2021 Volume 68 Issue 7 Pages 817-828
2021 Volume 68 Issue 7 Pages 817-828
Diabetic nephropathy (DN) seriously threatens the health of patients with diabetes. Moreover, it has been reported that mesenchymal stem cell (MSC)-derived exosomal miRNAs can modulate the progression of multiple diseases, including DN. It has been suggested that miR-125b is involved in DN. However, the biological functions of exosomal miRNAs, especially miR-125b, in DN are still unclear. To establish a DN model in vitro, we used a model of human embryonic kidney epithelial cells (HKCs) injury induced by high glucose (HG). Then, miR-125b was delivered to the model cells in vitro via MSC-derived exosomes (MSC-Exos), and the effect of exosomal miR-125b on HKCs apoptosis was evaluated by flow cytometry. qRT-PCR or western blotting was performed to measure miR-125b or tumour necrosis factor receptor-associated factor 6 (TRAF6) expression in HKC. The effect of MSC-Exos on HKCs apoptosis after miR-125b knockdown was determined by flow cytometry. Moreover, dual-luciferase reporter assays were used to determine the targeting relationship between miR-125b and TRAF6 in HKCs. Our data revealed that MSC-Exos increased HG-induced autophagy in HKCs and reversed HKCs apoptosis. Moreover, our study found that miR-125b was enriched in MSC-Exos and directly targeted TRAF6 in HKCs. In addition, exosomally transferred miR-125b inhibited the apoptosis of HG-treated HKCs by mediating Akt signalling. In summary, MSC-derived exosomal miR-125b induced autophagy and inhibited apoptosis in HG-treated HKCs via the downregulation of TRAF6. Therefore, our study provided a new idea for DN treatment.
DIABETIC NEPHROPATHY (DN) is a common diabetic complication that results from permanent uncontrolled diabetes, and it usually causes end-stage renal diseases [1, 2]. Moreover, previous studies have indicated that the induction of autophagy and inhibition of apoptosis could lead to the suppression of DN progression [3, 4]. Since DN seriously threatens the health of patients with diabetes, it is necessary to find new strategies for inducing autophagy and inhibiting apoptosis in DN.
Exosomes are membranous vesicles that regulate recipient cell functions by transferring mRNAs, miRNAs and soluble proteins in functional forms [5, 6]. Moreover, mesenchymal stem cell-derived exosomes (MSC-Exos) have been reported to exhibit suppressive effects on cancer and inflammation [7-9]. Wang D et al. showed that MSC-Exos targeting miR-125b could inhibit neointimal hyperplasia by activating myosin IE [10]. Moreover, the function of exosomes in cells provides multiple therapeutic applications, including cell growth and tissue injury repair following myocardial ischaemia reperfusion [11], neurological stroke [12] and inflammation [13]. In recent years, research on the mechanism of miRNA transfer, including exosomes, has attracted increasing attention [14, 15]. Zhu M et al. found that high glucose (HG)-induced exosomes derived from macrophages promoted macrophages to alleviate injury in kidney tissues during DN [16]. Furthermore, many studies have confirmed the function of exosomal miRNAs in DN [17, 18]. For example, urinary exosomal miR-21-5p and miR-30b-5p participate in DN [18]. MSCs can deliver exogenous miR-let7c via exosomes to attenuate renal fibrosis [19]. Furthermore, MSCs are known to have therapeutic effects on DN [20]. These data suggested that MSC-derived exosomal miRNAs might act as key mediators in DN. However, the function of exosome-mediated miRNA transfer in DN is largely unknown.
Previous studies have revealed some miRNAs that play important roles in the progression of DN. For instance, miR-143-3p can mediate the development of DN by targeting TGF-β1 [21]. In addition, Wang F et al. revealed that miR-21 can attenuate the symptoms of DN [22]. Moreover, miR-125b has been shown to be downregulated in diabetic neuropathy (DNP) [23]. Reports have indicated that miR-125b is downregulated in the exosomes found in the urine of patients with diabetes and diabetic kidney disease [18]. In addition, exosomal miR-125b derived from cardiovascular endothelial cells can induce cell autophagy to inhibit the progression of myocardial infarction [24]. However, the effect of MSC-Exo-transferred miR-125b on DN remains unclear.
