2021 Volume 68 Issue 4 Pages 387-398
2021 Volume 68 Issue 4 Pages 387-398
Intermittent fasting, which can effectively reduce obesity and improve the related metabolic syndrome has become an exciting research area in recent years. Adipose tissue is pivotal in regulating the metabolism and determining the occurrence of obesity. In the current study, we aimed to investigate the effects of acute fasting (AF) on fat tissue. Mice were subjected to AF for 36 h, receiving normal chow (low-fat diet [LFD]) or a high-fat diet (HFD), with free ad libitum access to drinking water, and those fed on free-diet counterparts without fasting serveding as controls. We found that AF obviously reshaped the morphology of fat tissue (WAT) and promoted the beiging of white adipose tissue in both LFD- and HFD-fed mice. AF principally improved the lipid metabolism, and increased the M2- polarization of macrophages in WAT white fat tissue of HFD-fed mice. Interestingly, we found that AF dramatically upregulated Sirt5 expression levels and fat tissue succinylation, suggesting that AF-induced beneficial effects on fat might occur via the regulation of Sirt5 levels and altered succinylation in fatty tissues. Our study clearly showed the remodeling function of adipose tissue during AF; in terms of mechanism, the regulation of succinylation levels by AF might provide new insights into the mechanism(s) underlying the improvement in fat metabolism by energy restriction.
ADIPOSE TISSUE is a central metabolic organ that regulates the energy balance. White and brown adipose tissue (WAT and BAT, respectively) are two major types of adipose tissue in humans. WAT plays a major role in energy (fat) storage when excess nutrients are present. It is also an important endocrine organ and can regulate energy expenditure, glucose homeostasis, appetite control, inflammation, insulin sensitivity, and tissue repair by releasing a variety of endocrine factors [1]. Beige adipocytes are induced thermogenic adipocytes that appear in WAT in response to various environmental stimuli [2]. BAT/beige fat is highly metabolically active, and it accumulates lipids for cold-induced adaptive heat production. BAT/beige fat thermogenesis plays an important role in body temperature balance, energy balance, glucose and lipid metabolism, and body-weight control. It has been reported that the BAT activation and associated heat production help in combating obesity and subsequent metabolic abnormalities [3]. New evidence suggests that WAT can be converted into energy-consuming tissue when white fat cells acquire beige/brown adipocyte characteristics; thus, it is of paramount importance to study the browning of white fat [4].
In recent years, improving obesity or metabolic disorders through caloric restriction or fasting has become an exciting area of study. Investigators have recently reported that intermittent fasting (IF) (36 hours of fasting and 10 hours of feeding), selectively induces beiging of inguinal WAT and ameliorates metabolic syndrome in obese mice by shaping the gut microbiota [5]. IF also increases the vascular endothelial growth factor (VEGF) level in WAT and induces angiogenesis, macrophage polarization in adipose tissue, and WAT browning/beiging, resulting in improved insulin sensitivity and reduced metabolic syndrome in mice [6]. Alternate-day fasting reduces fat mass (particularly trunk fat) and improves the markers of general health in healthy, middle-aged humans [7]. However, the beneficial effects of one-time acute fasting (AF) on adipose tissue have not been studied in detail, and the molecular mechanism underlying fasting-mediated adipose tissue remodeling remains elusive.
Sirtuin 5 (Sirt5), a desuccinylase that primarily exists in the mitochondrial matrix, plays important roles in glycolysis, the tricarboxylic acid cycle, fatty acid (FA) oxidation, electron transport chain, ketone body formation, nitrogenous waste management, and reactive oxygen species detoxification [8]. Sirt5-regulated uncoupling protein 1 (UCP1) is a key heat-producing protein in BAT, and the reduced function of UCP1 and other proteins in Sirt5-knockout (KO) BAT resulted in impaired mitochondrial respiration, defective mitophagy, and metabolic inflexibility [9]. Sirt5-knockout mice on a Sv129 background exhibited lesser subcutaneous WAT (sWAT) browning capacity than the controls, showing apparent cold intolerance [10]. Currently, whether a change in the fat tissue succinylation level is involved in fasting-induced metabolic improvement remains unclear, and the effects of fasting on fat tissue succinylation have not been reported.
In the present study, we demonstrated that AF reshaped fat tissue morphology and improved WAT beiging in mice on a low-fat diet (LFD, normal chow) (LFD) or high-fat diet (HFD). AF improved the lipid metabolism and increased macrophage M2-polarization in WAT of HFD-fed mice. Additionally, we found that AF upregulated Sirt5 levels in fat tissue, suggesting that fasting regulated the energy metabolism of fat tissue by regulating its succinylation level. Thus, our work will provide a novel understanding and conceptualization of the function and metabolic regulation of fat tissue by fasting.
