2021 Volume 68 Issue 6 Pages 729-738
2021 Volume 68 Issue 6 Pages 729-738
This review evaluated the risk of rheumatoid arthritis in patients with type 2 diabetes treated with dipeptidyl peptidase-4 inhibitors (Dpp-4i). The MEDLINE (via PubMed), Embase, the Cochrane Library databases and web of science were used to search the effects of Dpp-4i on rheumatoid arthritis in patients with type 2 diabetes from inception to 7 September, 2020. We included studies that met the following criteria:(i) A randomized controlled trial (RCT), prospective or retrospective cohort study examining the relationship between Dpp-4i and rheumatoid arthritis. Exclusion criteria included the following: Reviews and researches related to other diseases or subjects; and studies without data on the prevalence of rheumatoid arthritis were excluded. Risk of Bias table contained in Review Manager 5.3 and Newcastle-Ottawa scale (NOS) were used for quality assessment of included RCT and observational studies separately. Meta-analysis was used to estimate the risk of disease. We conducted a subgroup analysis of duration of follow-up, adjusted (adjusted RR or unadjusted RR), sample size and study design. A total of 10 independent studies assessing 1,420,414 patients were included in this analysis. In this meta-analysis, we found that there was nonsignificant increase of rheumatoid arthritis with Dpp-4 inhibitor exposure (RR 0.96, 95%CI (0.69–1.32)). Our results revealed that Dpp-4 inhibitors do not seem to increase the risk of rheumatoid arthritis. Long-term follow-up monitoring is necessary.
DIPEPTIDYL PEPTIDASE-4 INHIBITOR (DPP-4I) is an oral hypoglycemic drug increased insulin and C-peptide, decreased glucagon, and improved blood glucose control by increasing insulin (glucagon-like peptide-1 and glucagon-dependent insulin polypeptide) [1, 2]. Dpp-4 inhibitors were associated with a lower risk of hypoglycemia and had little effect on body weight compared with other second- or third-line treatments such as sulfonylureas or insulin by inhibiting the Dpp-4 enzyme [3]. However, it has been reported that inhibition of Dpp-4 enzyme may also lead to autoimmune related adverse events, such as bullous pemphigus [4], inflammatory bowel disease [5].
Rheumatoid arthritis (RA) is a chronic systemic inflammatory autoimmune disease characterized by symmetric and corrosive synovitis, which can lead to severe disability and premature death [6, 7]. Dpp-4 enzymes have been shown to be expressed in a variety of immune cells and to be involved in T cell activation and proliferation [8]. Dpp-4 enzymes have been shown to be involved in degrading pro-inflammatory molecules, such as IL-1, IL-2, and TNF-α, thereby playing an important role in anti-inflammatory immune response [9]. In the experimental model of arthritis, circulating levels of active pro-inflammatory chemokine stromal cell-derived factor-1 (SDF-1) was increased in Dpp4–/– mice , and this study also found that plasma DPPIV levels were significantly lower in patients with rheumatoid arthritis than in patients with osteoarthritis [10]. Several studies also confirmed the content and activity of Dpp4 enzyme in serum and immune cell surface isolated from synovial fluid were lower in patients with rheumatoid arthritis [11-13]. Currently, however, it is not clear whether Dpp-4i exposure increases the risk of rheumatoid arthritis in healthy individuals. In 2015, the US Food and Drug Administration (FDA) warned about the risk of joint pain associated with Dpp-4 inhibitors, and a recent meta-analysis also suggested that Dpp-4 inhibitors increase the risk of joint pain, and added joint pain as a label for potential adverse reactions to the product [14, 15]. Over the years, most case reports and observational studies have found an association between Dpp-4i exposure and RA risk [16, 17].
To fully understand the relationship between Dpp-4i and rheumatoid arthritis risk, we conducted a systematic review and meta-analysis to assess the impact of Dpp-4 inhibitors on rheumatoid arthritis risk in patients with type 2 diabetes.
