2021 Volume 68 Issue 7 Pages 849-856
2021 Volume 68 Issue 7 Pages 849-856
At the current time of rising demand for hospital beds, it is important to triage COVID-19 patients according to the treatment needed during hospitalization. The need for oxygen therapy is an important factor determining hospital admission of these patients. Our retrospective study was designed to identify risk factors associated with the progression to oxygen requirement in COVID-19 patients. A total of 133 patients with laboratory-confirmed COVID-19 were admitted to our hospital from February 22, 2020, to August 23. After excluding asymptomatic, non-Japanese, pediatric, pregnant patients and also those who needed oxygen immediately at admission, data of the remaining 84 patients were analyzed. The patients were separated into those who required oxygen after admission and those who did not, and their characteristics were compared. Age, body mass index (BMI), lymphocyte count, C-reactive protein (CRP), lactate dehydrogenase, estimated glomerular filtration rate, glucose intolerance, hypertension, and dyslipidemia were significantly different between the two groups. Multivariate analysis identified four significant and independent risk factors of oxygen requirement, including advanced age, obesity, glucose intolerance and lymphocytopenia. Dividing the patients into subgroups according to the number of these risk factors found in each patient indicated that the need for oxygen increased with higher number of these risk factors in the same individual. Our results suggest that the presence of higher number of these risk factors in COVID-19 patients is associated with future oxygen requirement and that this index can be potentially useful in triaging COVID-19 patients staying home in the context of need for hospitalization.
CORONAVIRUS DISEASE 2019 (COVID-19), caused by a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported in Wuhan, China, in December 2019 [1]. Since then, it has rapidly spread to result in worldwide pandemic. In Japan, the first confirmed case of SARS-CoV-2 infection was recorded on January 16, 2020 [2]. A few weeks later, in February, many of the passengers and crew members of the cruise liner “Diamond Princess”, which docked at the Port of Yokohama, were found to be infected, with the number of infected individuals reaching 712, 13 of whom had since died [3]. Then, the first wave of infection spread across Japan from the end of March and the Japanese Government declared a state of emergency on April 7 and asked to refrain from any nonessential and non-urgent outings throughout the country. The first wave was declared to be under control in mid of May and was followed by cancellation of the emergency state on May 21. However, a second wave of infection occurred at the beginning of July and the number of new confirmed cases per day reached a peak on August 7 [4]. The second wave settled at the end of September, but the number of newly infected people started to increase gradually again from the start of October, then steeply from November, reaching 2,595/day on November 21 [4], which is the highest number per day at the time of writing of this article. It can be said that Japan is in the midst of the third wave of infection at present and there is fear that the increasing number of new cases will overwhelm the public and private hospital system soon.
Our hospital is a medium-size clinical facility with 613 beds, including 14 in the Infectious Disease Unit, in the urban area of Osaka Prefecture, Japan [5]. It is also one of 351 designated medical institutions for type II infectious diseases in Japan, with a total of 1,758 beds. Designated medical institutions for type II infectious diseases are accepting the patients with type II infectious disease (poliomyelitis, tuberculosis, diphtheria, avian influenza, SARS and MERS). We have accepted patients with COVID-19 since February 2020 and expanded the number of beds for COVID-19 patients from 14 to 45 [5]. In March 30, 2020, Osaka Prefecture established the Osaka Prefecture Inpatient Follow-up Center to coordinate broad-based hospitalization based on patients’ symptoms. The center triaged patients with confirmed COVID-19 into four stages depending on the disease severity: (1) critical illness in need of mechanical ventilation or Intensive Care Unit (ICU) admission, (2) moderate diseases who have difficulty of breathing or pneumonia in image, and will need some medical treatment or oxygen, (3) mild diseases who have respiratory symptoms such as cough or fever but will not need hospitalization, (4) asymptomatic pathogen carriers [5]. Patients with critical illness are hospitalized at designated medical institutions for severe infectious diseases, university hospitals, or the National Hospital Organization; patients with moderate diseases are hospitalized in general hospitals (negative pressure room or special ward); patients with mild diseases or asymptomatic pathogen carriers are generally isolated in designated hotel rooms or at home, and hospitalized in some conditions. Our hospital is a designated general hospital for the treatment of mild to moderate patients. We provided basic supportive care including supplemental oxygen in necessary. In addition, we used some COVID-19 specific medications, including ciclesonide, hydroxychloroquine, favipiravir and betamethasone [5]. Patients who progressed to critical stage and required mechanical ventilation were moved to the hospitals accepting patients with critical illness. While about 10% of our patients progressed to a critical stage, about half of our patients did not need oxygen inhalation during hospitalization.