Tumour necrosis factor receptor-associated factor 6 (TRAF6) is considered a downstream modulator of IL-1β, which is involved in inflammation [25]. TRAF6 has been reported to act as a key mediator in DN [26, 27]. Moreover, in a study of DN-induced podocyte damage, it was found that inhibition of the Akt signalling pathway could lead to autophagy and inhibit cell apoptosis [28, 29]. In addition, it has been reported that TRAF6 regulates Akt signalling in prostate hyperplasia and promotes disease progression [30-32]. Based on this background, we conclude that the TRAF6 and Akt signalling pathways may play a crucial role in DN, and we speculate that TRAF6 affects the development of DN by regulating the Akt axis in DN.
In this study, we sought to confirm the biological function of MSC-Exos containing miR-125b in the progression of DN. We constructed an in vitro model of DN and added MSC-Exos containing miR-125b to detect changes in autophagy and apoptosis of the model cells. Our study confirmed that exosomes from MSCs expressing miR-125b significantly induced the autophagy and inhibited the apoptosis of HG-treated HKCs via mediation of the TRAF6/Akt axis. These findings will provide a new idea for DN treatment.
Bone marrow MSCs were extracted as previously described [33]. Total bone marrow cells were harvested from rats. The cells were then cultured in DMEM/F12 (HyClone, Logan, UT, USA) supplemented with 10% foetal bovine serum (FBS, Gibco, Carlsbad, CA, USA) at 37°C in 5% CO2.
Human embryonic kidney epithelial cells (HKCs) were purchased from ATCC (Manassas, VA, USA) and maintained in RPMI-1640 medium (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% FBS, 1% penicillin (Thermo Fisher Scientific) and 1% streptomycin (Thermo Fisher Scientific) at 37°C and 5% CO2. To establish an in vitro model of DN, HKCs were treated with high D-glucose (HG, Pepro Tech, Rocky Hill, NJ, USA) at a concentration of 35 mM for 48 h. Furthermore, mannitol, low glucose (LG) and lysosome inhibitor (Baf A1) were obtained from Pepro Tech (Rocky Hill, NJ, USA).
Tissue collectionFifteen pairs of DN tissues and adjacent normal tissues were collected from the Second Affiliated Hospital of Nanchang University between August 2019 and December 2019. The clinical and pathological data and tissues of these patients were collected with written informed consent. This research was approved by the Institutional Ethical Committee of the Second Affiliated Hospital of Nanchang University.
Construction of TRAF6 plasmid and cell transfectionThe TRAF6 overexpression plasmid TRAF6-pcDNA3.1 was constructed by inserting a cDNA clone of TRAF6 into the pcDNA3.1 vector. The TRAF6-pcDNA3.1 plasmid, miR-125b mimic/inhibitor or NC was directly added to the cells (at 50–60% confluence) and incubated for 24 h. The cells were transfected with Lipofectamine® 2000 (Thermo Fisher Scientific). Finally, the cells were selected with puromycin (2.5 μg/mL). The pcDNA3.1 vector, miR-125b mimic, miR-125b inhibitor and NC RNA were obtained from GenePharma (Shanghai, China).
Isolation of exosomesThe exosome pellet was placed on a carbon-coated copper grid, incubated for 5 min at 37°C, and then immersed in 2% phosphotungstic acid solution for 1 min. After washing with PBS, the preparations were captured using a transmission electron microscope (TEM; JEOL, Akishima, Japan) [34]. In addition, the efficiency of exosome isolation was further confirmed by western blotting with three exosome-specific molecular markers: TSG101, CD81 and CD63 (Abcam, Cambridge, UK).
HKCs co-cultured with MSC-ExosIn brief, 5 × 104 HKCs were seeded as previously described [35]. In addition, MSCs were first cultured for 48 h. Then, the cell supernatants were collected to isolate exosomes, which were placed in the lower chamber and cocultured with HKCs for 48 h.