To explore the effects of AF on body weight and fat tissue, we fed 8-week-old male C57BL/6 mice either normal chow (LFD) or HFD for 16 weeks. Mice were then fed or deprived of food. To subject the mice to AF, we placed the mice in cages with water, but no food, for 36 h. At the end of the 36-h food deprivation period, body weights of both the LFD- (Fig. 1A, 1B) and HFD-fed mice (Fig. 1E, 1F) were significantly reduced. By analyzing the body composition of the LFD- (Fig. 1C) and HFD-fed (Fig. 1G) mice, we found that the fat mass of both groups decreased, while the lean mass remained unchanged. AF mainly resulted in white fat (sWAT and visceral WAT [eWAT]) utilization in both LFD- (Fig. 1D) and HFD-fed (Fig. 1H). These observations suggested that AF reduced WAT storage and possibly improved adipose tissue morphology and metabolic performance.

Acute fasting (AF)-induced changes in body weight and body composition. After 36 h of fasting, low-fat diet (LFD)- mice and high-fat diet (HFD)-fed mice, compared to the controls, showed decreased body weights (A, B, E, F). Body composition analysis revealed reduced fat mass without changes in lean mass in both LFD and HFD mice (C, G). AF mainly consumed white fat in both LFD- and HFD-fed mice (Fig. 1 D, H). The asterisks represent the level of statistical significance calculated using a two-tailed Student’s t-test (* p < 0.05, ** p < 0.01,*** p < 0.001); data are presented as the mean ± standard error of mean. BAT, brown adipose tissue; sWAT, subcutaneous white adipose tissue; eWAT, visceral white adipose tissue.
Considering the correlation of fat cell size with cellular function and metabolic disease, we performed hematoxylin and eosin (H&E) staining of BAT and WAT to investigate the AF-induced morphological alterations in fat tissue induced by AF. After 36 h of fasting, the sWAT and eWAT in both LFD- and HFD-fed mice showed a large increase in the number of brown adipocyte-like cells as evidenced by multilocular lipid droplets (Fig. 2A, 2C). Consistent with H&E staining, the reduced relative area of sWAT and eWAT in both LFD- and HFD-fed mice revealed reduced white adipocyte size after AF (Fig. 2B, 2D). It has been suggested that large fat cells are considered to be poorly metabolized and associated with pathophysiological conditions, and a reduction in fat cell size is strongly associated with metabolic improvements [11, 12]. Collectively, these data indicated the potential health benefits of AF on fat metabolism and inflammation.

Acute fasting (AF)-induced morphological alterations in fat tissue. Hematoxylin & eosin (H&E) staining of subcutaneous and visceral white adipose tissue (sWAT and eWAT, respectively) and brown adipose tissue of low-fat diet (LFD)- and high-fat diet (HFD)-fasting and normal-diet mice (A, C). Magnification 20×. AF induced the reduction in the relative area of sWAT and eWAT in both LFD- and HFD-fed mice compared to the controls, respectively (Fig. 2B, 2D). The asterisks represent the level of statistical significance calculated using a two-tailed Student’s t-test (* p < 0.05, ** p < 0.01); data are presented as the mean ± standard error of mean.
Beiging of white fat is an important potential therapeutic measure for obesity and the related metabolic syndrome. To investigate the AF-induced beiging of WAT, we examined the mRNA levels of UCP1, a golden marker of BAT activation [13]. We found that UCP1 mRNA levels in sWAT and eWAT were increased in both LFD- and HFD-fed mice after AF (Fig. 3A, 3B), indicating that AF can strongly induce the beiging genesis of white fat tissue. Furthermore, we focused on AF-induced thermogenic gene expressions of peroxisome proliferator-activated receptor gamma coactivator 1 (PGC1)-α, PGC1-β, carnitine palmitoyltransferase 1 (CPT1)-α, CPT1-β, and PR domain containing 16 (PRDM16) induced by AF. It has been reported that PGC-1α/β exerts strong effects on FA oxidation and mitochondrial biogenesis [14, 15]. As the key rate-limiting enzyme in FA oxidation, CPT1 regulates fatty acid oxidation and facilitates adaptation to the environment [16]. The zinc- finger protein PRDM16 promotes browning of sWAT by activating the brown lipoid gene program [17]. Quantitative polymerase chain reaction (qPCR) analysis showed that AF induced the expression of FA oxidation and thermogenesis-related genes, including PGC1α, PGC1β, CPT1α, CPT1β, and PRDM16, in sWAT and eWAT of HFD-fed mice (Fig. 3C, 3D). Together, these results suggested that AF promoted the beiging of WAT, possibly contributing to the energy metabolism of fat tissue.
