2021 Volume 68 Issue 8 Pages 981-992
2021 Volume 68 Issue 8 Pages 981-992
Honokiol plays an important role in anti-oxidation, but its role in diabetic vascular complications is unclear. In this study, the effects of honokiol in high glucose/high fat (HG/HF)-induced human umbilical vein endothelial cells (HUVECs) were explored. After pre-treatment with honokiol, the cells were transferred to an HG/HF medium, and cell viability and apoptosis were respectively measured by methyl tetrazolium and flow cytometry. Moreover, the contents of reactive oxygen species (ROS), malondialdehyde (MDA), and superoxide dismutase (SOD) were measured. The expressions of C/EBP homologous protein (CHOP), glucose-regulated protein 78 (GRP78), phosphorylated-protein kinase RNA-like endoplasmic reticulum kinase (p-PERK), phosphorylated-inositol requiring enzyme-1α (p-IRE1α), cleaved caspase-3 and SIRT1 were determined by Western blot or quantitative reverse transcription PCR, respectively. Finally, the viability, apoptosis, and the contents of ROS, MDA, and SOD, as well as the expressions of CHOP, GRP78, p-PERK, p-IRE1α, cleaved caspase-3, Akt, p-Akt, and SIRT1 in the cells transfected with small interfering RNA SIRT1 (siSIRT1) were detected by the previously mentioned methods. Honokiol reversed the effect of HG/HF on promoting cell apoptosis, ROS and MDA contents, and the expressions of CHOP, GRP78, p-PERK, p-IRE1α and cleaved caspase-3, and also reversed the inhibitory effect of HG/HF on cell viability, SOD content and SIRT1 expression. However, siSIRT1 reversed the above effects caused by honokiol. Honokiol activated SIRT1 promoter. SIRT1 interacted with Akt, consequently promoting the activity of Akt. Therefore, honokiol activates the Akt pathway by regulating SIRT1 expression to regulate endoplasmic reticulum stress, promotes cell viability and inhibits the apoptosis of HG/HF-induced HUVECs.
DIABETES MELLITUS is a chronic disease with high blood glucose resulted from insufficient insulin secretion or insulin resistance [1]. In recent years, the incidence of diabetes mellitus has been steadily increasing [2]. Studies have shown that with the aggravation of insulin resistance and the extension of the course of disease in diabetic patients, diabetic complications, such as microvascular complications, macrovascular complications and neurological complications, will affect systemic vital organs, and that vascular complications are an important cause of death to diabetic patients [3-6]. Therefore, the prevention and treatment of diabetic vascular complications has become one of the hotspots in the field of diabetes-related research.
Endoplasmic Reticulum (ER) stress is an evolutionarily highly conserved cellular response mechanism and is one of the pathways through which cells undergo apoptosis. Hypoxia and oxidative stress responses can trigger the development of ER stress in endothelial cells and cancers [7-11]. Studies have shown that ER stress is involved in the development and progression of a variety of diseases [12]. Related studies have found that ER stress plays an important role in diabetes-related cardiovascular disorders and endothelial dysfunctions, and ER stress has the potential to serve as a targeted therapy for diabetic atherosclerosis [13, 14]. Specifically, in studies related to ER stress and atherosclerosis, salicylate was found to inhibit inflammation and ER stress by regulating the expressions of Heme oxygenase 1 (HO-1) and sirtuin 1 (SIRT1), which in turn improved atherosclerotic response [15]. SIRT1, which is a nicotinamide adenosine dinucleotide (NAD)+-dependent deacetylase, has been found to promote cardiomyocyte survival by attenuating the ER stress response; in addition, multiple studies demonstrated that SIRT1 is involved in regulating ER stress responses [16-19].
Honokiol is a plant active component with low molecular weight and high safety [20], exerting an important role in liver protection, anti-oxidation and glucose and lipid metabolism [21-23]. In addition, honokiol can reduce visceral adipose tissue weight and adipocyte size in mice, and prevent the development of insulin resistance. Moreover, honokiol could stimulate glucose transporter 4 translocation to the myocyte surface through the PI3K-Akt signaling pathway [24, 25]. Honokiol improved myocardial ischemia/reperfusion injury in diabetic rats by mediating SIRT1-Nrf2 signaling pathway and reducing oxidative stress [26]. However, it is unclear whether honokiol can improve high glucose/high fat (HG/HF)-induced human umbilical vein endothelial cells (HUVECs) function through SIRT1 regulation of ER stress.