When there is a rapidly increasing demand for hospital beds, it is important to triage COVID-19 patients staying at home or hotel rooms according to the potential need for in-hospital treatment, which mainly depends on the patients’ requirement for oxygen supplementation. The aim of the present retrospective study was to identify independent risk factors that could predict the progression to oxygen requirement in mild to moderate COVID-19 patients.
This study was a retrospective single-center cohort study that examined a total of 133 consecutive patients with laboratory-confirmed COVID-19 from February 22 to August 23, 2020. These patients were requested to be admitted to our hospital principally from their home, hotel rooms, or geriatric health services facility by Inpatient Follow-up Center or public health centers. In the present study, various RT-PCR assays were used, but all findings were based on the results of tests by public health centers in the Osaka area in Japan. After excluding asymptomatic, non-Japanese, pediatric, pregnant patients and also those who needed oxygen immediately at admission or before admission, data of the remaining 84 patients were analyzed. Our hospital had accepted 11 asymptomatic pathogen carriers exceptionally for considering their cohabitants’ conditions and we excluded them from the study subjects. We also excluded non-Japanese patients because the disease progression or the physique (or definition of obesity) might be different by ethnicity. We reviewed the medical records and examined the clinical factors associated with the progression to oxygen requirement state in patients with mild to moderate COVID-19.
Clinical and laboratory data were collected in all patients at hospital admission. All patients were admitted to our Infectious Disease Unit. Their height and body weight were measured by medical staff or obtained from their medical interview. The present study was conducted in accordance with the Declaration of Helsinki, and the Institutional Review Board of Toyonaka Municipal Hospital approval the study (#2020-05-12). The requirement for informed consent was waived through the opt-out method on our hospital website.
When admitted to our hospital, the patient was provided with basic support care, plus treatment with acetaminophen, antibiotics, oxygen and mechanical ventilation when indicated. With regard to COVID-19-specific medications, we used ciclesonide for patients with lung infiltrates on imaging, hydroxychloroquine for some patients with high fever (>38°C) or diarrhea, favipiravir for patients with moderate pneumonia, and betamethasone for patients with oxygen requirement.
Obesity was defined as body mass index (BMI) ≥25 kg/m2 [6]. We defined glucose intolerance as “random” glucose ≥7.8 mmol/L (≥140 mg/dL) or HbA1c(NGSP) ≥38 mmol/mol (≥5.7%) or treatment with glucose-lowering agents [7]. Diabetes was defined as being diagnosed before or “random” glucose ≥11.1 mmol/L (≥200 mg/dL) or HbA1c(NGSP) ≥43 mmol/mol (≥6.5%). Dyslipidemia represented HDL cholesterol <40 mg/dL, non-HDL cholesterol ≥150 mg/dL or treatment with drugs for dyslipidemia [8]. Hypertension was defined as treatment with anti-hypertensive medications or being diagnosed before. Moreover, oxygen was administered through the nostrils when peripheral arterial oxygen saturation at room air breathing was <93% continuously.
Categorical variables and continuous variables were presented as counts and mean ± SD, respectively. Categorical variables were compared using the χ2 test or Fischer exact test, if the cell counts were small. Continuous variables were compared using the Student t-test. Binomial (multiple) logistic regression analysis was used to identify independent factors associated with the oxygen requirement, using odds ratios (ORs) and 95% CIs. All reported p values were two-sided, and p < 0.05 was considered significant. All statistical analyses were performed using the JMP statistical software (ver. 14.3, SAS Institute, Inc., Cary, NC) or ‘EZR (Easy R)’ (R × 64 3.2.2) [9].