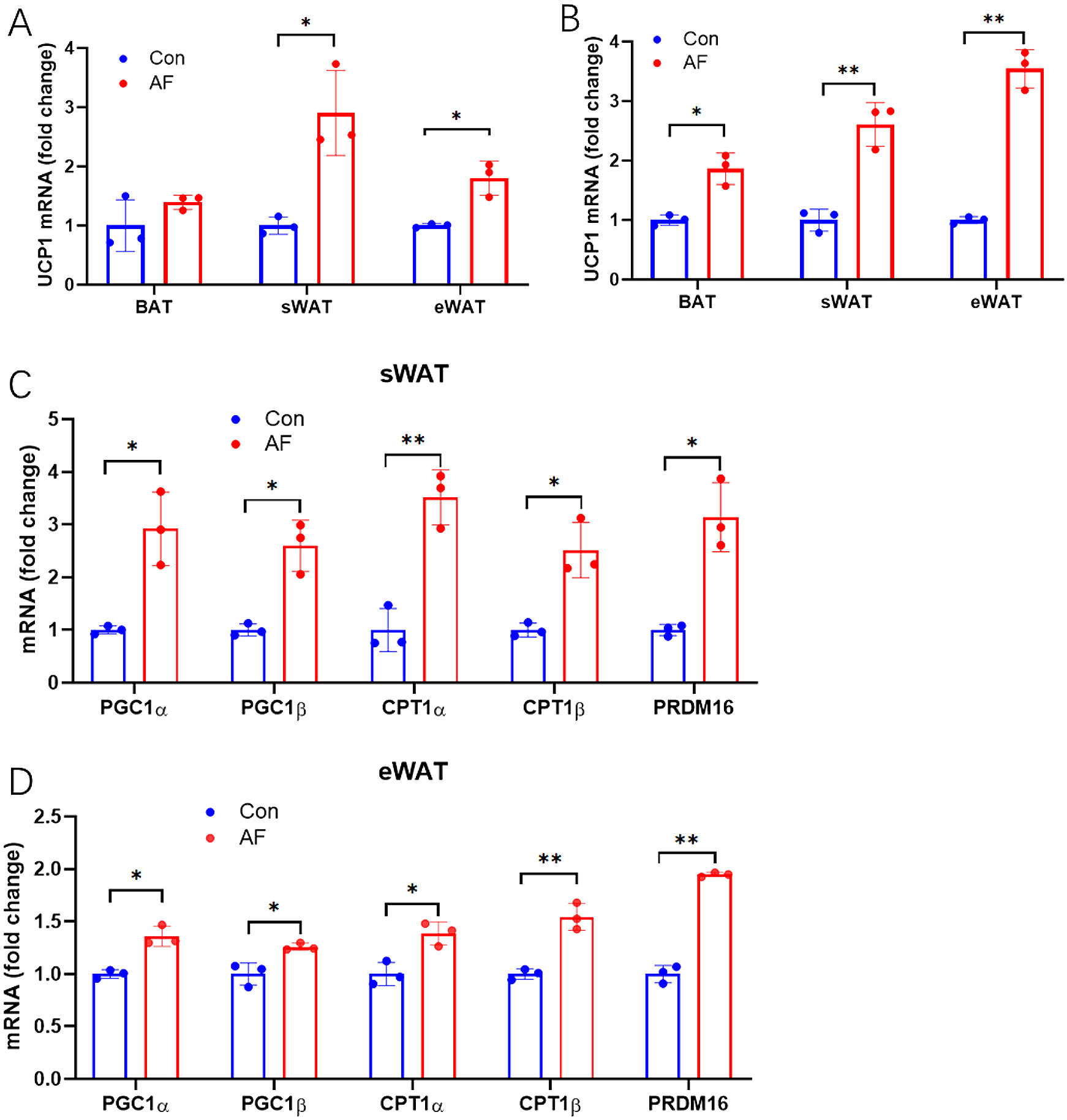
Acute fasting (AF) induced the browning of white adipose tissue (WAT). (A) RT-qPCR revealed that AF increased the expression of the brown/beige adipocyte marker UCP1 in WAT of LFD-fed mice, with no changes in UCP1 expression in BAT of LFD-fed mice. (B) AF increased UCP1 expression in BAT and WAT of HFD-fed mice. After 36 h of fasting, the mRNA expression of PGC1α, PGC1β, CPT1α, CPT1β, and PRDM16 were increased in sWAT(C) and eWAT(D) of HFD mice. The asterisks represent the level of statistical significance calculated using a two-tailed Student’s t-test (* p < 0.05, ** p < 0.01); data are presented as the mean ± standard error of mean.