Reverse transcription-quantitative polymerase chain reaction (qRT-PCR)Total RNA was extracted from HKCs or MSC-Exos using TRIzol reagent (TaKaRa, Tokyo, Japan). First-strand cDNA was synthesized by the PrimeScript RT reagent Kit (Takara). RT-qPCR was performed in an ABI7500 system using SYBR Green methods. RT-qPCR was performed in triplicate with the following protocol: 2 min at 95°C, followed by 35 cycles (30 s at 95°C and 45 s at 60°C). The specific primers used were miR-125b F: 5'-GGGGAACATTCAACGCTGT-3', R: 5'-CTCAACTGGTGTCGTGGAGTC-3'; U6, F: 5'-CTCGCTTCGGCAGCACAT-3', R: 5'-AACGCTTCACGAATTTGCGT-3'; TRAF6 F: 5'-GGGGAACATTCAACGCTGT-3', R: 5'-CTCAACTGGTGTCGTGGAGTC-3'; β-actin, F: 5'-CATCATCCCTGCCTCTACTGG-3', R: 5'-GTGGGTGTCGCTGTTGAAGTC-3'. The 2–ΔΔCt method was used to quantify the data.
Dual-luciferase reporter assayThe 3'-UTR of TRAF6 containing the putative binding sites of miR-125b was synthetized and obtained from Sangon Biotech (Shanghai, China) and then cloned into the pmirGLO Dual-Luciferase Expression Vector (Promega, Madison, WI, USA) to construct the wild-type or mutation-type reporter vector TRAF6 (WT/MT). TRAF6 (WT/MT) was transfected into HKCs together with the control, vector-control (NC) or miR-125b mimic using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s instructions. The relative luciferase activity was analysed by the Dual-Glo Luciferase Assay System (Promega).
Western-blot detectionTotal protein was isolated from HKCs by using RIPA buffer. The protein concentration was quantified by a BCA protein kit (Thermo Fisher Scientific). Then, the proteins were separated with SDS-PAGE gel (10%) and transferred to PVDF membranes. After that, the membranes were incubated with primary antibodies after blocking with 3% nonfat milk for 1 h. Subsequently, the membranes were incubated with secondary anti-rabbit antibodies (1:5,000) for 1 h. Finally, the membranes were visualized by an enhanced chemiluminescence (ECL) kit (Thermo Fisher Scientific) and scanned by using an Odyssey Imaging System, and the data were analysed with ImageJ software. Each experiment was repeated three times (Supplementary Fig. 1). The primary antibodies were as follows: anti-TRAF6 (ab33915, 1:2,000), anti-LC3 I (ab192890, 1:2,000), anti-LC3 II (ab63817, 1:1,000), anti-p62 (ab109012, 1:10,000), anti-Akt (ab8805, 1:1,000), anti-p-Akt (ab38449, 1:1,000) and anti-β-actin (ab8227, 1:1,000). β-actin was used as an internal control. All the antibodies were purchased from Abcam (Cambridge, UK).
Cell apoptosis analysisFlow cytometry was conducted using an Annexin V-FITC/PI apoptosis detection kit (MA0220, Dalian Meilun Biotech Co., Ltd., Dalian, China). HKCs (2 × 105 cell/mL) were subjected to a 5-min centrifugation at 500 g. The cells were resuspended in 195 μL binding buffer, incubated in the dark with 5 μL Annexin V-FITC for 10 min and 10 μL PI (20 μg/mL) for 5 min and detected by a flow cytometer.
Statistical analysisAll the experiments are expressed as the mean ± standard error (SD). RT-qPCR, flow cytometry and western blotting were repeated in triplicate. One-way analysis of variance (ANOVA) and Tukey’s tests were carried out for multiple group comparisons. P < 0.05 was considered statistically significant. In addition, regression analysis was performed to analyse the correlation.
