2021 Volume 68 Issue 11 Pages 1347-1357
2021 Volume 68 Issue 11 Pages 1347-1357
Chronic pancreatitis (CP) is a chronic inflammatory and fibrotic disease of the pancreas, and activated pancreatic stellate cells (PSCs) play a vital role in the progression of pancreatic fibrosis in CP. It has been reported that long non-coding RNA small nucleolar RNA host gene 11 (SNHG11) is highly expressed in chronic pancreatitis (CP) patients. However, the role of SNHG11 in CP progression is unclear. The purport of the study was to survey the role of SNHG11 in CP. We employed transforming growth factor (TGF)-beta1 (TGF-β1) to activate human pancreatic stellate cells (PSCs). Expression of SNHG11 was assessed with qRT-PCR. Loss-of-function experiments were executed to evaluate the effects of SNHG11 on the proliferation and migration of TGF-β1-treated PSCs. Some protein levels were detected by western blotting. The regulatory mechanism of SNHG11 was verified by the dual-luciferase reporter and RIP assays. As a result, SNHG11 was upregulated in plasma of CP patients and TGF-β1-treated PSCs. Also, SNHG11 inhibition reduced TGF-β1-induced proliferation, migration, and ECM accumulation in PSCs. Mechanistically, SNHG11 regulated leukemia inhibitory factor (LIF) expression by sponging miR-34b. Furthermore, miR-34b inhibitor abolished SNHG11 silencing-mediated effects on TGF-β1-treated PSC proliferation, migration, and ECM accumulation. LIF overexpression counteracted the repressive influence of miR-34b mimic on proliferation, migration, and ECM accumulation of TGF-β1-treated PSCs. In conclusion, SNHG11 knockdown reduced TGF-β1-induced PSC proliferation, migration, and ECM accumulation by the miR-34b/LIF axis.
CHRONIC PANCREATITIS (CP) is a progressive disease characterized by inflammation of the pancreas, which causes fibrosis and destroys exocrine and endocrine tissues [1]. Pancreatic fibrosis is the main risk factor for pancreatic cancer [2], which is caused by the proliferation of fibroblasts and the deposition of extracellular matrix (ECM), containing collagen fibers [3]. Pancreatic stellate cells (PSCs) are essential for maintaining normal pancreas structure [4], and activated PSCs play a key role in the development of pancreatic fibrosis [5, 6]. Therefore, it is of great significance to explore the mechanisms related to pancreatic fibrosis in CP.
Long non-coding RNAs (lncRNAs) are a type of transcripts that are longer than 200 nucleotides and do not have protein-coding capability [7]. According to the competing endogenous RNA (ceRNA) hypothesis, lncRNAs can adsorb microRNA (miRNA) by miRNA response elements (MREs), thus affect miRNA affinity with mRNA [8]. It has been proved that lncRNAs participate in diverse biological functions and disease processes [9] and act as potential biomarkers for disease diagnosis [10, 11]. Moreover, lncRNAs were revealed to be connected with pancreatic disease [12-14]. For instance, lncRNA MIAT regulated the fibrosis process of CP by modulating the activation of PSCs [15]. Small nucleolar RNA host gene 11 (SNHG11), also termed as NCRNA00101, is an lncRNA. SNHG11 has been uncovered as a latent biomarker for colorectal cancer early diagnosis [16]. Moreover, SNHG11 can contribute to the progression of hepatocellular carcinoma [17] and lung cancer [18]. However, the biological function of SNHG11 in CP was unclear.
In the current study, we explored the biological role of SNHG11 in CP. Our results suggested that SNHG11 inhibition decreased TGF-β1-induced proliferation, migration, and ECM accumulation through the miR-34b/LIF pathway in PSCs.
The research was authorized and supervised by the Ethics Committee of The First Affiliated Hospital of Bengbu Medical College of the Joint Service Support Force. 60 clinical plasma samples were obtained from The First Affiliated Hospital of Bengbu Medical College of which 30 were from CP patients and 30 were from healthy controls. The collected venous blood samples were placed in tubes containing ethylene diamine tetraacetic acid. Next, plasma was obtained by centrifugation at 1,600 × g for 10 min. Written informed consents were obtained from all participants.