A number of database resources, including MEDLINE (via PubMed), Embase, web of science and the Cochrane Library databases were searched from the inception of each resource to 7 September, 2020. The search strategy included the following terms: ‘Dipeptidyl Peptidase-4 Inhibitors’, ‘Dpp-4 inhibitors’, ‘gliptin’, ‘sitagliptin’, ‘vildagliptin’, ‘saxagliptin’, ‘linagliptin’, ‘alogliptin’ AND ‘rheumatoid arthritis’, ‘arthritis’, ‘arthritis rheumatoid’. Two authors independently screened the title, abstract and full text of the selected study. Pretesting and discussion were conducted prior to formal screening to ensure consistency among reviewers. The two reviewers resolved any differences through discussion or negotiation with the third reviewer. We included studies that met the following criteria: A randomized controlled trial, prospective or retrospective cohort study examining the relationship between Dpp-4i and rheumatoid arthritis. Exclusion criteria included the following: reviews and researches related to other diseases or subjects; and Studies without data on the prevalence of rheumatoid arthritis were excluded.
Data extractionThe data extraction was done by two reviewers independently, and the standard data extraction template they used contained the following information: basic information (first author, publication year, country) and contents of studies (study design, sample size, mean or median age, duration of follow-up and adjusted (adjusted RR or unadjusted RR)).
Quality assessmentTwo reviewers used the Newcastle Ottawa scale (NOS) [18] to assess the quality of observational studies. Each study was scored on a scale of 0–9. We gave a score of 0–3, 4–6 and 7–9 for low, medium and high-quality research, respectively. We used the Risk of Bias table contained in Review Manager 5.3 to assess the quality of included RCTs. Data extraction and quality evaluation were completed independently by two authors. Information was independently reviewed and determined by another author who referred to the original study.
Statistical analysisWe examined the relationship between Dpp-4i and the risk of RA based on the published effect assessment of each study and its 95% confidence interval (CI). Risk Ratio were expressed by 95% CIs, directly extracted or calculated indirectly. Between studies heterogeneity was investigated using I2 statistic, the percentage of I2 indicated the degree of heterogeneity. I2 percentages of 25%, 50%, and 75% indicated a low, moderate, and high degree of heterogeneity, respectively and we believed that p value <0.05 indicated significant heterogeneity. Meta regression analysis was used to determine whether covariates at study level might explain heterogeneity. Study designs, duration of follow-up, sample size, adjusted (adjusted RR or unadjusted RR) and regions were contained in multivariable model. Subgroup analyses were carried out based on the following variables: study design (Cohort study, case-control and RCT), duration of follow-up (>2 years, <2 years), sample size (≤10,000 or >10,000), adjusted (adjusted RR or unadjusted RR) and regions (Asia, North America and Multiple regions). In addition, sensitivity analyses were performed by omitting one study at a time to investigate the impact of each individual study on the pooled results. Funnel plot, Egger’s test and Begg’s test were used to evaluate publication bias. Statistical analyses were performed on random effect model by using STATA software (version 15.0; Stata Corporation, College Station, TX, USA).
The search strategy yielded 333 records, including 27 studies from PubMed, 258 from Embase, 2 from the Cochrane Library databases and 46 from web of science. 287 studies were assessed for eligibility after excluding 46 replicates. We further excluded 281 irrelevant studies, after reading the titles and abstracts and reviewing the full texts. Furthermore, an additional 4 records were identified. In brief, 10 were included [19-23, 16, 24-27]. The flow chart of the research screening process is shown in Fig. 1. Table 1 shows the characteristics of the included studies. Five studies [19, 20, 24, 26, 27] were RCTs, three [22, 16, 25] were cohort studies and two [21, 23] were case–control studies. These studies were conducted in the following countries: USA(n = 3) [21-23], Japan (n = 2) [24, 26], South Korea (n = 1) [25], Turkey (n = 1) [16], and there are three studies were conducted in multiple regions [19, 20, 27]. Sample sizes ranged from 180 to 1,140,784. The average age of the participants studied in most of the studies was about 60. The duration of follow-up ranged from 0.25 to 3.4 years. All RCTs were with low risk and high quality in Fig. 2. Each observational study was with a NOS score of ≥6, indicating that all included studies were of medium or high quality (Table 2).