All COVID-19 consecutive patients hospitalized at Toyonaka Municipal Hospital between February 22 and August 23, 2020, were enrolled in this study. They included 133 patients with laboratory-confirmed COVID-19. Among these patients, we excluded 11 asymptomatic patients, 5 readmitted patients, 3 patients transferred from another hospital, 5 non-Japanese patients, 3 pregnant patients, 1 patient using Home Oxygen Therapy, and 2 patients younger than 16 years of age. We also excluded 19 patients who needed and received oxygen before admission or immediately at admission before laboratory sampling. Fig. 1 shows a flow chart of patient enrollment. By the end of the follow-up period, all patients had been discharged, and we ultimately enrolled 84 patients with newly diagnosed, laboratory-confirmed, mild to moderate COVID-19 without oxygen therapy at admission (Fig. 1).
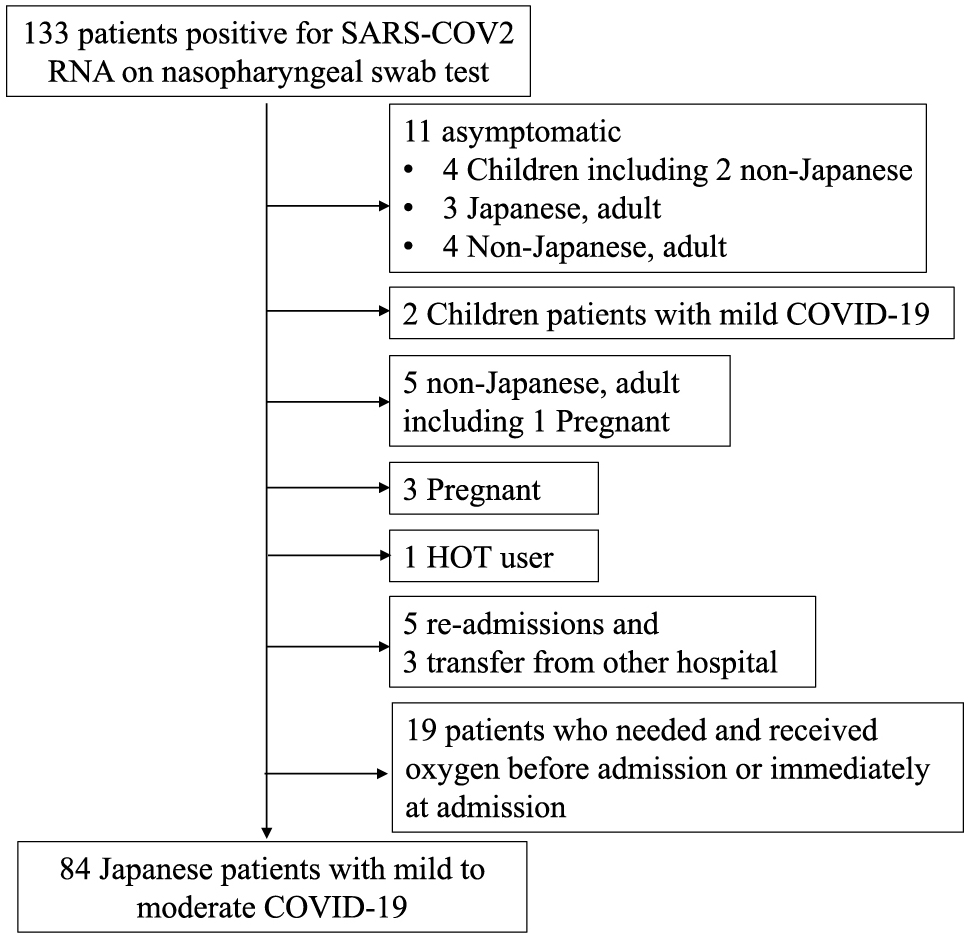
A flow chart of patient enrollment
133 patients with laboratory-confirmed COVID-19 were hospitalized at Toyonaka Municipal Hospital between February 22 and August 23, 2020. Among these patients, we excluded 11 asymptomatic patients, 5 readmitted patients, 3 patients transferred from another hospital, 5 non-Japanese patients, 3 pregnant patients, 1 patient using Home Oxygen Therapy, and 2 patients younger than 16 years of age. We also excluded 19 patients who needed and received oxygen before admission or immediately at admission before laboratory sampling.