Based on the above research background, we explored the mechanism of honokiol on the HG/HF-induced HUVECs. The results of this experiment provide a reliable theoretical basis for the treatment and prevention of diabetic vascular complications.
HUVECs (PCS-100-013) were purchased from American Type Culture Collection (ATCC; Maryland, USA), and the cells were cultured in Vascular Cell Basal Medium (PCS-100-030, ATCC, Maryland, USA) according to the manufacturer’s instructions. The components contained in the Endothelial Cell Growth Kit-VEGF (PCS-100-041, ATCC, Maryland, USA) were added to the medium. Afterwards, the seeded cells were cultured in an incubator (BC-J80, Boxun, Shanghai, China) with 5% CO2 at 37°C, and the medium was changed every 2 days.
Cells transfectionThe specific small interfering RNA (siRNA) targeting SIRT1 (siSIRT1 target sequence: 5'-GTCGCTTTAAAATAAGTTTCTCT-3') and siSIRT1 negative control (siNC; 5'-UUCUCCGAACGUGUCACGUTT-3') were purchased from GenePharma Company (Shanghai, China). The HUVECs at the logarithmic growth phase were collected, washed with Phosphate-Buffered Saline (PBS; 10010031, Thermo Fisher, Massachusetts, USA), and then the cell concentration was adjusted to approximately 1 × 105 cells/mL. Next, the cells were transferred to a 6-well plate for continued culture. When the cells confluence reached approximately 80%, siSIRT1 and siNC were transfected into HUVECs for 48 h, respectively, according to the operating instructions of LipofectamineTM 2000 Transfection Reagent (11668019, Thermo Fisher, Massachusetts, USA).
Cells treatmentTo determine the treatment concentration of honokiol (H4914, Sigma-Aldrich, Missouri, USA), honokiol was dissolved in dimethyl sulfoxide (DMSO; D2650, Sigma-Aldrich, Missouri, USA) mother liquor, according to the previous experimental method [27, 28]. Then the cells were collected and divided into the following five groups: (1) Control group: HUVECs were incubated in medium for 2 h and then cultured in culture medium containing normal amount (5 mmol/L) of glucose (G8270, Sigma-Aldrich, Missouri, USA) for 24 h; (2) HG/HF group: HUVECs were cultured in medium containing high glucose (25 mmol/L) and high saturated free fatty acid palmitate (500 μmol/L, 1170460001, Sigma-Aldrich, Missouri, USA) for 24 h after incubation in medium for 2 h; (3) HG/HF + Honokiol (5 μmol/L) group: HUVECs were pretreated with medium containing 5 μmol/L Honokiol for 2 h, and then transferred to medium containing HG/HF cultured for 24 h; (4) HG/HF + Honokiol (20 μmol/L) group: HUVECs were pretreated with medium containing 20 μmol/L Honokiol for 2 h, and then transferred to medium containing HG/HF cultured for 24 h; (5) HG/HF + Honokiol (80 μmol/L) group: HUVECs were pretreated with medium containing 80 μmol/L Honokiol for 2 h, and then transferred to medium containing HG/HF cultured for 24 h.
The transfected HUVECs were collected and cultured in normal medium or HG/HF medium for 24 h, or the transfected cells were pretreated with 80 μmol/L Honokiol for 2 h and then transferred to medium containing HG/HF cultured for 24 h.
Detection of cell viabilityThe viability of the HUVECs was determined by Methyl tetrazolium (MTT) cell viability Assay Kit (V13154, Thermo Fisher, Massachusetts, USA). The cells were collected and transferred to 96-well plates after adjusting cell concentration (5 × 103 cells/well). After the cells in each group were appropriately treated, an appropriate amount (10 μL) 12-mM MTT stock solution was added to each well according to the operating instructions, and then the cells were incubated at 37°C for 4 h. Next, 100 μL SDS-HCl solution was added to each well and the cells were incubated for 4 h. Finally, the absorbance of each sample well at 570 nm was detected with a microplate reader (SpectraMax iD3, Molecular Devices, Calif, USA).