Literature search PRISMA consort diagram.
| First author | Year | Region | Study design | age | male, No. (%) | Duration of follow-up (years) | Sample size | Adjustments | Adjusted (adjusted RR or unadjusted RR) |
|---|---|---|---|---|---|---|---|---|---|
| Seoyoung C Kim | 2014 | USA | Cohort study | 55.5 | 39.3 | 0.74 | 236,990 | Age, sex, smoking, comorbidities such as obesity, thyroid disease and other cardiovascular diseases, medications including calcium channel blockers, β-blockers, anticonvulsants, antipsychotics, procainamide, quinidine, hydralazine, methyldopa, thiazides, systemic steroids and non-steroidal anti-inflammatory drugs and healthcare use factors including visits to various specialists. | adjusted RR |
| Jong Mi Seong | 2019 | South Korea | Cohort study | 57.7 | 58.4 | 1.68 | 1,140,784 | Age; sex; year of cohort entry; diabetes mellitus-related hospitalization; the number of oral hypoglycemic drugs at cohort entry; and microvascular complications of diabetes mellitus (retinopathy, neuropathy, and nephropathy). | adjusted RR |
| Khalaf Kridin | 2018 | USA | Case-control | 74.5 | 42.8 | 3.0 | 5,988 | Sex, age, and ethnicity. | adjusted RR |
| Niranjan Kathe | 2017 | USA | Nested Case–control | 60.7 | 37 | 0.5 | 12,640 | Age, sex, and event date. | adjusted RR |
| Takeda | 2013 | Multiple regions | RCT | 60.9 | 67.9 | 3.4 | 5,380 | — | unadjusted RR |
| AstraZeneca | 2013 | Multiple regions | RCT | 65 | 66.9 | 2.9 | 16,492 | — | unadjusted RR |
| Boehringer Ingelheim | 2010 | Multiple regions | RCT | 59.8 | 60.2 | 1.1 | 1,551 | — | unadjusted RR |
| Takeda | 2008 | Japan | RCT | 60.2 | 71.9 | 1.1 | 180 | — | unadjusted RR |
| Z.A. Sayiner | 2018 | Turkey | Cohort study | 55.5 | 42.5 | 1.95 | 200 | — | unadjusted RR |
| Yutaka Seino | 2012 | Japan | RCT | 60.3 | 65.6 | 0.25 | 209 | — | unadjusted RR |
RCT, Randomized Controlled Trial; USA, the United States of America.

The quality assessment of included RCTs.
| Author name | Study design | Selection | Comparability | Outcome | Final score | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on basis of the design or analysis | Assessment of outcome | Whether the follow-up period was more than 0.25 years | Adequacy of follow up of cohorts | |||
| Jong Mi Seong 2019 | Cohort | * | * | * | * | ** | * | * | — | 8 |
| Seoyoung C Kim 2014 | Cohort | * | * | * | * | ** | * | * | * | 9 |
| Z.A. Sayiner 2018 | Cohort | * | * | * | * | * | * | * | — | 7 |
| Author name | Selection | Comparability | Exposure | Final score | ||||||
| Is the case definition adequate? | Representativeness of the cases | Selection of Controls | Definition of Controls | Comparability of cohorts on basis of the design or analysis | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non-Response rate | |||
| Khalaf Kridin 2018 | Case-control | * | * | * | * | ** | — | * | — | 7 |
| Niranjan Kathe 2017 | Nested Case–control | * | * | * | * | * | — | * | — | 6 |
One star represents a score of 1, and a study can be awarded a maximum score of 9 in total.