Cell culture, treatment, and transfectionHuman pancreatic stellate cells (PSCs) were bought form Procell (Wuhan, China) and cultured in DMEM/F-12 (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS (fetal bovine serum) (Thermo Fisher Scientific) and 1% penicillin/streptomycin (Sigma, St. Louis, MO, USA) in a humidified atmosphere with 5% CO2 at 37°C. For transforming growth factor (TGF)-beta1 (TGF-β1) treatment, PSCs were stimulated with TGF-β1 (10 ng/mL, Sigma) for 48 h.
Small interference RNA targeting SNHG11 (si-SNHG11) and corresponding negative control (si-NC), miR-34b mimic and inhibitor (miR-34b and anti-miR-34b), and their matching negative controls (miR-NC and anti-NC) were obtained from GenePharma (Shanghai, China). Cell transfection was performed using Lipofectamine 3000 reagent (Life Technologies, Carlsbad, CA, USA). For the pcDNA-LIF (LIF) (Accession: NM_002309) plasmid, the sequence of LIF was synthesized by GenePharma and then cloned into the pcDNA3.1 vector (Thermo Fisher Scientific) using the EcoRI and XhoI restriction sites.
Quantitative real-time polymerase chain reaction (qRT-PCR)The RNAiso Plus (Takara, Shiga, Japan) was applied to extract total RNA. A High-Capacity complementary DNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) or miRNA First-Strand Synthesis Kit (Takara) was utilized to carry out the reverse transcription. qPCR was carried out with the SYBR Premix Ex TaqTM II kit (Takara) in the CFX96 Real-time PCR Detection System (Bio-Rad, Hercules, CA, USA). The 2–ΔΔCt method was utilized to figure the levels of SNHG11, miR-34b, and LIF mRNA, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or U6 small nuclear RNA (snRNA) was used as an internal control. The primer sequences used in the research were displayed as below: SNHG11 (F: 5'-TGGGAGTTGTCATGTTGGGA-3', R: 5'-ACTCGTCACTCTTGGTCTGT-3'), miR-34b (F: 5'-CGCGTAGGCAGTGTCATTAGC-3', R: 5'-AGTGCAGGGTCCGAGGTATT-3'), LIF (F: 5'-AGATCAGGAGCCAACTGGCACA-3', R: 5'-GCCACATAGCTTGTCCAGGTTG-3'), GAPDH (F: 5'-GAAGGTGAAGGTCGGAGTC-3', R: 5'-GAAGATGGTGATGGGATTTC-3'), and U6 snRNA (F: 5'-GCTTCGGCAGCACATATACTAAAAT-3', R: 5'-CGCTTCACGAATTTGCGTGTCAT-3').
Cell counting kit-8 (CCK-8) assayTransfected PSCs (5 × 103 cells/well) were seeded in 96-well plates for 24 h, 48 h, or 72 h. Then, CCK-8 solution (Beyotime, Jiangsu, China) was added to each well and kept for 4 h. The microplate reader (Bio-Rad) was applied to analyze the absorbance at 450 nm.
Wound healing assayThe transfected PSCs (2 × 105 cells/mL) were seeded in 6-well plates. When grown to 90% confluence, the cell monolayers were scraped with a sterile micropipette tip. The wound gaps were imaged with a light microscope (Olympus, Tokyo, Japan) at 0 h and 48 h.
Transwell assayCell migration ability was assessed with the transwell chamber (Costar, Cambridge, MA, USA). In short, 200 μL cell medium containing PSCs (1 × 105 cells) was added to upper chambers. The bottom chamber was added with cell medium (600 μL) containing FBS (10%). After culture for 24 h, the migrating cells were fixed with paraformaldehyde (5%), followed by staining with crystal violet (0.5%, Sigma). The number of migrated cells was determined by an inverted microscope (Olympus) under 100× magnification.