To investigate the role of miR-125b and TRAF6 in DN, RT-qPCR was performed. As shown in Fig. 1A, the expression of miR-125b was significantly downregulated in DN tissues compared to normal tissues. In contrast, the level of TRAF6 in DN tissues was much higher than that in normal tissues (Fig. 1B). In addition, miR-125b was negatively correlated with TRAF6 (Fig. 1C). Taken together, miR-125b was downregulated in DN, while TRAF6 was upregulated.

MiR-125b was downregulated in DN tissues, while TRAF6 was upregulated. (A) The expression of miR-125b in DN tissues or adjacent normal tissues was detected by RT-qPCR. (B) The expression of TRAF6 in DN tissues or adjacent normal tissues was detected by RT-qPCR. (C) Regression analysis was performed to investigate the correlation between miR-125b and TRAF6. n = 15. * p < 0.05, ** p < 0.01.
To assess the function of MSC-Exos in DN, MSC-Exos were isolated. The exosomes were approximately 50–100 nm in diameter (Fig. 2A). In addition, exosomal markers (CD81, CD63 and TSG101) were highly expressed in the exosomes (Fig. 2B). Altogether, exosomes were stably isolated from MSCs and had high purity.

Exosomes were successfully isolated from MSCs. (A) Exosome pellets were placed on a carbon-coated copper grid, incubated for 5 min at 37°C, and then immersed in 2% phosphotungstic acid solution for 1 min. After washing with PBS, TEM micrographs were captured. (B) The expression of CD81, CD63, TSG101 and β-actin in MSC-Exos or MSC cell lysates was assessed by western blot. n = 3. * p < 0.05, ** p < 0.01, *** p < 0.001.
To detect gene expression, qRT-PCR was performed. As demonstrated in Fig. 3A, the expression of miR-125b in HKCs was downregulated by HG, while it was upregulated in MSC-Exos. In addition, the HG-induced decrease in miR-125b expression in HKCs was reversed when the HKCs were cocultured with MSC-Exos (Fig. 3A). However, mannitol or LG had a very limited effect on miR-125b expression (Fig. 3A). Moreover, the expression of miR-125b in HKCs was notably upregulated in the presence of MSC-Exos, while this phenomenon was obviously reversed by GW4869 (exosome inhibitor) (Fig. 3B). In addition, HG notably decreased LC3 II/LC3 I expression in HKCs (Fig. 3C). In contrast, the p62 level in HKCs was upregulated by HG (Fig. 3C). However, the effect of HG on these proteins was partially rescued by MSC-Exos, and the effect of MSC-Exos was also reversed by Baf A1 (Fig. 3C). Furthermore, HG-induced HKCs apoptosis was significantly inhibited when the HKCs were cocultured with MSC-Exos, while LG or mannitol limited cell apoptosis (Fig. 3D). On the other hand, the miR-125b levels in MSC-Exos were greatly reduced when the cells were incubated with the miR-125b inhibitor (Fig. 3E). When HG-treated HKCs were cocultured with MSC-Exos with reduced miR-125b levels, the miR-125b levels in HKCs did not increase (Fig. 3F). Furthermore, inhibiting miR-125b did not change the autophagy and apoptosis of HKCs (Fig. 3G–3H). Taken together, MSC-Exos induced autophagy and inhibited apoptosis in HKCs treated with HG by transferring miR-125b.

MSC-Exos inhibited HKCs apoptosis by inducing autophagy. (A) HG-treated HKCs were cocultured with MSC-Exos. MiR-125b expression in HKCs was measured by qRT-PCR. (B) MSCs were treated with GW4869 and cocultured with HG-treated HKCs. Then, the miR-125b levels in the HKCs were detected by qRT-PCR. (C) The LC3 I, LC3 II and p62 levels in HKCs were measured by western blot. The relative expression levels were quantified by normalizing to β-actin. (D) Flow cytometry was performed to test cell apoptosis. (E) MSCs were transfected with NC or miR-125b inhibitor. Exosomes were derived from MSCs. Then, miR-125b levels in MSC-Exos were analysed by qRT-PCR. (F) MSCs were transfected with the miR-125b inhibitor, and exosomes were derived from the MSCs. Then, HG-induced HKCs were cocultured with MSC-Exos. MiR-125b expression in HKCs was detected by qRT-PCR. (G) LC3 I, LC3 II and p62 expression in HKCs was measured by western blot. The relative levels were quantified by normalization to β-actin. (H) Cell apoptosis was examined by flow cytometry. n = 3. * p < 0.05, ** p < 0.01, *** p < 0.001.
