2021 Volume 68 Issue 11 Pages 1359-1365
2021 Volume 68 Issue 11 Pages 1359-1365
Evidence suggests that exercise can regulate skin functions such as promoting wound healing and inhibiting aging. Physical exercise modulates the secretion of proteins and peptides from skeletal muscles, called myokines, which play a role in transmitting exercise signals throughout the body. Therefore, exercise-regulated myokines may play a role in controlling skin functions; however, the precise mechanisms remain elusive. In this study, we focused on the recently identified CXC motif chemokine ligand 10 (CXCL10), an exercise-reduced myokine, and attempted to elucidate its role in regulating collagen synthesis in dermal fibroblasts. Mouse C2C12 myotubes were stimulated with or without electrical pulse stimulation (EPS) to induce contraction for 24 h, and conditioned medium was collected (EPS-CM or Ctrl-CM, respectively). The reduction in CXCL10 concentration by EPS was confirmed using ELISA. Next, mouse dermal fibroblasts were isolated from the dorsal skin of C57BL6/J mice (2 weeks old) and were stimulated with Ctrl-CM or EPS-CM for 24 h. EPS-CM treatment significantly increased collagen production compared to Ctrl-CM treatment. Even in the Ctrl-CM condition, the addition of an antagonist for CXCR3 (CXCL10 receptor) increased collagen production. In contrast, recombinant CXCL10 abolished EPS-CM-dependent collagen induction. Overall, this study raises the possibility that CXCL10 secretion from skeletal muscles may control collagen production in mouse dermal fibroblasts.
PHYSICAL EXERCISE modulates skeletal muscle-derived peptides and proteins, termed myokines [1, 2]. Some of these myokines are believed to send exercise signals to other tissues/organs, thereby regulating exercise-dependent adaptive processes in the whole body [3-5]. For example, the secretion and expression of interleukin-6 (IL-6), one of the earliest identified myokines, from skeletal muscles is increased by exercise [6], followed by regulation of glucose incorporation into skeletal muscles [7] and enhancing insulin action to synthesize glycogen [8]. Although various exercise-dependent myokines have been newly identified [9-11], their physiological functions remain elusive. Therefore, one of the major goals of myokine research is to estimate and identify their precise roles in exercise-regulated physiological changes.
We have identified several proteins as novel exercise-regulated myokines using an in vitro contractile model composed of C2C12 myotubes and electrical pulse stimulation (EPS) [12-15]. C-X-C motif chemokine ligand 10 (CXCL10), also known as interferon gamma-induced protein 10 (IP-10), is a newly identified myokine whose expression is reduced due to muscle contraction [14]. CXCL10 is secreted from monocytes and endothelial cells in response to interferon-gamma (IFNγ) or tumor necrosis factor-alpha (TNFα) [16]. CXCL10 exerts chemotaxic activity on monocytes and T lymphocytes [17] and contributes to the activation of T and B lymphocytes, natural killer cells, dendritic cells, and macrophages [18, 19]. Moreover, CXCL10 possesses potent angiostatic activity by promoting apoptosis of endothelial cells [20], which may contribute to its anti-tumor activity [21, 22]. It can be speculated that as CXCL10 is multifunctional, the reduction of CXCL10 from skeletal muscle through exercise may have a wide range of effects on the whole body.
Recent studies have strongly suggested that physical exercise regulates skin function. One example is the relationship between exercise and cutaneous wound healing. It has been shown that forced treadmill running in rats or endurance training in mice significantly alters cutaneous wound healing processes [23, 24]. A similar line of evidence was provided by studies using obesity models displaying delayed wound healing, skin thinning, and reduction of collagen synthesis [25], all of which were attenuated by physical exercise [25]. Moreover, exercise may lower cancer risk by inhibiting insulin-like growth factor-1 (IGF-1) signals [26, 27]. In a skin cancer model, exercise appears to activate p53, followed by induction of p21, insulin-like growth factor-binding protein 3 (IGFBP3), and phosphatase and tensin homolog (PTEN), which results in a reduction of IGF-1 signals [27]. More recently, it has been reported that interleukin-15 (IL-15), which is secreted from skeletal muscles, is upregulated by exercise and may improve aging-related collagen reduction and decreased mitochondrial activity in mouse dermis [23, 28]. However, the involvement of myokines other than IL-15 in regulating exercise-dependent cutaneous function have not yet been clarified.