NEWCASTLE - OTTAWA QUALITY ASSESSMENT SCALE COHORT STUDIES
Note: A study can be awarded a maximum of one star for each numbered item within the Selection and Outcome categories. A maximum of two stars can be given for Comparability
Selection
1) Representativeness of the exposed cohort
a) truly representative of the average Dipeptidyl Peptidase-4 Inhibitors users in the community *
b) somewhat representative of the average Dipeptidyl Peptidase-4 Inhibitors users in the community *
c) selected group of users e.g. nurses, volunteers
d) no description of the derivation of the cohort
2) Selection of the non-exposed cohort
a) drawn from the same community as the exposed cohort *
b) drawn from a different source
c) no description of the derivation of the non-exposed cohort
3) Ascertainment of exposure
a) secure record (e.g. Hospital records) *
b) structured interview *
c) written self-report
d) no description
4) Demonstration that outcome of interest was not present at start of study
a) yes *
b) no
Comparability
1) Comparability of cohorts on the basis of the design or analysis
a) study controls for age and sex *
b) study controls for any additional factor *
Outcome
1) Assessment of outcome
a) independent blind assessment *
b) record linkage *
c) self-report
d) no description
2) Was follow-up long enough for outcomes to occur
a) yes (We select a follow up period of 0.25 years for outcome of interest )*
b) no
3) Adequacy of follow up of cohorts
a) complete follow up - all subjects accounted for *
b) subjects lost to follow up unlikely to introduce bias - small number lost - >90% follow up, or description provided of those lost) *
c) follow up rate <90% and no description of those lost
d) no statement
NEWCASTLE - OTTAWA QUALITY ASSESSMENT SCALE CASE CONTROL STUDIES
Note: A study can be awarded a maximum of one star for each numbered item within the Selection and Exposure categories. A maximum of two stars can be given for Comparability.
Selection
1) Is the case definition adequate?
a) yes, with independent validation *
b) yes, e.g. record linkage or based on self-reports
c) no description
2) Representativeness of the cases
a) consecutive or obviously representative series of cases*
b) potential for selection biases or not stated
3) Selection of Controls
a) community controls *
b) hospital controls
c) no description
4) Definition of Controls
a) no history of disease (endpoint) *
b) no description of source
Comparability
1) Comparability of cases and controls on the basis of the design or analysis
a) study controls for age and sex *
b) study controls for any additional factor *
Exposure
1) Ascertainment of exposure
a) secure record (e.g. Hospital records) *
b) structured interview where blind to case/control status*
c) interview not blinded to case/control status
d) written self-report or medical record only
e) no description
2) Same method of ascertainment for cases and controls
a) yes*
b) no
3) Non-Response rate
a) same rate for both groups*
b) non respondents described
c) rate different and no designation
Ten studies showed a relationship between Dpp-4i and rheumatoid arthritis in participants with diabetes. We extracted RR from four studies [21-23, 25] directly and six studies [19, 20, 16, 24, 26, 27] were used four-layer table data to calculate RR. Comprehensive integration and analysis showed that there was a nonsignificant correlation between Dpp-4i use and risk of rheumatoid arthritis (RR 0.96, 95%CI (0.69–1.32), with moderate heterogeneity (I2 = 51.5%; p = 0.03; Fig. 3).

Forest plot of studies showing the pooled risk ratio (RR) for the association between Dpp-4i exposure and rheumatoid arthritis.
To study the effects of different study characteristics on the pooled RR, subgroup analysis was performed by subgroups. In subgroup analyses, analyses were performed based on duration of follow-up, adjusted (adjusted RR or unadjusted RR), study design and sample size, no statistical significances were discerned for the overall effect. By univariate and multivariate meta-regression analysis, the overall effect of each subgroup was not statistically significant. More detailed data are shown in Table 3. Sensitivity analysis is shown in Fig. 4, the association is stable. The funnel plot was roughly symmetrical (Fig. 5). Egger’s test (p = 0.79) or the Begg’s test (p = 0.72) showed there was no publication bias.