To detect the expression of TRAF6 in HKCs, as shown in Fig. 4A and 4B, TRAF6 expression in HG-treated HKCs was dramatically downregulated in the presence of MSC-Exos. These results were partially rescued by miR-125b inhibitors. Additionally, online prediction by TargetScan 7.2 found that the 3'UTR of TRAF6 contained a direct binding site for miR-125b (Fig. 4C). Moreover, decreased luciferase activity was present in HKCs following cotransfection with wt-TRAF6 and miR-125b mimic, and the luciferase activity increased after cotransfection with miR-125b inhibitor. However, when the miR-125b mimic or inhibitor was cotransfected with mut-TRAF6, the luciferase activity in HKCs remained unchanged. (Fig. 4D). In addition, the expression of TRAF6 was increased in HKCs after overexpression of TRAF6 and coculture with MSC-Exos (Fig. 4E and 4F). After overexpressing TRAF6 in HKCs, autophagy in HKCs was significantly decreased (Fig. 4G). In addition, the antiapoptotic effect of MSC-Exos on HG-treated HKCs was partially reversed when the cells were transfected with the TRAF6 overexpression vector (Fig. 4H). In summary, miR-125b from MSC-Exos reversed the HG-induced autophagy and apoptosis of HKCs by targeting TRAF6.
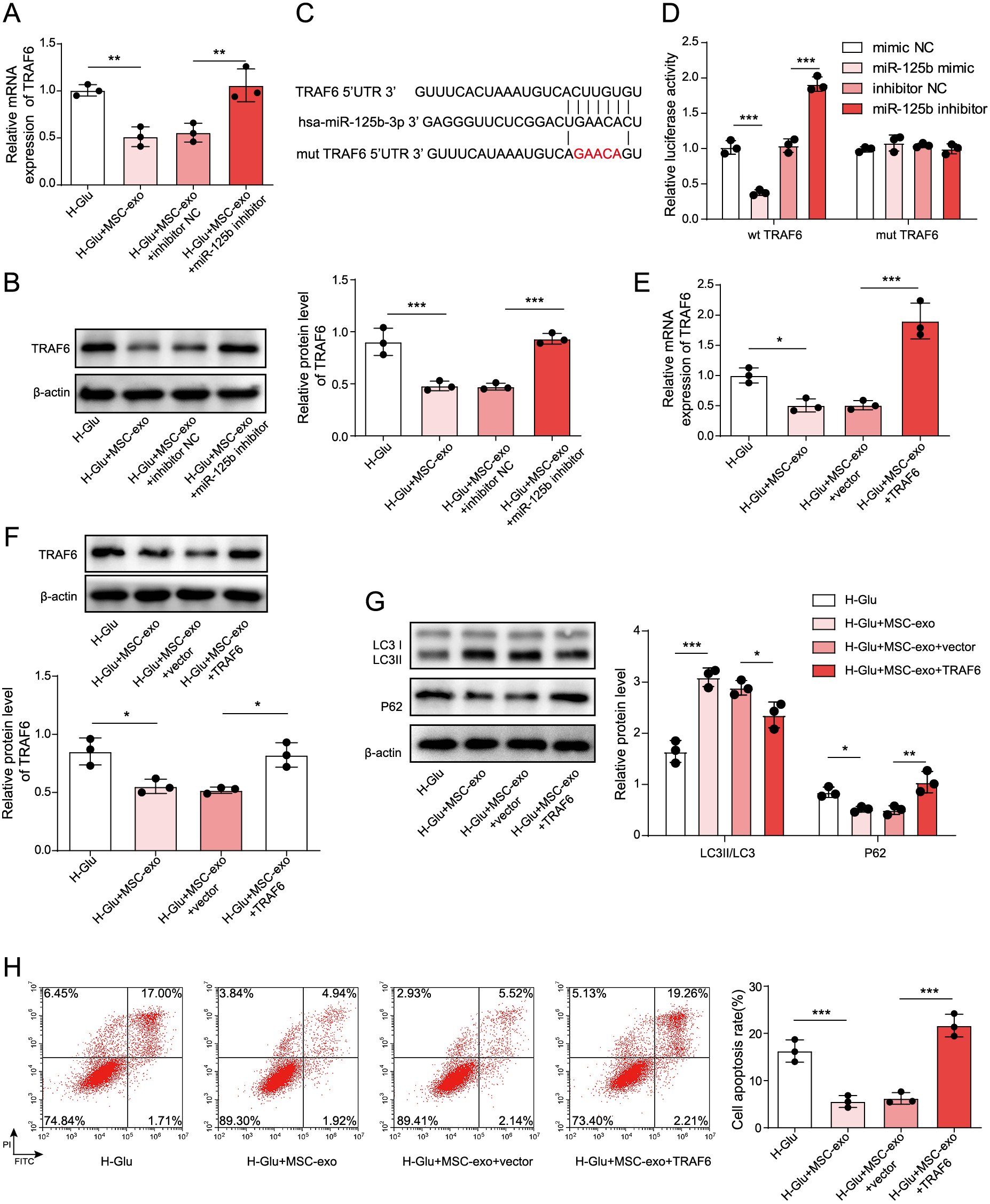
MiR-125b directly targeted TRAF6 in HKCs. (A) The expression of TRAF6 in HKCs was detected by qRT-PCR. (B) TRAF6 expression in HKCs was measured by western blot. The data were quantified by normalizing to β-actin. (C) The gene structure of TRAF6 revealed a target site of miR-125b in its 3'UTR. (D) A dual-luciferase reporter assay was used to confirm the correlation between miR-125b and TRAF6. (E) The TRAF6 levels in HKCs were measured by qRT-PCR. (F) TRAF6 expression in HKCs was detected by western blot. The data were quantified by normalizing to β-actin. (G) LC3 I, LC3 II and p62 expression in HKCs was measured by western blot. The data were quantified by normalization to β-actin. (H) Cell apoptosis was examined by flow cytometry. n = 3. * p < 0.05, ** p < 0.01, *** p < 0.001.