HOT: Home Oxygen Therapy
We identified 30 patients who progressed to oxygen requirement state after admission and 8 of these later progressed to critical illness that required mechanical ventilation. The 8 critically ill patients were then transferred to other hospitals which could respond to the clinical management of critically ill patients and 2 of them subsequently died. The clinical characteristics of the patients are summarized in Table 1. The mean (±SD) age of the patients was 52.9 ± 19.4 years (range, 19–90) and 53.6% were men. The mean BMI was 24.2 ± 5.1 kg/m2 (range, 14.2–46.1) and 62.2% were non-obese (BMI <25) while 37.8% were obese (BMI ≥25). When the frequency of obese subjects was calculated by gender, it was 42.2% in males and 32.4% in females, both of which were higher than those in the Japanese general population [10].
| n | 84 | ||
| Age (years) | 52.9 ± 19.4 | (19–90) | |
| Sex (males/females) | 45/39 | ||
| Body weight (kg) | 65.6 ± 17.1 | (29.1–130.0) | (83) |
| Body mass index, BMI (kg/m2) | 24.2 ± 5.1 | (14.2–46.1) | (82) |
| Obesity (yes/no) | 31/51 | ||
| Leukocyte count (/μL) | 5,855 ± 2,657 | (1,800–17,000) | (76) |
| Neutrophil count (/μL) | 4,268 ± 2,668 | (747–15,640) | (71) |
| Lymphocyte count (/μL) | 1,227 ± 565 | (373–2,883) | (71) |
| CRP (mg/dL) | 3.87 ± 5.09 | (0.02–22.3) | (75) |
| AST (IU/L) | 35.2 ± 19.8 | (13–122) | (76) |
| ALT (IU/L) | 35.4 ± 29.2 | (9–183) | (76) |
| LDH (IU/L) | 272 ± 109 | (123–638) | (73) |
| Cre (mg/dL) | 1.4 ± 2.2 | (0.4–12.2) | (76) |
| eGFR (mL/min/1.73 m2) | 70.6 ± 29.2 | (2.9–149.8) | (76) |
| Hemoglobin A1c, HbA1c (%) | 6.6 ± 1.8 | (5.2–13.5) | (44) |
| Casual serum glucose (mg/dL) | 131.4 ± 61.0 | (73–410) | (72) |
| Glucose intolerance (yes/no) | 40/44 | ||
| Diabetes (yes/no) | 24/60 | ||
| Total cholesterol (mg/dL) | 171.1 ± 53.6 | (100–395) | (48) |
| High-density lipoprotein-cholesterol (mg/dL) | 45.9 ± 13.5 | (27–82) | (44) |
| Non-high-density lipoprotein-cholesterol (mg/dL) | 127.6 ± 55.3 | (73–351) | (43) |
| Dyslipidemia (yes/no) | 36/48 | ||
| Statin use (yes/no) | 16/58 | ||
| Hypertension (yes/no) | 27/56 | ||
| ARB or ACEi use (yes/no) | 15/59 |
Data are mean ± SD (range). Numbers in the parenthesis (n) are number of tested subjects. CRP, C-reactive protein; AST, aspartate aminotransferase; ALT, alanine transaminase; LDH, lactate dehydrogenase; Cre, creatine; eGFR, estimated glomerular filtration rate; ARB, angiotensin II receptor blocker; ACEi, angiotensin converting enzyme inhibitor.
Next, we compared various clinical factors between the oxygen requirement group and the no requirement group. Age (p < 0.001), BMI (p = 0.019), lymphocyte count (p = 0.001), serum C-reactive protein (CRP) (p < 0.001), lactate dehydrogenase (LDH) (p = 0.002), estimated glomerular filtration rate (eGFR) (p < 0.001), glucose intolerance (p < 0.001), diabetes (p = 0.002), hypertension (p = 0.003), and dyslipidemia (p < 0.001) were significantly different between the two groups (Table 2).