In this study, we investigated the effect of exercise-dependent myokines on cutaneous functions, with particular focus on the exercise-reduced myokine CXCL10.
All experiments using animals were approved by the Animal Care Committee at Toyo University. Male C57BL6/J mice (8 weeks old) (Charles-River Japan, Inc., Kanagawa, Japan) were individually housed in a temperature-controlled room. Mice were fed chow (Nosan Corporation, Kanagawa, Japan) and provided with water ad libitum, and were acclimatized to a 12 h light cycle. The mice were subjected to forced treadmill running. (15 cm/s for 30 min). After treadmill running, the mice were anesthetized and sacrificed immediately. An area of approximately 6.0 cm2 (3.0 × 2.0 cm) was shaved on the back of each mouse with electric clippers, and approximately 2.0 cm2 (2.0 × 1.0 cm) of whole dorsal skin was collected.
Cell cultureMouse skeletal muscle cell line C2C12 cells were maintained in growth medium (Dulbecco’s modified Eagle medium (DMEM) low glucose (LG; 1.0 g/L) (Nacalai Tesque, Kyoto, Japan) + 10% fetal bovine serum (FBS) (Biowest, Nuaillé, France) + 1% penicillin-streptomycin mixed solution (Nacalai Tesque)) in a 75 cm2 flask (Corning, NY, USA). Cells were cultured at 37°C under a 5% CO2 atmosphere. The medium was changed every 48 h. For all experiments, C2C12 myoblasts were seeded into 8 well- plates (Thermo Fisher Scientific, Waltham, MA, USA) with 2 mL of growth medium. When C2C12 cells reached 100% confluency, differentiation was induced by changing to differentiation medium (DMEM high glucose (HG; 4.5 g/L) (Nacalai Tesque) + 2% calf serum (Biowest) + 1% penicillin-streptomycin mixed solution). The medium was replaced every 24 h. Induction of differentiation was performed for approximately 10 days until myotubes were formed.
Mouse dermal fibroblasts were isolated from the dorsal skin of the mice. Mice (2 weeks old) were euthanized by cervical dislocation. The dorsal skin was cut to 10 mm2 and stuck on a 6 well-plate (Corning). After drying, 2 mL of media (Minimum Essential Medium Eagle (Thermo Fisher Scientific) + 10% FBS (Biowest) + 1% penicillin-streptomycin (Nacalai Tesque) + 1% MEM Non-Essential Amino Acids Solution (100X) (Nacalai Tesque) + 1% sodium pyruvate solution (100X) (Nacalai Tesque)) were added. Approximately 10 days later, fibroblasts that had migrated to the peripheral parts of mouse dorsal skin slices were collected using TrypLETM Express (1X) (Thermo Fisher Scientific) and seeded in 75 cm2 flasks (Corning) with growth medium.
Electrical pulse stimulation (EPS)Differentiated C2C12 myotubes were cultured in an 8 well-plate and placed in a chamber for electrical pulse stimulation (C-Dish; IonOptix, Milton, MA, USA). EPS (20 V/30 mm, 1 Hz, 2 ms) was applied to the myotubes using a C-Pace Pulse generator (C-Pace 100; IonOptix). After incubation with EPS for 24 h, the culture supernatants were collected.
Real-time qPCRRNA from cells or tissues was isolated using TRIzol® reagent (Thermo Fisher Scientific) according to the manufacturer’s protocol, and the concentration was quantified using NanodropTM 2000/2000c (Thermo Fisher Scientific). Reverse transcription was performed using ReverTraAce® RNA (Macherey-Nagel, Düren, Germany) according to the manufacturer’s protocol. Quantitative PCR was performed using the KAPA SYBR FAST qPCR Kit Master Mix (2X) ABI PrismTM (Kapa Biosystems, Boston, MA, USA) with the following primer sets: mouse Gapdh, 5'-TGT GTC CGT CGT GGA TCT GA-3' and 5'-CGT GCT TCA CCA CCT TCT TGA-3', and mouse Col1a1, 5'-GCC AAG AAG ACA TCC CTG AAG-3' and 5'-TGT GGC AGA TAC AGA TCA AGC-3'.