To investigate the effects of AF on the lipid metabolism in WAT of HFD-fed mice, we measured the relative lipid gene levels. FA synthase (FASN) and acetyl-CoA carboxylase (ACC) are two major enzymes involved in de novo lipogenesis, whose expression and activity in adipose tissue, liver, and other key organs affect weight gain [18]. Sterol regulatory element-binding transcription factor 1 (SREBP1) can induce the expression of genes mainly involved in sterol and FA biosynthesis in response to cholesterol deprivation [19]; and adipose triglyceride lipase (ATGL) performs the first step of triglyceride (TG) hydrolysis, generating diglycerides (DGs) and FAs [20]. Hormone-sensitive lipase (HSL) then performs the second step, hydrolyzing DGs into monoglycerides (MGs) and FAs [21]. PPARα promotes the β-oxidation of fatty acids [22]. Expression levels of ACC, FASN, and SREBP1 genes associated with lipid synthesis were reduced in both eWAT and sWAT of HFD-fed mice after AF (Fig. 4A, 4C). In contrast, expression levels of HSL, ATGL, and PPARα genes associated with lipid catabolism and oxidation were increased in both eWAT and sWAT of HFD-fed mice after AF (Fig. 4B, 4D). Additionally, we measured the blood lipid levels in mice after AF. We found that TGs, low-density lipoprotein cholesterol (LDL), and non-esterified free FAs (NEFAs) were decreased in both LFD- and HFD-fed mice after AF (Fig. 4E, 4F). Collectively, these results demonstrated that AF improved the lipid metabolism in WAT of in HFD-fed mice.

The effects of acute fasting (AF) on the lipid metabolism of white adipose tissue (WAT) in high-fat diet (HFD)-fed mice. AF increased the expression of lipid catabolism and oxidation-related genes in eWAT (B) and sWAT (D) of HFD-fed mice. Both low-fat diet- (E) and HFD-fed (F) mice showed decreased blood lipid levels after AF. The asterisks represent the level of statistical significance calculated using a two-tailed Student’s t-test (* p < 0.05, ** p < 0.01); data are presented as means ± SEM. ACC, acetyl-CoA carboxylase; FASN, fatty acid synthase; SREBP1, sterol regulatory element-binding transcription factor 1; HSL, hormone-sensitive lipase; ATGL, adipose triglyceride lipase; PPARα, peroxisome proliferator-activated receptor α; CHO, cholesterol; TG, triglycerides; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; NEFA, non-esterified free fatty acids.
Fasting can influence immune responses. Macrophages are the primary components of innate immunity, and they can be differentiated into the M1 or M2 phenotype in response to environmental stimulation. M1 macrophages can cause adipose tissue inflammation, whereas M2 macrophages can promote reshaping of WAT into BAT morphology, also known as the WAT browning/beiging, thereby enhancing insulin sensitivity and metabolic health [6, 23-27]. Therefore, we focused on macrophage polarization in WAT of HFD-fed mice after AF. AF induced downregulation of the pro-inflammatory phenotype M1 marker gene, chemokine (C-C motif) ligand 5 (CCL5), and up-regulation of the anti-inflammatory phenotype M2 marker genes, arginase 1 (ARG1) and chitinase-like 3 (CHIL3), in sWAT (Fig. 5A). AF induced the same changes, including the decrease in the expression levels of M1 marker genes (CCL5, cluster of differentiation 68 [CD68], and inducible nitric oxide synthase [iNOS]) and the increase in the expression levels of M2 marker genes (ARG1 and CHIL3), in eWAT (Fig. 5C). M1 macrophages produce pro-inflammatory cytokines; consistent with the changes in the macrophage polarization marker genes, the mRNA expression of inflammatory cytokines (interleukin [IL]-6, IL-1b, and tumor necrosis factor [TNF]-α) was decreased in both sWAT and eWAT after AF (Fig. 5B, 5D). Collectively, the above results suggested that AF promoted M2-polarization of macrophages in WAT of HFD-fed mice.