Western blottingTotal protein was extracted with the RIPA lysis buffer (Beyotime). After quantification with the BCA Assay Kit (Pierce, Rockford, IL, USA), the extracted total protein was separated by using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10%, SDS-PAGE). Afterward, the separated proteins were transferred to the polyvinylidene difluoride (PVDF, Millipore, Billerica, MA, USA) membranes and then blocked with Tris Buffered Saline Tween (TBST) buffer with 5% skims milk. Next, the PVDF membranes were incubated with primary antibodies at 4°C overnight, including anti-COL1A1 (ab90395, 1:500, Abcam, Cambridge, MA, USA), alpha-smooth muscle actin (α-SMA) (ab32575, 1:1,000, Abcam), LIF (ab235908, 1:1,000, Abcam), and anti-GAPDH (ab8245, 1:500, Abcam). Next, the PVDF membranes were incubated with goat anti-mouse immunoglobulin G (IgG) (ab6708, 1:3,000, Abcam) or anti-rabbit IgG (ab97051, 1:2,000, Abcam). Finally, the immunoblot was visualized through enhanced chemiluminescence solution (Beyotime).
Nuclear-cytoplasmic fractionation assayThe PARIS kit (Life Technologies) was utilized for nuclear and cytoplasmic RNAs extraction. The levels of SNHG11 in the nuclear and cytoplasmic RNAs were analyzed via qRT-PCR, and U6 snRNA or 18S ribosomal RNA (rRNA) was used as a control for nucleus and cytoplasm, respectively. The primer sequences of 18S rRNA were F: 5'-GTGGTGTTGAGGAAAGCAGACA-3' and R: 5'-TGATCACACGTTCCACCTCATC-3'.
Dual-luciferase reporter assayThe targets of SNHG11 were predicted by miRcode and GSE25820 databases. The targets of miR-34b were predicted using starbase and GSE91035 databases. The sequences of wild type SNHG11 (SNHG11-wt), mutant SNHG11 (SNHG11-mut), LIP 3'UTR (Untranslated Region)-wt, and LIP 3'UTR-mut were synthesized and inserted into the psiCHECK-2 plasmids (Promega, Madison, WI, USA), respectively. PSCs were transfected a luciferase reporter and miR-34b mimic and miR-NC with Lipofectamine 3000. The luciferase activities were assessed with the dual-luciferase reporter assay system (Promega).
RNA pull-down assay with biotinylated SNHG11 probeThe biotinylated SNHG11 probe and Oligo probe were synthesized by Sangon Biotech (Shanghai, China). In brief, the lysates of PSCs were incubated with probe-coated beads (Sigma), which were obtaining by co-incubating biotinylated SNHG11 probe or Oligo probe with streptavidin magnetic beads (Thermo Fisher Scientific). The enrichment of miR-34b, miR-184, and miR-373 was analyzed by qRT-PCR.
RNA pull-down assay with biotinylated miR-34b probePSCs were transfected with biotinylated miR-34b probe (Bio-miR-346) or Oligo probe (Sangon Biotech). 48 h later, the lysates of PSCs were incubated M-280 streptavidin magnetic beads (Sigma), which were blocked with yeast tRNA (Sigma). The abundances of PALLD, LIF, VEGFA, GJC1, CALD1, MRVI1, CST1, RGS5 were analyzed by qRT-PCR.
RNA immunoprecipitation (RIP) assayThe specific binding between SNHG11 or LIP and miR-34b was verified by RIP assay with the EZ-Magna RIP Kit (Millipore). The lysate of PSCs was incubated with protein A/G sepharose beads conjugated with anti-IgG or anti-Argonaute2 (Ago2) antibodies (Sigma). The enrichment of SNHG11, LIP, or miR-34b was analyzed by qRT-PCR.
Statistical analysisAll experiments were repeated at least 3 times, and each experiment was performed in triplicate. Data were expressed as mean ± standard deviation. SPSS 20.0 software (IBM Corporation, Armonk, NY, USA) was utilized to carry out the statistical analysis. Independent Student’s t test was utilized to evaluate the differences between two groups. Analysis of variance with Tukey’s multiple comparisons test was employed to compare the difference among multiple groups. Differences were deemed significant if p < 0.05.
To verify the expression pattern of SNHG11 in CP, we detected SNHG11 expression in plasma of 30 CP patients and 30 healthy controls. qRT-PCR exhibited that SNHG11 was highly expressed in patients with CP relative to healthy controls (Fig. 1A). Moreover, SNHG11 expression was higher in TGF-β1-treated PSCs in contrast to the control group (Fig. 1B). To validate the function of SNHG11 in CP, we performed loss-of-function experiments. The knockdown efficiency of si-SNHG11 in PSCs was validated by qRT-PCR (Fig. 1C). As exhibited in Fig. 1D–1F, the silence of SNHG11 reduced TGF-β1-induced proliferation and migration in PSCs. Also, the upregulation of COL1A1 and α-SMA in PSCs caused by TGF-β1 stimulation was overturned after SNHG11 knockdown (Fig. 1G). Together, these results indicated that SNHG11 might be related to the fibrosis process of CP.