Measurement of collagen secretionMouse dermal fibroblasts were treated with the conditioned medium obtained from C2C12, stimulated with or without electrical pulse, in the presence or absence of CXCR3 antagonists (NBI74330, TOCRIS Bioscience, Bristol, United Kingdom). Collagen secretion from dermal fibroblasts was analyzed using the Sirius Red Collagen Detection Kit (Chondrex, Inc., Redmond, WA, USA). In brief, the culture supernatant of dermal fibroblasts was concentrated, and Sirius Red solution was added to the concentrated samples and collagen standards (500, 250, 125, 63, 31.5, 16, and 8 μg/mL); it was then incubated at room temperature for 30 min. The absorbance of each reaction was measured using xMarkTM microplate spectrophotometer (Bio-Rad, Hercules, CA, USA).
Statistical analysisAll experiments were repeated at least three times. Statistical analyses were performed using GraphPad Prism (GraphPad Software, CA, USA). Student’s t-test or one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test was used. Differences were considered significant at * p < 0.05.
The metabolism of collagen is accelerated by physical training in young mice [29]. The effect of physical exercise is not limited to regulating collagen synthesis, as it also regulates collagen degradation by controlling the expression of matrix metalloproteinases and their inhibitors [30]. More recently, Safder et al. demonstrated that endurance exercise suppressed thinning of the dermis in polymerase-gamma mutator mice [31]. Overall, reports strongly suggest that physical exercise has the potential to control the collagen content in connective tissues.
To confirm whether a single bout of exercise in mice regulates collagen expression in the skin, we used a forced treadmill running model, as previously described [14, 32]. Forced exercise significantly increased Col1a1 gene expression in the dorsal skin by approximately 3.5-fold compared to the sedentary control (* p < 0.05, n = 5–6, t-test) (Fig. 1). These results suggested that a single bout of forced exercise in mice was sufficient to induce Col1a1 expression in the skin.
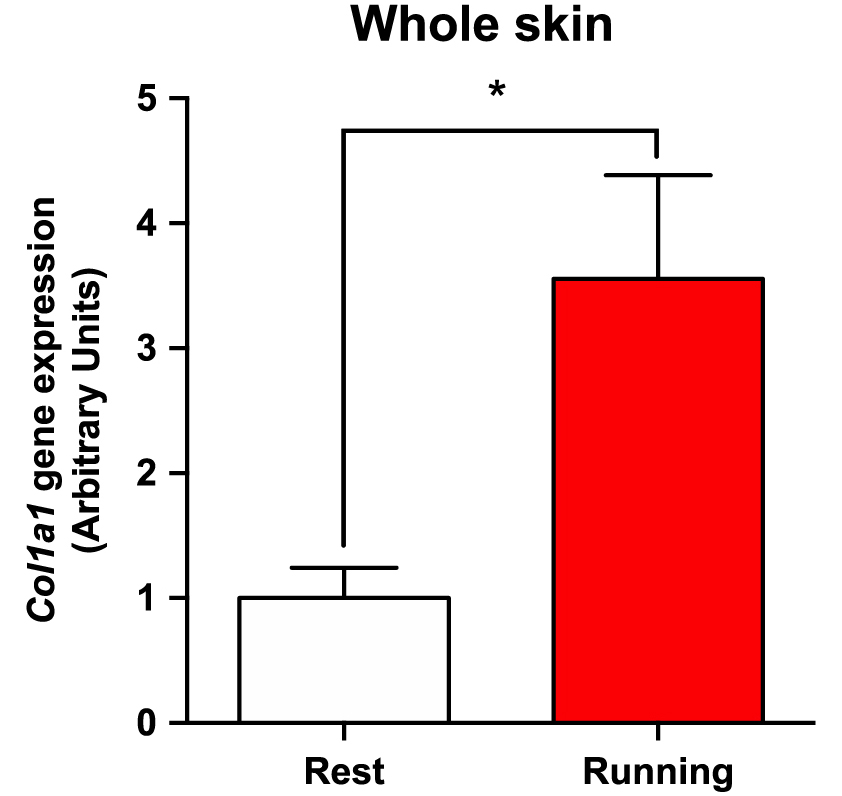
A single bout of exercise increases Col1a1 expression in mouse dorsal skin. Male C57BL/6 mice were divided into a sedentary group and an exercise group. Exercise group underwent forced treadmill running for 30 min. Gene expression of Col1a1 in the dorsal skin was analyzed by qPCR. The gene expression levels were normalized by Gapdh gene expression. The graphs represent mean ± SEM (* p < 0.05, n = 5–6).