| n | oxygen requirement group (n = 30) | no requirement group (n = 54) | p-value | |
|---|---|---|---|---|
| Age (years) | 84 | 64.7 ± 14.3 | 46.4 ± 18.8 | <0.001 |
| Advanced age (more/less than 65) | 84 | 16/14 | 10/44 | 0.001 |
| Sex (males/females) | 84 | 19/11 | 26/28 | 0.254 |
| Body mass index, BMI (kg/m2) | 82 | 25.9 ± 3.9 | 23.2 ± 5.5 | 0.019 |
| Obesity (yes/no) | 82 | 17/13 | 14/38 | 0.010 |
| Leukocyte count (/μL) | 76 | 6,193 ± 2,510 | 5,647 ± 2,749 | 0.387 |
| Neutrophil count (/μL) | 71 | 4,784 ± 2,611 | 3,933 ± 2,681 | 0.191 |
| Lymphocyte count (/μL) | 71 | 947 ± 445 | 1,409 ± 563 | <0.001 |
| CRP (mg/dL) | 75 | 6.8 ± 6.4 | 2.0 ± 2.9 | <0.001 |
| AST (IU/L) | 76 | 38.6 ± 18.7 | 33.1 ± 20.3 | 0.246 |
| LDH (IU/L) | 73 | 321 ± 122 | 241 ± 88 | 0.002 |
| eGFR (mL/min/1.73 m2) | 76 | 56.6 ± 24.0 | 79.3 ± 29.0 | <0.001 |
| Glucose intolerance (yes/no) | 84 | 23/7 | 17/37 | <0.001 |
| Diabetes (yes/no) | 84 | 15/15 | 9/45 | 0.002 |
| Hypertension (yes/no) | 83 | 16/14 | 11/42 | 0.003 |
| Dyslipidemia (yes/no) | 84 | 21/9 | 15/39 | <0.001 |
n, number of available data; CRP, C-reactive protein; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; eGFR, estimated glomerular filtration rate.
These parameters were entered into binominal logistic regression analysis (Table 3). The continuous variables (age, BMI, CRP, lymphocyte count, LDH, and eGFR) were converted to categorical ones described as before [5]. Since age is considered to be the most important risk factor for severe disease in COVID-19 [11] and correlates with some of the other factors, the same analysis was repeated after adjustment for age and gender. The results identified BMI, low lymphocyte count, glucose intolerance, and dyslipidemia as significant risk factors for oxygen requirement (Table 3). In addition, we also performed the same analysis after adjustment for age, gender, and BMI, since BMI is known to correlate with glucose intolerance and dyslipidemia. The last identified low lymphocyte count, and glucose intolerance as significant risk factors for oxygen requirement (Table 3).
| No adjustment | Adjusted for gender and age | Adjusted for gender, age and BMI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p | |
| Gender (male: 1, female: 0) | 1.860 | 0.745–4.640 | 0.183 | — | — | — | — | — | — |
| Age (1 = ≥65/0 = <65) | 5.030 | 1.860–13.600 | 0.001 | — | — | — | — | — | — |
| Obesity (1 = BMI ≥25 kg/m2/0 = BMI <25) |
3.550 | 1.380–9.150 | 0.009 | 7.330 | 2.000–26.900 | 0.003 | — | — | — |
| CRP (1 = ≥3/0 = <3) | 4.640 | 1.710–12.600 | 0.003 | 2.380 | 0.776–7.300 | 0.130 | — | — | — |
| Lymphocytopenia (1 = <1,000/0 = ≥1,000) |
4.650 | 1.680–12.900 | 0.003 | 3.180 | 1.020–9.930 | 0.047 | 8.320 | 1.950–36.600 | 0.004 |
| LDH (1 = ≥300/0 = <300) | 2.260 | 0.805–6.370 | 0.121 | — | — | — | — | — | — |
| eGFR (0 = <30/1 = 30~60/2 = ≥60) | 0.370 | 0.173–0.793 | 0.011 | 0.570 | 0.252–1.290 | 0.177 | — | — | — |
| Hypertension (1 = yes/0 = no) | 4.360 | 1.640–11.600 | 0.003 | 1.770 | 0.571–5.510 | 0.322 | — | — | — |
| Glucose intolerance (1 = yes/0 = no) | 7.150 | 2.570–19.900 | <0.001 | 4.520 | 1.480–13.800 | 0.008 | 3.810 | 1.140–12.700 | 0.030 |
| Dyslipidemia (1 = yes/0 = no) | 6.070 | 2.270–16.200 | <0.001 | 4.300 | 1.450–12.800 | 0.009 | 2.330 | 0.702–7.750 | 0.167 |
BMI, body mass index; CRP, C-reactive protein; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; eGFR, estimated glomerular filtration rate.