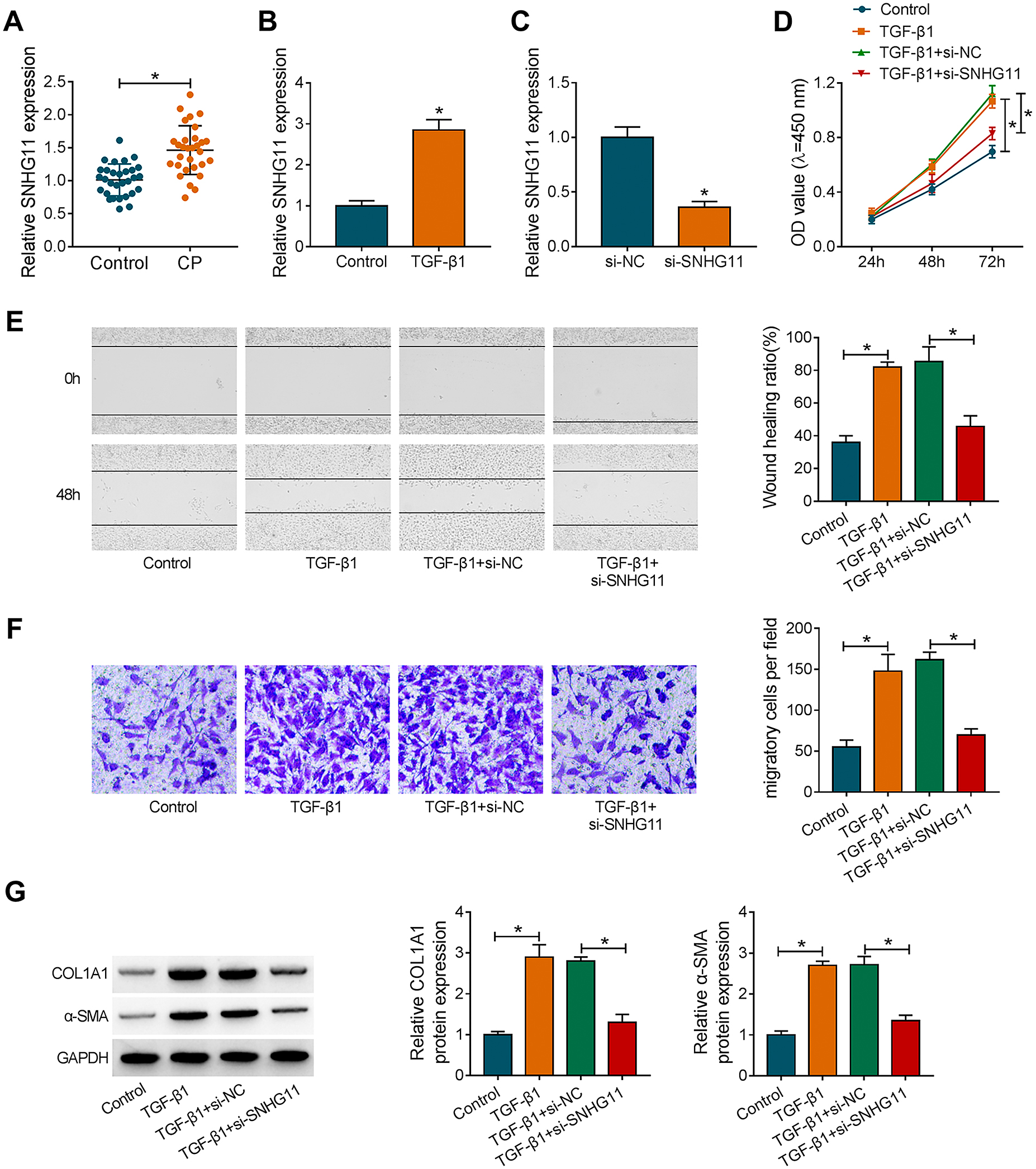
SNHG11 silencing reduced TGF-β1-induced proliferation, migration, and ECM accumulation in PSCs. (A) qRT-PCR analysis of SNHG11 expression in the plasma of CP patients and healthy controls. (B) qRT-PCR analysis of SNHG11 expression in TGF-β1-treated PSCs. (C) qRT-PCR validated the knockdown efficiency of si-SNHG11 in PSCs. (D–F) Influence of SNHG11 knockdown on proliferation and migration of TGF-β1-treated PSCs was analyzed by CCK-8, wound healing, or transwell assays. (G) Western blotting analysis of protein levels of COL1A1 and α-SMA in PSCs transfected with si-NC or si-SNHG11 under TGF-β1 treatment. * p < 0.05.