To explore the mechanism by which MSC-Exos mediated the development of DN, we conducted further investigations. As expected, the levels of phosphorylated Akt in HG-treated HKCs were significantly downregulated by MSC-Exos, while TRAF6 overexpression reversed the effect of MSC-Exos on the phosphorylation of Akt (Fig. 5A). In HG-induced HKCs cocultured with MSC-Exos, the addition of MK-2206 (Akt inhibitor) had a very limited effect on TRAF6 and miR-125b expression (Fig. 5B). However, inhibiting miR-125b significantly increased the phosphorylation of Akt in HKCs treated wtih HG, while this phenomenon was reversed by MK-2206 (Fig. 5C). Additionally, MK-2206 significantly reversed the autophagy inhibitory effect induced by the downregulation of miR-125b (Fig. 5D). In contrast, the downregulation of miR-125b notably induced the apoptosis of HG-induced HKCs cocultured with MSC-Exos, while the effect of miR-125b knockdown on apoptosis was reversed in the presence of MK-2206 (Fig. 5E). In summary, exosomes from MSCs expressing miR-125b suppressed the development of DN via regulation of the TRAF6/Akt axis.

Exosomes from MSCs expressing miR-125b suppressed the development of DN via regulation of the TRAF6/Akt axis. (A) The Akt and p-Akt levels in HKCs were measured by western blot. (B) The expression of TRAF6 and miR-125b in HKCs was detected by qRT-PCR. (C) Western blotting was used to measure the TRAF6 levels in HKCs, and β-actin was used for normalization. The expression of Akt and its phosphorylation level were examined by western blotting. (D) The LC3 I, LC3 II and p62 levels in HKCs were assessed by western blot. The data were quantified by normalization to β-actin. (E) Cell apoptosis was examined by flow cytometry. n = 3. * p < 0.05, ** p < 0.01, *** p < 0.001.