In the next step, we used multiple logistic regression analysis to identify independent risk factors among the above significant factors including advanced age and obesity. As shown in Table 4, advanced age, obesity, lymphocytopenia, and glucose intolerance were independent and significant risk factors for oxygen requirement also in multiple logistic regression analysis. The odds ratios listed in Table 4 confirmed the significance of each variable. Based on this result, we found 71 patients who had no missing data and showed, none, one, two, three or four of these variables. They were categorized into five subgroups according to the number of these risk factors in each patient. As shown in Table 5, the proportion of patients requiring oxygen increased according to the number of these variables, with none requiring oxygen in those having none of these variables, to 100% of the patients who met the conditions set for all the variables.
| OR | 95%CI | p | |
|---|---|---|---|
| Advanced age (1 = ≥65 years/0 = <65) | 8.80 | 1.62–47.80 | 0.012 |
| Obesity (1 = BMI ≥25 kg/m2/0 = BMI <25) | 26.0 | 3.54–190.00 | 0.001 |
| Lymphocytopenia (1 = <1,000/μL/0 = ≥1,000) | 29.4 | 4.08–212.00 | <0.001 |
| Glucose intolerance (1 = yes/0 = no) | 7.46 | 1.46–38.00 | 0.016 |
BMI, body mass index.
| Number of risk factors | patients who required oxygen | Frequency of patients who required oxygen | p | |
|---|---|---|---|---|
| yes | no | |||
| 0 | 0 | 12 | 0% | <0.001 |
| 1 | 2 | 16 | 11% | |
| 2 | 11 | 13 | 46% | |
| 3 | 14 | 1 | 93% | |
| 4 | 1 | 0 | 100% | |
At the current time of rising demand for hospital beds, it is important to triage COVID-19 patients staying at home or hotel rooms according to the type of treatment needed during hospitalization. Because the need for oxygen therapy is an important factor determining hospital admission of these patients, we tried to find which factors would be independent risk factors of oxygen requirement in the patients with mild to moderate COVID-19.
The two main findings of this study were that advanced age, obesity, glucose intolerance, and lymphocytopenia are independent risk host factors for oxygen requirement in Japanese COVID-19 patients. The second finding was that patients who were ≥65 years-of-age and were obese and glucose intolerant with lymphocytopenia were the most who needed oxygen, and in broader terms, the need for oxygen increased with the presence of larger number of these risk factors.
To date, various clinical factors and laboratory findings have been reported to be associated with progression to severe illness or mortality in COVID-19. Advanced age seems to be one such factor as confirmed in many studies worldwide [11]. In fact, in Japan also, the frequency of severe stage was reported to be about 8.5% in confirmed COVID-19 older than 60’s but only about 0.3% younger than 50’s [12]. The reason for old age vulnerability remains unclear at present.
Obesity has also been reported to be associated with increase odds of progressing to severe COVID-19. Cai et al. [13] reported that those patients who were overweight (BMI, 24.0–27.9 kg/m2) were at 1.84-fold (95% CI, 0.99–3.43) odds, while those who were obese (BMI, ~28.0 kg/m2) 3.40-fold (95%CI, 1.40–8.26) of developing severe COVID-19, after adjustment for various factors. The odds values estimated in our study seem to be larger than their results because the study outcome is different. Obese patients might need to allocate a disproportionately high percentage of total body oxygen consumption to the infected lung, and reduce functional residual capacity and expiratory volume [14]. In addition, obese people are at increased risk of developing pulmonary emboli or aspiration pneumonia [15] and such additional pathophysiologies could aggravate pneumonia in COVID-19, leading to severe illness. However, the exact mechanism(s) of the correlation between obesity and disease progression remains unclear.