| Subgroups | n | RR (95% CI) | p-value | I2 (p-value) | Meta-regression | |
|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||
| Total | 10 | 0.96 (0.69, 1.32) | 0.56 | 51.50% (0.03) | ||
| Duration of follow-up | 0.70 | 0.82 | ||||
| <2 years | 7 | 0.93 (0.64, 1.36) | 0.71 | 64.40% (0.01) | ||
| >2 years | 3 | 1.18 (0.60, 2.30) | 0.63 | 0.0% (0.52) | ||
| Study design | 0.83 | 0.60 | ||||
| Cohort study | 3 | 0.89 (0.51, 1.57) | 0.69 | 75.7% (0.02) | ||
| Case-control | 2 | 1.17 (0.95, 1.43) | 0.13 | 0.0% (0.69) | ||
| RCT | 5 | 0.78 (0.33, 1.87) | 0.58 | 0.0% (0.82) | ||
| Adjusted RR | 0.34 | 0.92 | ||||
| Yes | 4 | 0.88 (0.61, 1.27) | 0.49 | 74.80% (0.008) | ||
| No | 6 | 1.38 (0.72, 2.65) | 0.33 | 2.80% (0.40) | ||
| Sample size | 0.11 | 0.31 | ||||
| <10,000 | 6 | 1.55 (0.92, 2.60) | 0.10 | 0.0% (0.60) | ||
| >10,000 | 4 | 0.80 (0.55, 1.18) | 0.26 | 73.40% (0.01) | ||
| Region | 0.88 | 0.67 | ||||
| Asia | 4 | 1.08 (0.45, 2.60) | 0.87 | 64.80% (0.04) | ||
| North America | 3 | 0.98 (0.64, 1.50) | 0.92 | 68.00% (0.04) | ||
| Multiple regions | 3 | 0.82 (0.25, 2.69) | 0.74 | 0.0% (0.66) | ||
CI, confidence interval; RCT, Randomized Controlled Trial; RR, relative risk;

The result of sensitivity analysis.
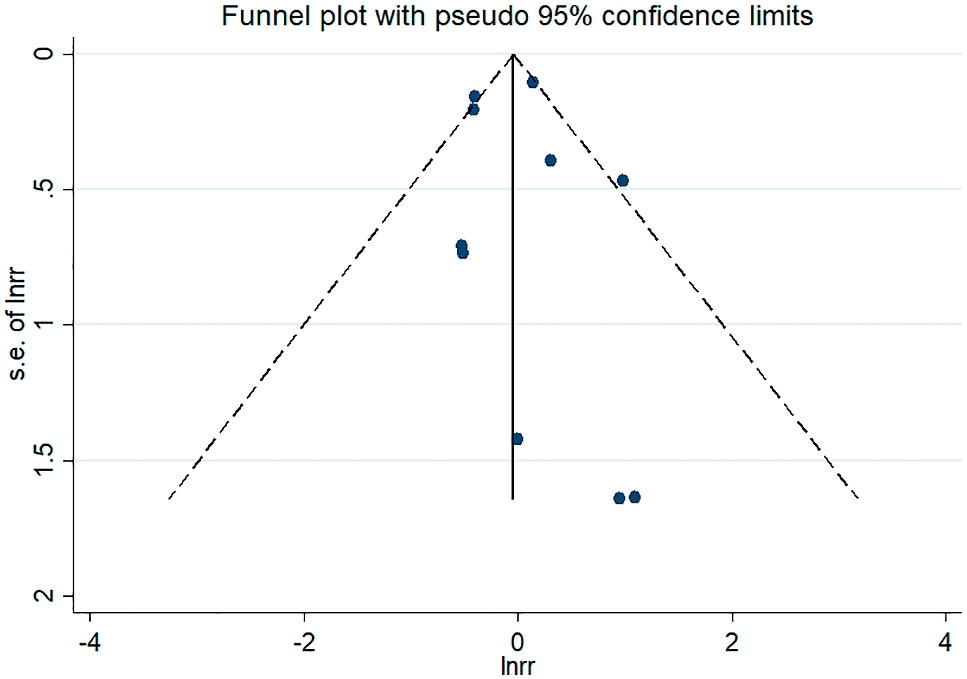
The funnel plot of included studies.
Dpp-4i exposure for rheumatoid arthritis risk is unclear. Although a previous meta-analysis [15] indicated the increased risk of arthralgia with Dpp-4i exposure. As we all know, arthralgia and rheumatoid arthritis are two different concepts. Arthralgia is merely a nonspecific symptom which can be induced by several factors, aged degenerative joint disease such as osteoporosis or osteoarthritis [28, 29], long-term prescriptions such as glucocorticoid or antituberculosis drugs [30, 31]. Rheumatoid arthritis is a chronic systemic inflammatory disease that must be confirmed by serological tests and clinical symptoms [32]. Studies had shown that only a small percentage of patients with joint pain developed rheumatoid arthritis after years of follow-up [33]. In addition, we suspect that this difference may be due to insufficient follow-up time in these studies for some patients to be identified. There was also a population-based cohort study found that Dpp-4i used in patients with type 2 diabetes during a 5-year follow-up period, regardless of gender or age, did not increase the incidence of severe joint pain or non-specific joint disease [34]. Therefore, the effect of Dpp-4i use on the risk of rheumatoid arthritis remains unclear.