Next, we investigated whether contraction-dependent alteration of myokine profiles in C2C12 cells affects collagen expression and secretion in dermal fibroblasts. Mouse dermal fibroblasts isolated from 2-week-old mice were treated with culture supernatants from C2C12 myotubes, stimulated with or without EPS for 24 h (EPS-CM and Ctrl-CM, respectively; see Fig. 2A). Collagen secretion was significantly induced by EPS-CM compared with Ctrl-CM (mean ± SEM: Ctrl-CM, 9.8 ± 1.3 μg/mL; EPS-CM, 15.2 ± 1.6 μg/mL) (Fig. 2B, * p < 0.05, n = 5, t-test). In addition, EPS-CM enhanced Col1a1 gene expression by approximately 1.5-fold compared to Ctrl-CM (Fig. 2C, * p < 0.05, n = 5, t-test). These results suggest that collagen production in fibroblasts is regulated by EPS-regulated secreted factor(s), possibly myokine(s).

Effects of EPS-dependent changes of myokine secretion on mouse dermal fibroblasts. (A–C) Mouse C2C12 myotubes were incubated with or without electrical pulse stimulation (EPS) for 24 h, followed by collecting conditioned medium (EPS-CM or Ctrl-CM, respectively). Dermal fibroblasts were isolated and treated with either EPS-CM or Ctrl-CM for 24 hours (See (A) for more details). (B) Collagen production from fibroblasts was analyzed by Sirius red collagen detection kit. The graphs represent mean ± SEM (*: p < 0.05, n = 5). (C) Col1a1 gene expression was analyzed by qPCR. The graphs represent mean ± SEM (* p < 0.05, n = 5).
Several myokines are induced or reduced by EPS in C2C12 cells [12-15]. Of these, CXCL10 is implicated in the regulation of several skin functions. For example, cyclic GMP-AMP (cGAMP) promotes skin wound healing, which is mediated by CXCL10 secretion [33]. In addition, the CXCL10 receptor CXCR3 is expressed in both epidermal cells and dermal fibroblasts to regulate cell migration [34]. Moreover, high glucose or transforming growth factor-β induced collagen production in NIH/3T3 cells, which was significantly attenuated by administration of recombinant CXCL10 [35]. These results allowed us to hypothesize that the reduction of CXCL10 in EPS-CM contributes to enhancing collagen production in fibroblasts.
The reduction in CXCL10 concentration in EPS-CM was confirmed by ELISA (Fig. 3A, * p < 0.05, n = 3, t-test), and the expression of myokine-related receptors was quantified by qPCR. After demonstrating that EPS induced CXCL1 and CXCL5, whereas reduced CXCL10 and CCL5 secretion from C2C12 cells [13-15], we analyzed the corresponding receptors (CXCR2 for CXCL1 and CXCL5; CCR1, 3, and 5 for CCL5; CXCR3 for CXCL10). CCR1, CXCR2, and CXCR3 were observed in the mouse fibroblasts (Fig. 3B).

EPS-CM dependent attenuation of CXCL10-CXCR3 signaling contributes to increased collagen production in mouse dermal fibroblasts. (A) Mouse C2C12 myotubes were incubated with or without electrical pulse stimulation (EPS) for 24 h, followed by collecting conditioned medium (EPS-CM or Ctrl-CM, respectively). CXCL10 levels were analyzed by ELISA (* p < 0.05, n = 3). (B) The expression of each chemokine receptor was analyzed by PCR. (C) Mouse dermal fibroblasts were treated with Ctrl-CM for 24 h in the presence of different concentration of NBI74330 (0, 10, 20, and 30 ng/mL). Collagen production was analyzed as described (* p < 0.05, n = 3). (B) Mouse dermal fibroblasts were treated with Ctrl-CM or EPS-CM in the presence of recombinant CXCL10 (rCXCL10; 25 pg/mL). Again, collagen production was analyzed as described (* p < 0.05, n = 5–9). All graph represents mean ± SEM.