Diabetes mellitus was reported to be another risk factor for disease severity or mortality in COVID-19. Hyperglycemia at hospital admission, even in patients with undiagnosed diabetes, has also been reported to be a predictor of poor clinical outcome and mortality [16]. Thus, in this study, we defined glucose intolerance as casual plasma glucose ≥7.8 mmol/L (≥140 mg/dL) or HbA1c(NGSP) ≥38 mmol/mol (≥5.7%) including pre-diabetes [7, 16] or treatment with glucose-lowering agents at admission and analyzed with this factor. The definition included known diabetes and hyperglycemia as well as undiagnosed diabetes. Hypertension was not significant after adjustment for age and gender. Furthermore, dyslipidemia also proved not to be a significant risk factor after adjustment for BMI, in addition to age and gender. However, glucose intolerance was determined as a significant risk factor even after adjustment for age, gender, and BMI. This result seemed to be compatible with the recent report [17]. Among the obesity-related metabolic disorders, only glucose intolerance seemed to be an independent risk factor from obesity for COVID-19 disease progression.
To date, several laboratory findings have also been reported to be associated with worse outcome; markers of inflammation (e.g., low lymphocyte count), liver damage (e.g., AST and LDH), and kidney dysfunction (e.g., eGFR) [5]. However, in the present study, only a single marker of inflammation; low lymphocyte count, was found to be a significant independent risk factor. In this study, we identified four significant and independent risk factors (advanced age, obesity, glucose intolerance, and low lymphocyte count) that might predict oxygen requirement. Furthermore, we divided the patients into subgroups according to the presence of none to all these four risk factors and found that the need for oxygen is more likely to include patients with higher number of these risk factors. This finding suggests that the presence of large number of risk factors increases the likelihood of need for use of oxygen in COVID-19 patients and that this scheme can be potentially useful in triaging patients staying at home or hotel rooms. Actually, these four factors are primarily measurable factors and suitable for triaging.
These risk factors have already been reported in previous studies from Western countries and China [11, 13, 18, 19], but most of them were identified as risk factors for mechanical ventilation or critically illness. Also in Japan, there has been some reports which suggest that BMI, diabetes, older age, and lymphocytopenia can be risk factors for severe COVID-19 [20, 21], but their analyses were not multivariate and did not show whether they were independent or not. Our study, for the first time, clarified independent risk factors for oxygen requirement in Japanese patients with mild to moderate COVID-19.
Our study has certain limitations due to its retrospective nature. First, our patients have already been triaged for admission by the Osaka Prefecture Inpatient Follow-up Center considering their age, fever, difficulty of breathing, pneumonia, pre-existing disease, cohabitants’ conditions and also hospital beds at each time. Thus, they are more likely to have poor prognosis among COVID-19 patients. However, when the patients were restricted to those whose peripheral arterial oxygen saturation at admission was 95% or more, we obtained similar results to those in Table 5 (data not shown). Thus, we believe that the number of our identified risk factors in individuals could also predict oxygen requirement even in the milder COVID-19 patients. Second, this study included a relatively small number of patients and also missing data were encountered at least in some. In addition, we obtained information of height and body weight through interview in some patients. This was because we were not very accustomed to seeing COVID-19 patients and avoided unnecessary or urgent contact with the patients to reduce the risk of infection. Further prospective studies of larger cohorts in patients staying at home or hotel rooms are necessary to confirm the results of this study.
In conclusion, we reported that advanced age, obesity, glucose intolerance, and low lymphocyte count are independent host risk factors for disease progression to oxygen requirement stage, in Japanese COVID-19 patients. Furthermore, the proportion of patients requiring oxygen increased with the presence of larger number of these risk factors in the same individual. These data suggest that the number of these risk factors in individuals could predict oxygen requirement and could be potentially used to triage such in the context of future need for hospitalization.
We thank all the medical staff and physicians at Toyonaka Municipal Hospital. We also thank Word-Medex Pty Ltd for help in the English language editing of this manuscript.
All authors declare no conflicts of interest.
Y.O. and H.I. designed the study, analyzed the data and wrote the manuscript. K.M. collected and analyzed the data. T.N., K.S., A.K, N.I., O.M., Y.O., M.Y., and K.A. collected the data and reviewed the manuscript. All authors read and approved the final manuscript.