Lenvatinib | SD | None | Cachexia | 4.8 |
| 10 | 69/F | Rec. DTC | X | 1b | 1 | IVC | 3 | 5.2 | None | Lenvatinib | PR | None | Cachexia | 2.4 |
| 11 | 70/M | Primary | 4a | 0 | 0 | IVB | 1 | 2.4 | EBRT | Lenvatinib | PR | None | Bleeding | 18.2 |
| 12 | 68/M | Rec. DTC | X | X | 1 | IVC | 2 | 4.8 | Surgery (lung) | Lenvatinib | X | None | Cachexia | 10.3 |
| 13 | 40/M | Rec. DTC | X | X | 1 | IVC | 2 | 32.6 | None | Lenvatinib | SD | None | Bleeding | 2.0 |
1) Indication for treatment; Rec. ATC, recurrent disease after treatment of anaplastic thyroid cancer; Primary, primary tumor of anaplastic thyroid cancer; Rec. DTC, anaplastic transformation of recurrent disease after treatment of differentiated thyroid cancer, 2) classified by TNM classification 8th edition, 3) PI, prognostic index, 4) NLR, neutrophil-to-lymphocyte ratio, 5) TKI, tyrosine kinase inhibitor, 6) EBRT, external beam radiation therapy, 7) CT, chemotherapy with weekly paclitaxel, 8) PD, progressive disease, 9) SD, stable disease, 10) PR, partial remission
OS, overall survival; ATC, anaplastic thyroid cancer; DTC, differentiated thyroid cancer
The patients’ overall treatment status is shown in Fig. 1. The interval from ATC diagnosis to TKI therapy initiation varied from 0.2 to 16.7 (median 0.5) months. In five patients, treatment for ATC was initiated by methods other than TKI therapy, such as surgery, radiation or chemotherapy. The duration of TKI treatment ranged from 0.4 to 9.2 (median 1.9) months. The survival period following TKI treatment was zero to 5.3 (median 0.3) months.

Overall treatment status. C, chemotherapy; S, surgery; R, radiation therapy; PR, partial response; SD, stable disease; PD, progressive disease; one patient had no evaluable lesion.
Maximal response was observed, with none of the patients showing complete response (CR), three showing partial response (PR), five showing stable disease (SD), and four showing progressive disease (PD). One patient (case 12) operated on for a rapidly growing lung tumor after radioisotope therapy for multiple lung metastases from DTC. The resected lung tumor was revealed pathologically as ATC. There were still multiple metastases remained in the lung but had no evaluable ATC lesions. The overall RR was 25.0% (3/12), and the clinical benefit rate (CBR) was 66.7% (8/12). Adverse events were found in all the patients and were of a grade greater than 3 in 12 patients (92.3%). In six cases (46.1%), TKI treatment was terminated due to the presentation of adverse events, such as fatigue, liver dysfunction, skin reactions, rupture of the common carotid artery, perforation of the esophagus, or wound healing failure after spinal surgery.
The median OS was 4.7 (95% confidence interval: 2.0–11.5) months, and the 3, 6 and 12-month survival rates were 53.9 ± 13.8, 30.8 ± 12.8, and 15.4 ± 10.0%, respectively (Fig. 2). There were no differences in the OS according to age, sex, and T and M stage. Patients without nodal involvement demonstrated significantly better OS values than those with nodal metastasis (18.2 vs. 2.8 months, p = 0.02), although data on nodal status were available only in nine patients. Patients with treatment response tended to survive for longer durations than those without response (18.2 vs. 4.0 months, p = 0.07). The survival period from TKI therapy initiation was 2.9 (95% confidence interval: 1.8–4.5) months, and no difference between responders and non-responders (5.2 vs. 2.8 months, p = 0.16) was found. Twelve patients died of the disease: seven of cachexia, three of suffocation, one of esophageal bleeding, and one of cerebral bleeding. One patient (case 1) died from fall accident at home following the postoperative period for the metastatic lung tumor refractory to TKI treatment. Three patients (23.1%) survived for longer than 12 months. All three of them received conventional therapy before TKI treatment initiation. In two patients, the primary tumor was surgically resected, and in all three patients external beam radiation to the neck was provided before TKI treatment initiation (Table 1).