To explore the regulation mechanism of SNHG11 in CP, we first analyzed the distribution of SNHG11 in PSCs. nuclear-cytoplasmic fractionation experiments manifested that SNHG11 was mainly distributed in the cytoplasm of PSCs (Fig. 2A). Through analyzing miRcode and GSE25820 databases, we found that SNHG11 might be a decoy for 3 miRNAs (miR-34b, miR-184, and miR-373), which were among the top 100 miRNAs with the most significant downregulation in CP (Fig. 2B). Moreover, miR-34b and miR-184 were markedly pulled-down by SNHG11 probe compared to the Oligo probe, particularly miR-34b (Fig. 2C). There was an apparent downregulation of miR-34b in CP patients in the GSE25820 database (Fig. 2D). The putative binding sites of SNHG11 in miR-34b and a mutant sequence were exhibited in Fig. 2E. The overexpression efficiency of miR-34b mimic in PSCs was showed in Fig. 2F. Dual-luciferase reporter assay revealed that miR-34b overexpression could reduce the luciferase intensity of the SNHG11-wt reporter in PSCs, while there was no overt difference in the SNHG11-mut reporter (Fig. 2G). RIP assay exhibited that miR-34b and SNHG11 could be co-enriched in the anti-Ago2 group in comparison to the control IgG (Fig. 2H). Also, miR-34b expression was apparently decreased in TGF-β1-treated PSCs and the plasma of CP patients (Fig. 2I and 2J). Pearson’s correlation analysis presented that there was a negative correlation between miR-34b and SNHG11 expression in plasma of CP patients (Fig. 2K). Collectively, these data indicated that SNHG11 was a sponge of miR-34b in PSCs.

SNHG11 acted as a decoy for miR-34b in PSCs. (A) The distribution of SNHG11 in the cytoplasm or nuclear of PSCs was determined via the nuclear-cytoplasmic fractionation assay. (B) Schematic illustration showed the overlapping targets of SNHG11 in miRcode and GSE25820 databases. (C) qRT-PCR presented the abundances of miR-34b, miR-184, and miR-373 in Oligo probe and SNHG11 probe group. (D) Schematic illustration exhibited the expression trend of miR-34b in CP patients in the GSE25820 database. (E) Schematic illustration presented the binding bites between SNHG11 and miR-34b. (F) qRT-PCR verified the transfection efficiency of miR-34b mimic in PSCs. (G) Dual luciferase reporter assay analyzed the luciferase intensities of SNHG11-wt and SNHG11-mut reporters in PSCs transfected with miR-34b or miR-NC. (H) After RIP assay, the enrichment of miR-34b and SNHG11 was evaluated by qRT-PCR. (I and J) Expression trend of miR-34b in TGF-β1-treated PSCs and the plasma of CP patients was determined by qRT-PCR. (K) The correlation between miR-34b and SNHG11 in plasma of CP patients was analyzed by Pearson’s correlation analysis. * p < 0.05.
Subsequently, we explored whether SNHG11 regulated TGF-β1-induced proliferation, migration, and ECM accumulation in PSCs by adsorbing miR-34b. QRT-PCR confirmed the knockdown efficiency of anti-miR-34b in PSCs, as exhibited in Fig. 3A. We observed that the introduction of anti-miR-34b reversed the repressive effect of SNHG11 knockdown on proliferation and migration of TGF-β1-treated PSCs (Fig. 3B–3D). Also, miR-34b inhibitor overturned the downregulation of COL1A1 and α-SMA in SNHG11-silenced PSCs under TGF-β1 stimulation (Fig. 3E). These findings revealed that SNHG11 adsorbed miR-34b to regulate TGF-β1-induced proliferation, migration, and ECM accumulation in PSCs.