The correlation between HG and miR-125b has been reported. For example, dexmedetomidine could protect neurons from HG-induced injury by inhibiting miR-125b [36]. In addition, knockdown of MALAT1 inhibited the proliferation, migration, tube formation and vascular permeability of hRMECs induced by HG through upregulation of miR-125b [23]. Thus, HG might play a crucial role in the regulation of miR-125b. Thus, the underlying mechanism remains to be further explored.
It has been previously reported that MSC-Exos are involved in multiple diseases [37-39]. In the current research, we found that MSC-Exos inhibited the progression of DN by inducing autophagy. In addition, miR-125b carried by MSC-Exos suppressed the progression of DN via the TRAF6/Akt axis. Our findings increased our understanding of the function of MSC-Exos in DN, suggesting that MSC-Exos could useful in the study of DN. It has been confirmed that autophagy participates in the progression of DN [40, 41]. The induction of autophagy might inhibit the development of DN [42, 43]. Moreover, exosomes are known to regulate the progression of inflammation induced by DN [16, 44]. Our data were similar to these previous reports. However, the correlation between exosomes and autophagy remains largely unknown. Our research confirmed the relationship between MSC-Exos and autophagy in DN, suggesting that MSC-Exos could be developed as autophagy activators.
The relationship between exosomes and miRNAs was verified. For example, Cai X et al. exosome-transmitted microRNA-133b suppressed bladder cancer cell proliferation by activating dual-specificity protein phosphatase 1 [14]. In addition, exosomal miR-146a derived from MSCs could enhance the sensitivity of ovarian cancer cells to chemotherapy via PI3K/Akt signalling [34]. On the other hand, miRNAs have been confirmed to play key roles in DN [45, 46]. Moreover, MSC-Exos delivering miR-125b could induce autophagy to attenuate the symptoms of myocardial infarction [24]. Consistently, our study revealed that miR-125b from MSC-Exos could induce autophagy to inhibit HG-induced HKCs apoptosis via Akt signalling, increasing our understanding of the mechanism by which miR-125b mediates the progression of DN.
TRAF6 is known to play key roles in cell growth [47-49]. Furthermore, it has been verified that TRAF6 could induce the progression of DN [50, 51]. Moreover, it has been previously reported that TRAF6 could modulate Akt signalling in tumours and inflammation [52, 53]. In addition, inactivation of TRAF6 might lead to cell autophagy [54]. Our results were similar to these previous data. The function of TRAF6 in cell growth may result in a similar phenomenon between these previous studies and our findings. Taken together, our study first suggested that MSC-Exos inhibited the progression of DN via regulation of the TRAF6/Akt axis. On the other hand, it has been revealed that HG could inactivate the Akt pathway in podocytes [55]. In addition, PC-12 cells treated with HG exhibited suppressed expression of Akt [56]. Therefore, HG could act as an inhibitor of the Akt pathway, and the potential mechanism by which HG mediates the Akt pathway needs to be explored in the future.
In summary, MSC-Exos delivering miR-125b inhibited the progression of DN via TRAF6/Akt signalling. Our findings provide a new strategy for the treatment of DN.
We would like to give our sincere gratitude to the reviewers for their constructive comments.
These authors declared no competing interests in this research.
Not Applicable.
Ethical approvalNot Applicable. This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publicationNot Applicable.
Availability of data and materialAll data generated or analyzed during this study are included in this article. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributionConception and study design: Xia Cai;
Data acquisition: Fang Zou;
Data analysis: Rui Xuan;
Manuscript drafting: Xia Cai;
Manuscript revising: Xiao-Yang Lai

western blot image three times (A) Fig. 2B western blot image. (B) Fig. 3C western blot image. (C) Fig. 3G western blot image. (D) Fig. 4B western blot image. (E) Fig. 4G western blot image. (F) Fig. 4F western blot image. (G) Fig. 5A western blot image. (H) Fig. 5C western blot image. (I) Fig. 5D western blot image.