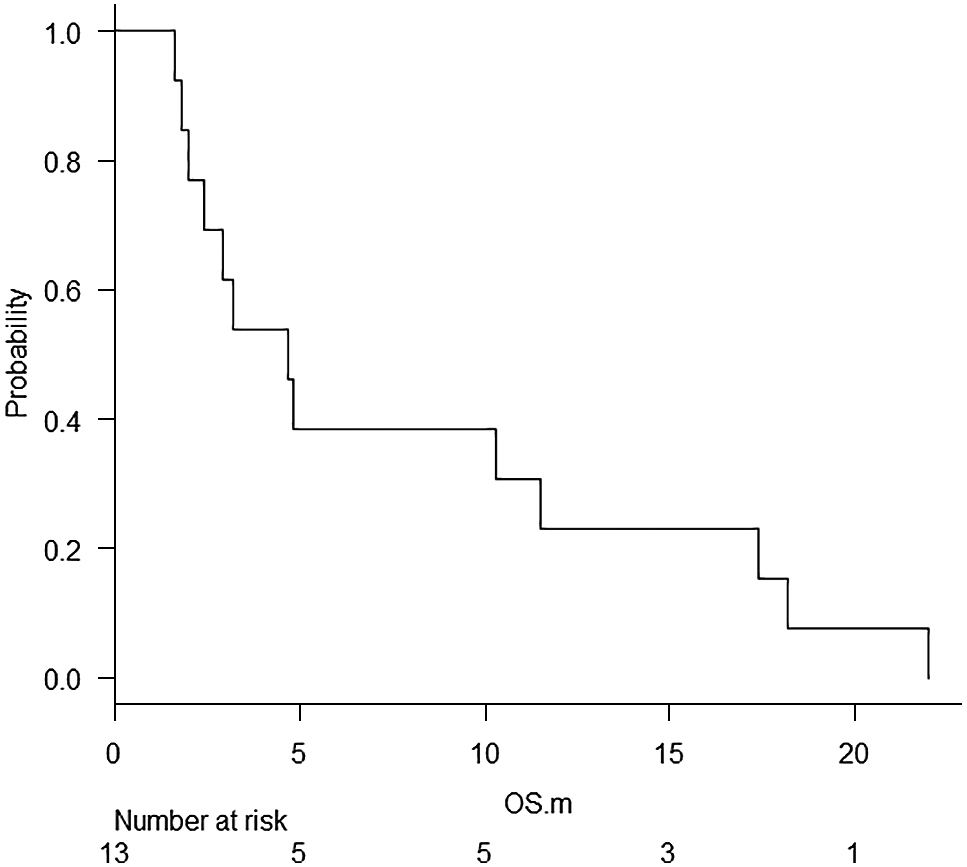
Overall survival period of all patients treated with tyrosine kinase inhibitors.
OS, overall survival
Five deaths occurred within 30 days after the start of TKI. In all cases, tumor size, PI score, and NLR increased, indicating death from poor disease control. There were no treatment-related deaths.
The PI ranged from 1 to 4, with a median of 2. Patients with low PI scores (0 or 1) demonstrated longer survival periods than those with high PI scores (2 to 4) (17.4 vs. 3.8 months, p = 0.37) (Fig. 3); however, the difference was not statistically significant. The NLR ranged from 2.1 to 32.6 (median 4.7) before TKI treatment initiation. A rapid decrease in the NLR was observed within a month from the initiation of TKI therapy in 11 patients (84.6%). The NLR remained low during successful TKI treatment in two patients (cases 12 and 13). The NLR rose again at the time of the termination of TKI treatment in all the patients investigated (Fig. 4).

Overall survival period of patients stratified by prognostic index.
OS, overall survival; PI, prognostic index.