To our knowledge, this is the first meta-analysis to assess RA risk after Dpp-4i exposure. In the present meta-analysis, we identified a nonsignificant role of Dpp-4i use on development of RA (RR 0.96, 95%CI (0.69–1.32), this association was stable in sensitivity analysis. There was no publication bias detected in our analyses. By pooling five RCTs, the impact of Dpp-4i was nonsignificant (RR 0.78, 95%CI (0.33–1.87)). Furthermore, the impact of Dpp-4i remained nonsignificant for multiple subgroups. The current results show that the use of Dpp-4i at least does not increase the risk of RA. Further drug epidemiology and pathology studies are needed.
Dipeptidase 4 (Dpp-4) is called the lymphocyte surface protein CD26. It is not only present in serum, plasma, urine, semen, cerebrospinal fluid and synovial fluid, but also in T cells, NK cells, and B cells. It is expressed on the surface of myeloid cells and participates in the development and stimulation of T cells. In recent years, people have paid more attention to the role of CD26 in the immune system [35]. Differences in the expression and activity of serum CD26 have been observed in many autoimmune diseases, including rheumatoid arthritis (RA), type 1 diabetes, systemic lupus erythematosus (SLE) and inflammatory bowel disease (IBD) [8], which suggests it might be related to the pathogenesis of autoimmune diseases. Therefore, more experimental studies on Dpp-4 and rheumatoid arthritis are necessary.
This systematic review and meta-analysis included studies from several different populations and provided larger sample of patients. We included events approximately 1,420,000 patients. We intentionally developed strict inclusion criteria so that we could explore heterogeneity among study populations and draw conclusions relevant to the majority of patients. In addition, the analysis incorporates unpublished data from the American clinical trial registry. However, there seem to be some limitations. Firstly, the current study was not registered in PROSPERO. Therefore, it may exist small bias, but we still strictly followed the steps of Cochrane Handbook for Systematic Reviews of Interventions (https://training.cochrane.org/handbook/current). In theory, the studies included in the meta-analysis should have identical design and protocols. In our analysis, both RCTs and observational studies were included, which resulted in a moderate heterogeneity (I2 = 51.5%; p = 0.03). We addressed this by using random-effects model, subgroup analysis, and sensitivity analysis (rather than exclusion) in the preliminary analysis. Although there is no publication bias in Egger’s test, the unpublished results may have changed our meta-analysis results.
Based on a random-effect analysis, exposure to Dpp-4i does not appear to increase the risk of RA (RR 0.96, 95%CI (0.69–1.32). However, considering (1) potential mechanism of Dipeptidase 4 involvement in autoimmune diseases, (2) relationship between drug exposure time and delayed development of rheumatoid arthritis, (4) limited reports on RA incidence in published studies, and (5) lacking of long-term clinical trials designed to identify the incidence of RA, we believe that larger observational trials and strong post-marketing surveillance programs are necessary to assess the association of Dpp-4 inhibitors with RA.
The authors declare no competing or financial interests in this work.
Miao Wang and Muqin Li carried out the literature search, selection, validity assessment, data abstraction, and data analysis. Ying Xie, Miao Wang, and Muqin Li wrote the paper and incorporated the comments from other authors and peer reviewers. Ying Xie and Miao Wang had the original idea for the paper, formulated the protocol, and contributed to data abstraction and analysis. All authors reviewed and approved the final draft of the paper.
This work was supported by grants from the National Natural Science Foundation of China (grant number 81670742).
Dpp-4i, Dipeptidyl peptidase-4 inhibitors; RA, Rheumatoid arthritis; CI, Confidence interval; RCT, Randomized Controlled Trial; RR, Risk ratio