Acute fasting (AF) promoted M2-polarization of macrophages in white adipose tissue (WAT) of high-fat diet (HFD)-fed mice. (A) Gene analysis revealed that the M1 marker gene CCL5 decreased and M2 marker genes ARG1 and CHIL3 increased in sWAT of HFD mice after AF. (C) In the eWAT of HFD mice, mRNA expression of M1 marker (CCL5, CD68 and iNOS) were downregulated and M2 marker genes (ARG1 and CHIL3) were upregulated by AF. AF induced the reduction in inflammatory cytokine (IL6, IL1b, and TNFα) mRNA expression in sWAT(B) and eWAT(D) of HFD mice. The asterisks represent the level of statistical significance calculated using a two-tailed Student’s t-test (* p < 0.05, ** p < 0.01); data are presented as the mean ± standard error of mean. CCL5, chemokine (C-C motif) ligand 5; CD68, cluster of differentiation 68; iNOS, inducible nitric oxide synthase; ARG1, arginase 1; CHIL3, chitinase-like 3; STAT6, signal transducer and activator of transcription 6; IL6, interleukin 6; IL1b, interleukin 1b; TNFα, tumor necrosis factor α.
We explored the underlying mechanisms of AF-induced WAT beiging and macrophage M2-polarization in HFD-fed mice. We focused on Sirt5 because Sirt5 is associated with macrophage M2 (anti-inflammatory)-polarization and WAT beiging [10, 28]. We measured the Sirt5 levels in BAT, eWAT, and sWAT of HFD-fed mice; and our data showed that both Sirt5 mRNA and protein levels were significantly elevated in the 3 types of adipose tissue after AF (Fig. 6A, 6B). Additionally, AF markedly upregulated the succinylation of fat tissue in HFD-fed mice (Fig. 6C). These observations indicated that Sirt5 might play a role in AF-induced beneficial effects on fat tissue.

Acute fasting (AF) upregulated Sirt5 expression levels and fat tissue succinylation in high-fat diet (HFD)-fed mice. (A) RT-qPCR showed that the mRNA level of Sirt5 was increased in BAT, sWAT, and eWAT of HFD mice after AF. (B) AF induced the upregulation of Sirt5 protein levels in eWAT, sWAT, and BAT of HFD mice. (C) AF induced the succinylation of fat tissues in HFD mice. The asterisks represent the level of statistical significance calculated using a two-tailed Student’s t-test (* p < 0.05); data are presented as the mean ± standard error of mean.
Metabolic diseases caused by excess nutrition and obesity are increasing globally. The latest epidemiological study showed that nearly 1 in 2 adults in America will be obese and the prevalence of obesity will be higher than 50% in 29 states and not below 35% in any state [29]. Adipose tissue plays an important role in whole-body metabolism [30]; and improvements in metabolism from energy restriction, especially IF, have been widely studied [5-7, 31-34]. However, the effects of AF on adipose tissue have not been fully elucidated. We demonstrated herein that AF decreased body weight, fat mass, and WAT cell size in LFD- and HFD-fed mice; and promoted the WAT browning in HFD-fed mice. Besides, AF improved lipid metabolism and increased M2-polarization of macrophages in WAT of HFD-fed mice, and upregulated the Sirt5 levels in fat tissue.
Lacerda et al. (2019) fed male BALB-c mice with a chow diet (lean mice) or a high-carbohydrate refined (mildly obese mice) diet for 8 weeks and subjected the animals to 24 h of fasting [35]. They found body weight decreased in lean mice but not in mildly obese mice at 24 h after fasting. Since type of mice and duration of fasting we employed were different from those in the aforementioned study, our study showed that 36 h of fasting significantly reduced the body weight of both LFD- and HFD-fed C57BL/6J mice. Thus, the loss in body weight of mice may depend on the strain of the mice and the duration of fasting. Since the early stage of fasting, during which the body adapts to the lack of nutrients, lasts for 24 h [36], we measured the weight of each organ after 36 h of fasting, and indicated that 36 h of fasting decreased fat mass and mainly resulted in the utilization of white fat (sWAT and eWAT) in both LFD- and HFD-fed mice. Ding et al. showed that 24 h of fasting mobilized more visceral fat than subcutaneous fat, and we likewise found that 36 h of fasting mobilized more eWAT than iWAT—a phenomenon that was more pronounced in HFD-fed mice [37]. Furthermore, we found that the liver weight was also diminished in HFD mice after 36 h of fasting (p = 0.08).