Detection of cell apoptosisCell apoptosis was detected by Annexin V-FITC/Propidium Iodide Apoptosis Detection Kit (BMS500FI-100, Thermo Fisher, Massachusetts, USA). According to the protocol, the treated HUVECs in each group were collected, washed with PBS, and then resuspended in Binding Buffer to adjust the cell concentration to 5 × 105 cells/mL. Subsequently, 5 μL Annexin V-FITC was added to the cell suspension, and treated for 10 min at room temperature. Then the cells washed with Binding Buffer, and resuspended in Binding Buffer. Finally, the cells were treated with the 10 μL Propidium Iodide and then analyzed by flow cytometry (CytoFLEX, Beckman Coulter, Calif, USA).
Detection of intracellular reactive oxygen species (ROS)Intracellular ROS level was detected by ROS Detection Kit (BB-47053-1, BestBio, Shanghai, China). According to the kit instruction, the treated HUVECs in each group were collected, and transferred to 6-well plates. DCFHDA was first diluted with serum-free medium at a ratio of 1:1,000, and then added into each well to culture the cells in an incubator for 20 min. Next, the cells were washed again with serum-free medium, and the fluorescence intensity of the samples was measured with a fluorospectro photometer (83061-01, Cole-Parmer, Illinois, USA). The ROS level was analyzed based on the fluorescence intensity of the samples.
Determination of malondialdehyde (MDA) and superoxide dismutase (SOD) contentsCell MDA content was measured by Lipid Peroxidation MDA Assay Kit (S0131S, Beyotime, Shanghai, China). After collecting the treated HUVECs in each group, the cells were lysed with cell lysate (P0013, Beyotime, Shanghai, China), and then centrifuged (Avanti J-15R, Beckman Coulter, Calif, UAS) at 10,000 × g for 10 min before the supernatant had been taken for subsequent measurements. Subsequently, the protein concentration was measured with a BCA Protein Assay Kit (P0009, Beyotime, Shanghai, China). Then the TBA stock solution and MDA detection working solution as well as the diluted standards were prepared according to the manufacturer’s instructions. Finally, the absorbance of the samples was measured with a microplate reader at 532 nm and the content of MDA was calculated.
The SOD levels of HUVECs were measured by a SOD Assay Kit (ml076328, Enzyme-linked Biotechnology Co., Ltd., Shanghai, China). The treated cells in each group were collected into centrifuge tubes, and an appropriate amount of extract was added to the centrifuge tubes. After the cells were broken by ultrasonic waves, and then centrifuged at 8,000 × g for 10 min at 4°C. Afterwards, the supernatant was taken to detect the SOD content of the samples.
Quantitative reverse transcription PCR (RT-qPCR)The treated HUVECs in each group were collected into centrifuge tubes, and washed with PBS after centrifugation, and then mRNAs were extracted from the cells by mRNA preparation Kit (MRN70, Sigma-Aldrich, Missouri, USA). Afterwards, the purity of mRNAs was determined by UV spectrophotometer (DU-720, Beckman Coulter, Calif, USA), and the integrity of the mRNAs was examined by agarose gel (ST004Q, Beyotime, Shanghai, China) electrophoresis. The cDNA was synthesized using a cDNA Reverse-transcription Kit (4374966, Thermo Fisher, Massachusetts, USA), followed by PCR amplification. In the experiment, the expression of SIRT1 was detected by a RT-qPCR system (CFX96 Touch Real-Time PCR, Bio-Rad, Calif, USA), with SYBRTM Green Master Mix (A25742, Thermo Fisher, Massachusetts, USA). The primer synthesis sequences were as follows: SIRT1 (F: 5'-TAGCCTTGTCAGATAAGGAAGGA-3', R: 5'-ACAGCTTCACAGTCAACTTTGT-3'), GAPDH (F: 5'-GGAGCGAGATCCCTCCAAAAT-3', R: 5'-GGCTGTTGTCATACTTCTCATGG-3'). In this experiment, GAPDH was a reference gene and the results were analyzed by the 2–ΔΔct method.