Change in the neutrophil-to-lymphocyte ratio (NLR) before, during and at the termination of treatment with tyrosine kinase inhibitors. A case (#13) with an extraordinarily high NLR (32.6, 21.9, 29.6) was omitted from the plot.
NLR, neutrophil-to-lymphocyte ratio
Successful treatment with multidisciplinary treatment involving surgery, radiation, and chemotherapy [8, 25] could be achieved only in select cases without distant metastasis [26, 27]. Surgery and radiation may control local disease and prevent suffocation temporarily. Still, a majority of patients die of locally recurrent and/or disseminated disease [28]. Systemic treatment is, thus, important for disease management. Iyer et al. demonstrated the efficacy of targeted therapies with lenvatinib as well as dabrafenib plus trametinib in ATC patients. The RR and CBR was 30% (3/10) and 70% (7/10), respectively, for lenvatinib. Median OS after the initiation of TKI therapy was 3.9 months for the patients treated with lenvatinib [13]. Iwasaki et al. described their experiences on 23 stage IVC ATC patients treated with lenvatinib. The RR and CBR was 17.4% (4/23) and 45.3% (10/23), respectively. Median OS was 5.5 months including the period for initial surgery in 9 patients, and it was 4.3 months for 14 patients treated with lenvatinib only [18]. In the present study, we also could observe RR of 25% and CBR of 66.7%. These were in line with the observation reported above [13, 18] and in the clinical trial [10]. The effects could be observed soon after treatment initiation. Unlike the clinical trial, the effect of lenvatinib lasted for less than a median of 3 months in our series and the Iwasaki’s [18]. Salvage therapy could not be administered due to the rapid deterioration of patients’ general condition owing to disease progression. Therefore, we could not demonstrate a significant overall effect of TKI therapy on survival prolongation as was also failed to demonstrate in the previous report [13, 18]. Still, there certain was an extension of survival for approximately 2 months by employing TKI. At the same time, long-term survival for periods longer than 12 months was observed in three patients. TKI therapy was indicated for cases with recurrent disease after initial successful local control. In addition, two more patients who had undergone surgical resection of the primary tumor survived for longer than 10 months. As both the TKIs used were administrated orally, local control at the neck was very important. It was indicated that, for the exhibition of the maximal effect of TKI treatment, successful local control in the neck with conventional multidisciplinary therapy was necessary.
The PI is a simple and useful indicator for the evaluation of the prognoses of ATC patients [8, 19, 20, 29]. Our findings demonstrated the possible utility of PI in the prediction of TKI treatment efficacy; however, further investigation with a larger number of cases is necessary. As observed in this study, the elevated NLR values in most of the ATC patients decreased following the introduction of TKI therapy, and was maintained at low levels during successful treatment and then increased at treatment termination. These observations suggest the utility of the NLR in the evaluation of treatment effect and the sustainability of the treatment in individual patients.
This study has several limitations. ATC diagnosis was determined clinically by the evaluation of extraordinarily aggressive progression in 1 month without pathological confirmation in two patients (cases 3 and 13). The analyzed patients were enrolled retrospectively, and the initiation and termination criteria for TKI treatment were not standardized. The maximal effect was determined at a single time-point without the confirmation of the consecutive effect. Salvage treatment after sorafenib use could not be attempted in two patients (cases 2 and 3) because lenvatinib had not been approved for use at that time.
In conclusion, the response rate to TKI therapy in ATC patients was around 30% in practice. Although the duration to treatment failure was short with the use of TKI therapy alone, several patients demonstrated long survival durations through the administration of TKI treatment as a part of successful multidisciplinary treatment. The decreases observed in the initially elevated NLR values during treatment in individual patients may suggest the continuation of the effect of TKIs. There is a need for the development of evolutional treatment strategies for the management of patients with this aggressive and lethal disease.
NO received honoraria and research funding from Eisai and Bayer. SN received research funding from Eisai. SK received honoraria and research funding from Eisai. SI, YT, TM, YA, TT and MO declare no competing financial interests.