To elucidate the potential involvement of the CXCR3 and CXCL10 receptors on collagen production in dermal fibroblasts, we tested the effect of a CXCR3 antagonist NBI74330. Mouse dermal fibroblasts were treated with Ctrl-CM, which contained substantial amounts of CXCL10, in the presence of 0–30 ng/mL of (±)-NBI74330. After 24 h of incubation, the collagen concentration in the culture supernatants was measured. As shown in Fig. 3C, 30 ng/mL of NBI74330 treatment induced collagen concentration (Ctrl, 10.1 ± 1.3 μg/mL; 30 ng/mL NBI74330, 17.6 ± 2.8 μg/mL) (Fig. 3C, * p < 0.05, n = 3, ANOVA), indicating that the attenuation of CXCR3 signaling induced collagen secretion in dermal fibroblasts.
We also examined whether administration of recombinant CXCL10 (rCXCL10) abolished EPS-CM-dependent collagen production. Dermal fibroblasts were treated with Ctrl-CM, EPS-CM, or EPS-CM supplemented with rCXCL10 (25 pg/mL), and collagen concentration was measured. As shown in Fig. 3D, the addition of recombinant CXCL10 (rCXCL10) partially abolished the EPS-CM dependent collagen production, whereas did not affect Ctrl-CM dependent collagen production (Fig. 3D, Ctrl-CM, 9.6 ± 0.6 μg/mL; Ctrl-CM with rCXCL10, 10.1 ± 1.1 μg/mL; EPS-CM, 16.4 ± 1.5 μg/mL; EPS-CM with rCXCL10, 13.1 ± 1.8 μg/mL) (Fig. 3D, * p < 0.05, n = 5–9, ANOVA). These results suggest that EPS-dependent CXCL10 reduction in EPS-CM attenuates CXCR3 signaling in dermal fibroblasts and may, in turn, contribute to EPS-CM-induced collagen production in dermal fibroblasts.
To our knowledge, IL-15 is the only reported exercise-regulated myokine that regulates collagen synthesis in the skin [28]. Although further studies are required, we propose that exercise-dependent alteration of CXCL10 secretion from skeletal muscles may also control collagen production in the dermis. We also noted other exercise-regulated myokines, which may be involved in regulating skin functions. Decorin, a small leucine-rich proteoglycan, was identified as an exercise-induced myokine [36] and has the capacity to bind collagen, which is believed to regulate collagen fibrillogenesis and the final diameter of fibrils [37]. Another myokine, CXCL12, also known as stromal cell-derived factor 1 (SDF-1), is also upregulated by physical exercise [38] and accelerates skin wound healing [39].
In addition to physical exercise, obesity has a considerable role in dermal connective tissues. For example, obesity-associated enlarged adipocytes secrete more free fatty acids (FFAs) [40], and the elevation of FFAs reduces Col1a1 gene expression in NIH/3T3 cells [41]. Lőrincz recently reported that voluntary exercise in mice improved obesity-dependent thinning of the dermis associated with the reduction of collagen density [25]. Moreover, we recently reported that CXCL10 secretion from skeletal muscles was induced when mice were given a high-fat diet for 10 weeks [42]. Therefore, the exercise-dependent reduction of the myokine CXCL10 may have a role in attenuating the obesity-dependent inhibition of collagen synthesis.
In conclusion, our present study raised the possibility that CXCL10 secretion from skeletal muscles controls collagen production in mouse dermal fibroblasts. Although it remains possible that the exercise directly stimulates the skin and increases the expression of collagen, the exercise-dependent CXCL10 reduction in skeletal muscle may, in part, contribute to exercise-induced regulation of skin function.
We thank Dr. Jérôme Lamartine (Université Claude Bernard Lyon 1, France), Dr. Keitaro Yamanouchi, and Dr. Takashi Matsuwaki (University of Tokyo, Japan) for their helpful comments. We also thank Ms. Ayano Yoshikawa for providing excellent technical assistance. This work was partially supported by a Grant-in-Aid for Scientific Research (C) #19K06442 to T.N. and Grant-in-Aid for JSPS Fellows #19J10894 to Y.I-S. from the Japan Society for the Promotion of Science (JSPS).
The authors have no conflicts of interest to declare.
Y.I-S. and T.N. designed the study. Y.I-S. performed the experiments and analyzed the data. Y.I-S. and T.N. wrote the manuscript. Both authors read and approved the final manuscript.