Western blotThe HUVECs in each group were collected and digested with Trypsin-EDTA Solution (C0201, Beyotime, Shanghai, China), and the cell concentration was adjusted to 5 × 105 cells/mL. After centrifugation, the supernatant was discarded and the cells were washed with precooled PBS, and then an appropriate amount of RIPA lysis and Extraction Buffer (89900, Thermo Fisher, Massachusetts, USA) was added to the centrifuge tube to extract total protein from the cells. Then the total protein concentration was measured with a BCA Protein Assay Kit (P0009, Beyotime, Shanghai, China). Polyacrylamide gel was prepared by SDS-PAGE Gel Kit (P1200, Solarbio, Beijing, China), and then equal amounts (25 μL) of protein samples were added to the sample wells of the gel, followed by electrophoresis. At the end of electrophoresis experiments, the gel was removed, and the protein was transferred to a PVDF membrane (YA1701, Solarbio, Beijing, China), which was blocked with Western Blocking Buffer (SW3010, Solarbio, Beijing, China) for 60 min at room temperature. After that, the membranes were washed with Tris Buffered saline Tween (TBST; T1085, Solarbio, Beijing, China) 3 times for 3 min each time. PVDF membrane was then treated overnight at 4°C in primary antibodies. After that, the PVDF membrane was washed with TBST solution 3 times for 3 min each time, and then the membrane was incubated in the secondary antibody for 1 h at room temperature. Afterwards, the membranes were washed with TBST, and then the membranes were completely immersed in ECL Plus working solution (PE0010, Solarbio, Beijing, China) for 3 min at room temperature in the dark. Finally, the gel was analyzed on a gel image processing system (FluorChem FC3, Alpha Innotech, Calif, USA). All the information and sources of antibodies were shown in Table 1.
| ID | Catalog number | Company (country) | Molecular weight | Dilution ratio |
|---|---|---|---|---|
| CHOP | ab11419 | Abcam (Cambridge, UK) | 31 kDa | 1/1,000 |
| GRP78 | ab21685 | Abcam (Cambridge, UK) | 75 kDa | 1/1,000 |
| p-PERK | DF7576 | Affinity Biosciences (Massachusetts, USA) | 125 kDa | 1/1,000 |
| p-IRE1α | ab48187 | Abcam (Cambridge, UK) | 110 kDa | 1/1,000 |
| Cleaved caspase-3 | ab2302 | Abcam (Cambridge, UK) | 17 kDa | 1/500 |
| SIRT1 | ab110304 | Abcam (Cambridge, UK) | 81 kDa | 1/1,000 |
| p-Akt | ab38449 | Abcam (Cambridge, UK) | 56 kDa | 1/1,000 |
| Akt | ab8805 | Abcam (Cambridge, UK) | 55 kDa | 1/500 |
| GAPDH | ab8245 | Abcam (Cambridge, UK) | 36 kDa | 1/1,000 |
| Mouse IgG | ab205719 | Abcam (Cambridge, UK) | 1/5,000 | |
| Rabbit IgG | ab205718 | Abcam (Cambridge, UK) | 1/5,000 |
Co-IP assay was performed to identify the direct interrelation between SIRT1 and Akt on HUVECs. Firstly, immunoprecipitation (IP) was performed on HUVECs using the Abcam immunoprecipitation kit (ab206996), following the manufacturer’s instructions. Briefly, non-denaturing lysis buffer was used to collect cell lysate. A small amount of cell lysate was used for Western blot analysis to detect the expressions of SIRT1 and Akt, and while other cell lysate were used for IP. Then, 300 μg of cell lysate was incubated overnight with 3 μg/mL of either control mouse IgG (sc-2025, Santa Cruz,) or SIRT1 mouse monoclonal antibody (ab110304, Abcam). Antibody- bound proteins were captured using protein A/G sepharose beads, eluted, and analyzed via Western blot.