SNHG11 regulated proliferation, migration, and ECM accumulation by sponging miR-34b in TGF-β1-treated PSCs. (A) Transfection efficiency of anti-miR-34b in PSCs was assessed by qRT-PCR. (B–E) PSCs were transfected with si-NC, si-SNHG11, si-SNHG11 + anti-NC, or si-SNHG11 + anti-miR-34b. (B–D) The proliferation and migration of TGF-β1-treated PSCs were determined through CCK-8, wound healing, or transwell assays. (E) The protein levels of COL1A1 and α-SMA in TGF-β1-treated PSCs were measured by western blotting. * p < 0.05.
To survey the downstream target of miR-34b in CP, we predicted the targets of miR-34b by starbase and GSE91035 databases. As displayed in Fig. 4A, 8 genes (PALLD, LIF, VEGFA, GJC1, CALD1, MRVI1, CST1, RGS5), which among the top 100 genes with the most significant upregulation in CP, might be targets of miR-34b. Furthermore, only 2 genes (LIF and VEGFA) could be pulled down by the miR-34b probe in the 8 predicted genes (Fig. 4B). The upregulation of LIF in CP patients in the GSE91035 database was showed in Fig. 4C. The predicted binding sequence between miR-34b and LIF 3'UTR were presented in Fig. 4D. Moreover, the luciferase activity of the LIF 3'UTR-wt reporter was reduced in miR-34b-overexpressed PSCs, but the luciferase activity of the LIF 3'UTR-mut reporter did not change (Fig. 4E). Also, miR-34b and LIF were prominently enriched in the Ago2 antibody group (Fig. 4F). As expected, miR-34b mimic repressed the levels of LIF mRNA and protein in PSCs, but miR-34b inhibitor had a reverse influence (Fig. 4G and 4H). In addition, the mRNA and protein levels of LIF were upregulated in TGF-β1-treated PSCs and the plasma of CP patients (Fig. 4I–4L). The expression of LIF mRNA was negatively correlated with miR-34b in the plasma of CP patients (Fig. 4M). These results suggested that LIF severed as a target for miR-34b in PSCs.

LIF was a downstream target of miR-34b. (A) Schematic illustration presented the overlapping targets of miR-34b in starbase and GSE91035 databases. (B) qRT-PCR analysis of the enrichment of the predicted genes in Oligo probe and miR-34b probe groups. (C) Schematic illustration showed the expression of LIF in CP patients in the GSE91035 database. (D) Schematic illustration presented the predicted binding sequence between miR-34b and LIF 3'UTR. (E) The luciferase activities of LIF 3'UTR-wt and LIF 3'UTR-mut reporters in PSCs were determined by dual-luciferase reporter assay. (F) The combination between LIF and miR-34b was analyzed by RIP assay. (G and H) Influence of miR-34b overexpression and knockdown on the levels of LIF mRNA and protein was analyzed by qRT-PCR and western blotting. (I–L) Relative expression levels of LIF mRNA and protein in TGF-β1-treated PSCs and the plasma of CP patients were measured by qRT-PCR and western blotting. (M) The correlation between LIF mRNA and miR-34b in the plasma of CP patients was evaluated by Pearson’s correlation analysis. * p < 0.05.
Based on the above findings, we further explored whether LIF was involved in miR-34b mimic-mediated PSC proliferation, migration, and ECM accumulation under TGF-β1 stimulation. After LIF transfection, the levels of LIF mRNA and protein were upregulated in PSCs (Fig. 5A and 5B). As expected, the forcing expression of LIF reversed the inhibitory effect of miR-34b mimic on proliferation and migration of TGF-β1-treated PSCs (Fig. 5C–5E). Moreover, miR-34b mimic decreased the protein levels of COL1A1 and α-SMA in TGF-β1-treated PSCs, but this decrease was restored after LIF overexpression (Fig. 5F). Together, these results indicated that miR-34b regulated TGF-β1-induced proliferation, migration, and ECM accumulation by targeting LIF in PSCs.