In our study, we found that 36 h of fasting not only reduced the weight of WAT, but also, more importantly, promoted the browning of WAT. This finding was in agreement with the results of previous studies. Liu et al. (2019) fed male C57 BL/6 J mice chow or a HFD for 8 weeks before subjecting them to IF for an additional 8 weeks [38]. They showed that IF increased energy expenditure and UCP1 mRNA levels in the inguinal and gonadal fat depots, and the UCP1 protein levels in the inguinal fat in both the diet-fed groups on the fed days [38]. Kim et al. (2017) found that IF significantly upregulated the levels of beige/brown adipose markers (i.e., Ppargc1a, Cidea, and UCP1) in perigonadal WAT of HFD mice [6]. IF was shown to selectively activate beige-fat development in inguinal WAT, most likely by re-shaping the gut microbiota, stimulating acetate and lactate accumulation and increased beiging [5]. Besides, Ding et al. showed that 24 h of fasting downregulated the expression of UCP1 and prdm16 in sWAT form chow diet BALB-c mice [37]. In our study, we performed the acute fasting for 24 hours to check the expression of thermogenic genes in HFD mice. Interestingly, the result showed that even a 24-hour fasting also can significantly increase the expression of UCP1 and PGC1a (Fig. S1 A and B). However, the expression of PRDM16 had a marginal difference (Fig. S1 C). This result indicated that 24hs fasting is enough to reshape the sWAT to transform the beige formation in sWAT form HFD mice. Acute fasting may bring more beneficial effects in WAT on high-fat diet animals rather than chow diet animals. Also, the different animal model in metabolism study may bring some different phenotypes. Combining the results of our and previous studies, we could draw a preliminary conclusion that both AF and IF could effectively induce browning of white fat; however, the mechanism(s) involved remains unclear.
Consistent with the morphologic alterations in fat tissue induced by 36 h of fasting, fat metabolism was also observed in our study. The expression levels of the genes associated with lipid synthesis were reduced, while the expression levels of the genes associated with lipid catabolism and oxidation were increased in WAT of HFD mice after 36 h of fasting. These results suggest that WAT is broken down and burned to fuel in the body during food deprivation.
Moreover, 36 h of fasting promoted M2 polarization of fat macrophages in HFD mice. Obesity leads to low levels of chronic inflammation in WAT, which in turn leads to insulin resistance and metabolic syndrome [39-41]. Restoring M2 macrophages to obese individuals by various means helps to resolve the related inflammation and insulin resistance [42, 43]. Our study proved that 36 h fasting activated M2 macrophage polarization and alleviated inflammation in WAT of HFD-fed mice. This finding was in agreement with the result of a study by Kim et al. (2017) [6], which showed that both one-time fasting for 24 h and IF were sufficient to induce macrophage M2-polarization as evidenced by increased M2 macrophages and their marker gene expression (e.g., Clea10a, Il10, Ym1 and Arg1), as well as decreased expression of M1 marker genes. This suggests that the beneficial changes induced by AF are repeated during IF, thereby facilitating its metabolic benefits.
The mitochondrial sirtuin, Sirt5, regulates the activity of important mitochondrial enzymes and drives metabolic cycles in response to fasting and caloric restriction [44]. In this study, we observed that fasting for 36 h upregulated Sirt5 levels and decreased the succinylation modification level in fat tissue. Sirt5 is required for brown adipocyte differentiation and regulates brown adipogenic gene activation at least partially through an indirect effect on histone modifications [10]. Sirt5 partially suppresses the pro-inflammatory response in macrophages at least in part by regulating pyruvate kinase M2 succinylation, activity, and function [28]. Therefore, we speculated that the increase in Sirt5 level contributed to the browning and remodeling of white fat by promoting M2-polarization of macrophages. However, more studies are needed to corroborate this hypothesis.
For decades, the beneficial effects of IF on metabolic diseases in humans and animals have been demonstrated, but very few articles have attempted to clarify the underlying mechanism [5, 6]. There are few studies on AF, compared to IF—let alone on the underlying mechanism of AF. Additionally, the beneficial effects of AF can only be maintained for a short time after refeeding, but the beneficial effects of IF or intermittent energy restriction (IER) can be well maintained; this may be a promising treatment for metabolic diseases in the future. Thus, the purpose of our study on AF was to observe the extent of this beneficial effect since IER can be used to maintain physical fitness by refeeding, thereby maintaining the beneficial effects of AF during re-fasting. Systematic observation of AF is currently lacking, and the present study entailed some intriguing concepts. However, more in-depth and detailed research must be performed in the future.
Our study had a few limitations. First, the mechanism of AF-induced adipose tissue remodeling was not adequately studied. Second, the inclusion of only male mice showed results that cannot be extrapolated to female mice. The reason we did not use female C57BL/6J mice was that they are resistant to HFD-induced obesity. Third, we did not evaluate the glucose metabolism (using glucose tolerance test [GTT] and insulin tolerance test [ITT]), but this was immaterial since we performed AF for 36 h, which exceeded the conventional fasting time for GTT (16 h) and ITT (4 h). Thus, this might have affected the phenotype, and it was difficult to determine whether the test was appropriate. However, we believe that the blood glucose metabolism may be improved after fasting, but it may take a longer duration of IF to maintain these beneficial effects.