MiR-34b regulated proliferation, migration, and ECM accumulation by targeting LIF in TGF-β1-treated PSCs. (A and B) After pcDNA or LIF transfection, the levels of LIF mRNA and protein in PSCs were determined by qRT-PCR and western blotting. (C–F) PSCs were transfected with miR-34b, miR-NC, miR-34b + pcDNA, or miR-34b + LIF. (C–E) The proliferation and migration of PSCs under TGF-β1 treatment were determined via CCK-8, wound healing, or transwell assays. (F) Western blotting analyzed the protein levels of COL1A1 and α-SMA in TGF-β1-induced PSCs. * p < 0.05.
Given that SNHG11 acted as a decoy for miR-34b, which targeted LIF in PSCs. We further investigated whether SNHG11 functioned as a ceRNA to regulate LIF expression by sponging miR-34b. The results exhibited that the introduction of anti-miR-34b overturned the downregulation of LIF in si-SNHG11-transfected PSCs (Fig. 6A and 6B). Also, there was a positive correlation between LIF mRNA and SNHG11 in the plasma of CP patients (Fig. 6C). These results manifested that SNHG11 could regulate LIF expression by sponging miR-34b.
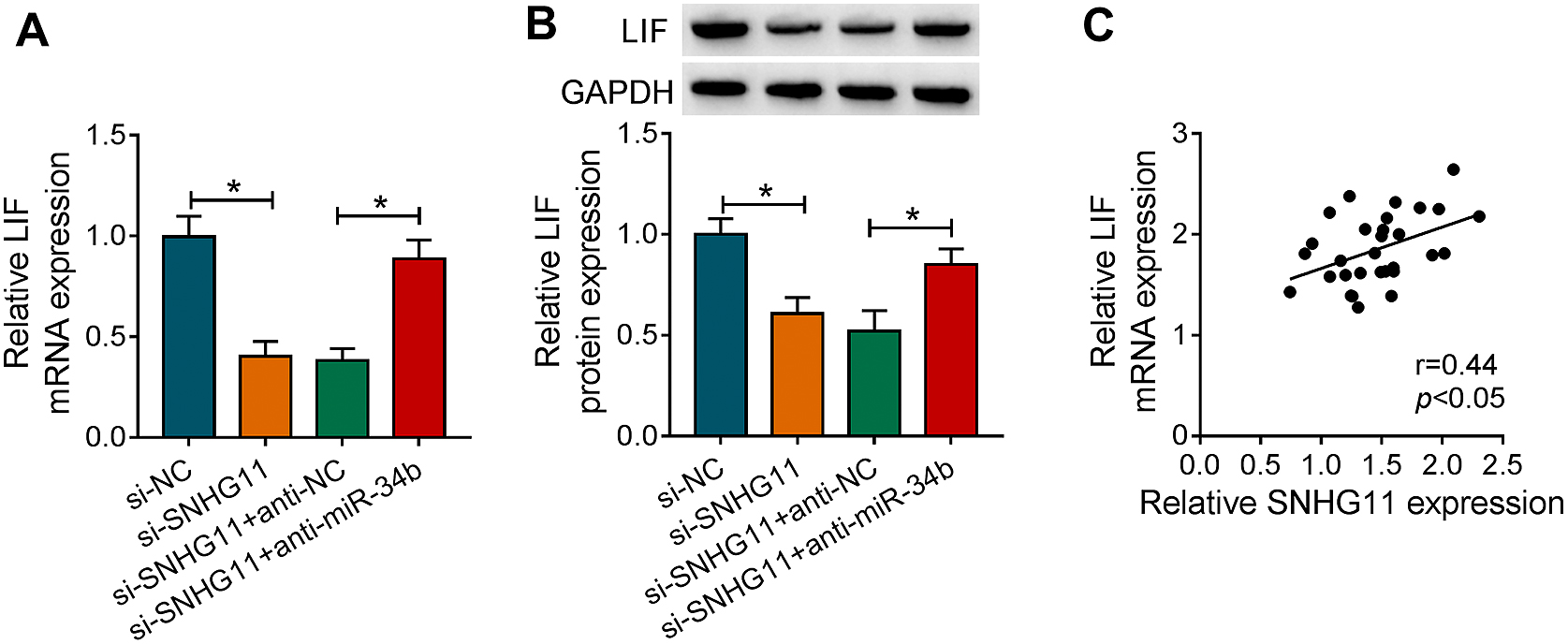
SNHG11 regulated LIF expression by adsorbing miR-34b in PSCs. (A and B) The mRNA and protein levels of LIF in PSCs transfected with si-NC, si-SNHG11, si-SNHG11 + anti-NC, or si-SNHG11 + anti-miR-34b were detected using qRT-PCR and western blotting. (C) Pearson’s correlation analysis revealed the correlation between SNHG11 and LIF mRNA in the plasma of CP patients. * p < 0.05.