In conclusion, our study demonstrated that AF altered adipose tissue morphology of mice on LFD and HFD-fed mice, and promoted WAT browning. Additionally, AF improved lipid metabolism and increased macrophage M2 polarization and the Sirt5 expression in WAT of HFD-fed mice.
Eight-week-old male C57BL/6J mice were randomly divided into six groups: (1) mice fed a LFD for 16 weeks and for 36 h (LFD and AF control, n = 5); (2) mice fed a LFD for 16 weeks and subjected to 36 h of fasting (LFD and AF, n = 5); (3) mice a fed high-fat diet for 16 weeks and 36 h (HFD and AF control, n = 5); (4) mice fed a HFD for 16 weeks and subjected to 36 h of fasting (HFD and AF, n = 5); (5) mice fed high-fat diet for 16 weeks and 5 days (HFD and PF control, n = 5); and (6) mice fed a high-fat diet for 16 weeks and subjected to fasting for 5 d (HFD and PF, n = 5). Mice were housed in ventilated cages with controlled environmental settings (22℃, 12-h light/dark cycles) in a specific pathogen-free facility. LFD (H10010, 10% fat; 3.85 kcal/g) and HFD (H10060, 60% fat; 5.24 kcal/g) were purchased from Research Diets (HFK Bioscience, Beijing, China). All mice had free access to water. All experimental procedures and use of animals were conducted according to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health and approved by the Animal Ethics Committee of Nanjing Agricultural University, Nanjing (approval ID, SYXK2017-0007).
RNA isolation and reverse transcription (RT)-qPCRTotal RNA was extracted from frozen adipose tissues using TRIzol reagent according to the manufacturer’s instructions. The purity and concentration of the RNA were determined using a NanoDrop spectrophotometer (ND-1000, Thermo Fisher Scientific, MS, USA). Total RNA (1ug) was reverse transcribed using a cDNA Synthesis SuperMix (Biotool.com). Real-time PCR was performed in an ABI 7900HT Fast Real-Time PCR System (AB Applied Biosystems, Warrington, UK) with SYBR Green PCR Master Mix (Applied Biosystems) and gene-specific primers. The sequence and GenBank accession number for the forward and reverse primers used to quantify mRNA are listed in the Table 1. The following conditions were used for real-time PCR: 95°C for 10 min, then followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. The 2–ΔΔCt (Ct, threshold cycle) method was used to analyze the relative changes in gene expression normalized against β-actin mRNA expression. Primer sequences are available upon request.
| Gene | Forward primer (5'→3') | Reverse primer (5'→3') |
|---|---|---|
| Mouse-Cyclophilin | CAAATGCTGGACCAAACACAA | GCCATCCAGCCATTCAGTCT |
| Mouse-Ucp1 | GGCAAAAACAGAAGGATTGC | TAAGCCGGCTGAGATCTTGT |
| Mouse-Prdm16 | GAAGTCACAGGAGGACACGG | CTCGCTCCTCAACACACCTC |
| Mouse-Cpt1α | GACTCCGCTCGCTCATTCC | GACTGTGAACTGGAAGGCCA |
| Mouse-Pgc1α | ACAGCTTTCTGGGTGGATTG | TGAGGACCGCTAGCAAGTTT |
| Mouse-Pgc1β | CGTATTTGAGGACAGCAGCA | TACTGGGTGGGCTCTGGTAG |
| Mouse-Pparα | AGCCTCAGCCAAGTTGAAGT | TGGGGAGAGAGGACAGATGG |
| Mouse-ACC | GCTGCTCGGATCACTAGTGAA | TTCTGCTATCAGTCTGTCCAG |
| Mouse-FASN | GCTGCTCGGATCACTAGTGAA | TGTAGCCCACGAGTGTCTCG |
| Mouse-SREBP1 | GGAGGGGTAGGGCCAACGGCCT | CATGTCTTCGAAAGTGCAATCC |
| Mouse-HSL | CTGAGATTGAGGTGCTGTCG | CAAGGGAGGTGAGATGGTAAC |
| Mouse-Atgl | GCGCCAGGACTGGAAAGAAT | TGAGAACGCTGAGGCTTTGAT |
| Mouse-Sirt5 | GCCACCGACAGATTCAGGTT | CCACAGGGCGGTTAAGAAGT |
| Mouse-IL6 | AGACAAAGCCAGAGTCCTTCAG | GCCACTCCTTCTGTGACTCCAG |
| Mouse-IL1β | TTCAGGCAGGCAGTATCACTC | GAAGGTCCACGGGAAAGACAC |
| Mouse-TNFα | TGGGCCTCTCATGCACCACC | GAGGCAACCTGACCACTCTCCCT |
| Mouse-Arg1 | CTCCAAGCCAA AGTCCTTAGAG | AGGAGCTGTCATTAGGGACATC |
| Mouse-iNOSF | GTTCTCAGCCCAACA ATACAAGA | GTGGACGGGTCGATGTCAC |
| Mouse-CCL5 | CCTGCTGCTTTGCCTACCTCTC | ACACACTTGGCGGTTCCTTCGA |
| Mouse-CD68 | GGCGGTGGAATACAATGTGTCC | AGCAGGTCAAGGTGAACAGCTG |
| Mouse-CHIL3 | TACTCACTTCCACAGGAGCAGG | CTCCAGTGTAGCCATCCTTAGG |
| Mouse-STAT6 | ACGACAACAGCCTCAGTGTGGA | CAGGACACCATCAAACCACTGC |
Total protein from adipose tissue was extracted using RIPA lysis buffer containing 1 mM PMSF. The homogeneous mixture was centrifuged at 10,000 g and the protein concentration of the supernatant was detected using a BCA Kit (P0011; Beyotime Biotechnology, Nantong, China). The protein solution was then mixed with the loading buffer and heated for 5 min in boiling water. An equal amount of each protein sample was loaded and separated by 12.5% SDS PAGE in an electrophoresis tank. Then, the protein of the sample was transferred from the gel to a PVDF membrane, which was then blocked with 5% bovine serum albumin (BSA). Western blotting was performed using the corresponding detection antibodies.