TGF-β1 is a member of TGF-beta superfamily, which plays a role in several biological processes [19]. It has been uncovered that TGF-β1 is a key regulator of PSC activation and ECM production [20]. A previous study revealed that TGF-β1 overexpression induced CP in murine pancreas [21]. Also, inhibition of the activation of TGF-β1 precursor reduced the severity of pancreatic fibrosis [22]. α-SMA is commonly used as a marker of fibrotic activity of activated tissue fibrotic cells [23]. COL1A1, a component of ECM, is usually upregulated during the process of fibrosis [24, 25]. Herein, SNHG11 was upregulated in plasma of CP patients and TGF-β1-treated PSCs. Moreover, SNHG11 inhibition reduced proliferation, migration, and protein levels of α-SMA and COL1A1 in TGF-β1-induced PSCs. Report of Liu et al. also pointed out that SNHG11 expression was enhanced in CP patients, and SNHG11 might be a biomarker for CP diagnosis [26]. Therefore, we concluded that TGF-β1-induced proliferation, migration, and ECM accumulation in PSCs partly depended on the mechanism regulated by SNHG11 in CP.
Increasing evidence revealed that lncRNAs could act as ceRNAs to regulate gene expression by adsorbing miRNAs in various diseases [27]. In this study, SNHG11 was mainly distributed in the cytoplasm of PSCs, implying that SNHG11 might function as a ceRNA. Though bioinformatics analysis, dual-luciferase reporter, and RIP assays, we verified SNHG11 as a decoy for miR-34b in PSCs. MiR-34b had been reported to play a tumor-suppressive role in a series cancers [28-30]. Liu et al. revealed that miR-34b severed as a tumor metastasis suppressor in pancreatic cancer through targeting Smad3 [31]. Circular RNA circ-BFAR sponged miR-34b to increase MET expression, resulting in accelerating the progression of pancreatic ductal adenocarcinoma [32]. Also, Munding et al. revered that miR-34b was downregulated in CP patients (GSE25820) [33]. In this study, we verified that miR-34b was reduced in the plasma of CP patients and TGF-β1-treated PSCs. Furthermore, miR-34b inhibitor revered SNHG11 inhibition-mediated influence on TGF-β1-induced proliferation, migration, and ECM accumulation in PSCs. These results indicated that miR-34b might play a protective role during fibrosis progression in CP, and SNHG11 modulated TGF-β1-induced proliferation, migration, and ECM accumulation in PSCs by sponging miR-34b.
In addition, we found that LIF acted as a target for miR-34b. LIF, a member of the interleukin-6 cytokine superfamily, has a wide range of biological activities that can affect bone metabolism, cachexia, neurodevelopment, embryogenesis, and inflammation [34]. Also, LIF derived from activated PSCs was related to the pathogenesis of pancreatic ductal adenocarcinoma [35] and LIF might be a potential therapeutic target for pancreatic ductal adenocarcinoma [36, 37]. Herein, LIF overexpression restored the suppressive impact of miR-34b mimic on TGF-β1-induced proliferation, migration, and ECM accumulation in PSCs, indicating that miR-34b targeted LIF to modulate TGF-β1-induced proliferation, migration, and ECM accumulation in PSCs. Notably, SNHG11 acted a ceRNA and regulated LIF expressed by sponging miR-34b in PSCs. Therefore, we concluded that SNHG11 regulated TGF-β1-induced proliferation, migration, and ECM accumulation in PSCs by the miR-34b/LIF pathway.
In conclusion, TGF-β1-induced SNHG11 sponged miR-34b to elevate LIF expression, thereby facilitating proliferation, migration, and ECM accumulation of PSCs. The research offered a new underlying mechanism by which SNHG11 regulated the fibrosis process of CP.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
None declared.
Thanks for all participants involved in this study.