Body composition measurementsThe total fat and lean masses of mice after a 7-week treatment with either vehicle or WECS were assessed with the Small Animal Body Composition Analysis and Imaging System (MesoQMR23-060H-I, Nuimag Corp., Shanghai, China), according to the manufacturer’s instructions.
Hematoxylin-Eosin (H&E) stainingTissues were fixed in PBS buffer containing 4% paraformaldehyde overnight at room temperature and washed on a shaking table at room temperature. The tissues were then stained according to standard protocols. Resin and cover glass were used to seal the section. We took images with an inverted microscope (DS-RI1; Nikon, Tokyo, Japan).
Statistical analysisWe used single-factor analysis of variance (ANOVA) followed by the 2-tailed Student’s t-test, for comparisons. We presented almost all the data as the mean ± 1 standard error of mean. Differences were considered significant at p < 0.05. We used Graph-PadPrism8 (GraphPad Software, SanDiego, CA,USA) for data analysis.
Conceptualization and project administration, T.M., and C.Z.; Resources, Q.W.; Data curation, formal analysis, investigation, software, visualization, writing of the original draft and writing the review or editing, T.M. and C.Z.; methodology, supervision, and validation, T.M., C.Z. and Q.W.
This work was supported by the China Scholarship Council State Scholarship Fund (201806275134 to T Mao) and the National Natural Science Foundation of China (31972565 to Q Wei and 81700684 to C Zhang).
AF, Acute fasting; IF, Intermittent fasting; PF, Prolonged fasting; WAT, White adipose tissue; BAT, Brown adipose tissue; sWAT, Subcutaneous white adipose tissue; vWAT, Visceral white adipose tissue; LFD, Low-fat diet (Normal chow); HFD, High-fat diet; UCP1, Uncoupling protein 1; IL-6, Interleukin-6; TNF-α, Tumor necrosis factor-alpha; CCL5, Chemokine (C-C motif) ligand 5; PGC1, Peroxisome proliferator-activated receptor gamma coactivator 1; CPT1, Carnitine palmitoyltransferase 1; PRDM16, PR domain containing 16; FASN, Fatty acid synthase; ACC, Acetyl-CoA carboxylase; SREBP1, Sterol regulatory element-binding transcription factor 1; PPARα/γ, Peroxisome proliferator-activated receptor alpha/gamma; HSL, Hormone-sensitive lipase; ATGL, Adipose triglyceride lipase; CHO, Cholesterol; TG, Triglyceride; HDL, High-density lipoprotein cholesterol; LDL, Low-density lipoprotein cholesterol; NEFA, Non-esterified free fatty acids; CD68, Cluster of differentiation 68; iNOS, Inducible nitric oxide synthase; ARG1, Arginase 1; CHIL3, Chitinase-like 3; STAT6, Signal transducer and activator of transcription-6; Sirt5, Sirtuin 5.
We express our gratitude to Prof. Emeritus Reinhold J. Hutz of the Department of Biological Sciences, University of Wisconsin-Milwaukee, USA, for reading the original manuscripts and offering valuable suggestions.
The authors declare that they have no conflict of interest.
Ethical approvalAll applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The Animal Ethical Committee of the Institutional Animal Care and Use Committee of Nanjing Agricultural University (No. SYXK2017-0007) approved all experimental procedures of this study.