Dual luciferase reporter analysisSIRT1 promoter sequences were amplified by PCR using upstream and downstream primers of SIRT1 promoter: F: 5'-CCGCTCGAGCTACACGCTCGCCACAAA-3'; R: 5'-CCCAAGCTT CCGCCATCTTCCAACTGC-3'. The human SIRT1 promoter sequences were located into the XhoⅠ/HindⅢ-digested pGL3-Basic reporter vector to construct the pGL3-Basic-SIRT1. Cells were subjected to transfection of pGL3-Basic-SIRT1 and internal reference PRL-TK plasmid, for 24 h and treated with honokiol at 5–80 μmol/L. Dual-luciferase reporter assay system (E1910, Promega, Madison, WI, USA) was applied for analysis of the luciferase activities. The promoter activities were calculated by normalization of the ratio between the activities of firefly luciferase and Renilla luciferase.
Statistical analysisThere are three biological replicates and three technical replicates for each biological replicate. Measurement data were described by mean ± standard deviation, and multiple groups were compared using One-way ANOVA. All statistical analyses were implemented using Graphpad 8.0 software. P < 0.05 was considered as statistically significant.
From Fig. 1A, it can be observed that HG/HF treatment prominently inhibited cell viability compared with the Control group (p < 0.001), however, after the cell pretreatment with honokiol at 5 μmol/L, 20 μmol/L and 80 μmol/L, honokiol notably reversed the inhibitory effect of HG/HF on cell viability as compared with the HG/HF group (p < 0.05). By detecting the apoptosis of HUVECs using flow cytometry, we found that HG/HF significantly promoted apoptosis compared with the Control group (p < 0.001), whereas after the cell pre-treatment with honokiol, honokiol reduced the effect of HG/HF on promoting apoptosis as compared with the HG/HF group (Fig. 1B–C, p < 0.01).

Honokiol reversed the inhibitory effect of HG/HF on cell viability and SOD content; also reversed the effect on promoting apoptosis and the contents of ROS and MDA
(A) The viability of HG/HF-induced HUVECs after pre-treatment with different concentrations (5, 20 and 80 μmol/L) of honokiol was examined by MTT. (B–C) The apoptosis of HG/HF-induced HUVECs after pre-treatment with different concentrations (0, 5, 20 and 80 μmol/L) of honokiol was examined by flow cytometry. (D) The ROS contents of HG/HF-induced HUVECs after pre-treatment with different concentrations (5, 20 and 80 μmol/L) of honokiol were examined. (E) The MDA content of HG/HF-induced HUVECs after pre-treatment with different concentrations (5, 20 and 80 μmol/L) of honokiol was examined. (F) The SOD content of HG/HF-induced HUVECs after pre-treatment with different concentrations (5, 20 and 80 μmol/L) of honokiol was examined. *** p < 0.001 vs. Control; # p < 0.05, ## p < 0.01, ### p < 0.001 vs. HG/HF. (HG/HF, high glucose/high fat; HUVECs, human umbilical vein endothelial cells; MMT, Methyl tetrazolium; ROS, reactive oxygen species; MDA, malondialdehyde; SOD, superoxide dismutase)
As shown in Fig. 1D and 1E, the ROS level was based on fluorescence intensity of the samples, and MDA content in HUVECs was detected. The results showed that HG/HF obviously increased the contents of ROS and MDA in the cells as compared with the Control group (p < 0.001), whereas honokiol clearly inhibited the effect of HG/HF on increasing the contents of ROS and MDA in HUVECs when compared with the HG/HF group (p < 0.05). In addition, we found that HG/HF inhibited the contents of SOD in comparison with the Control group (p < 0.001), but honokiol markedly promoted the contents of SOD in HUVECs compared with the HG/HF group (Fig. 1F, p < 0.01).
Honokiol reversed the effect of HG/HF on promoting the expressions of ER stress markers and cleaved caspase-3 as well as the inhibitory effect on SIRT1 expression in HUVECsAs shown in Fig. 2A and 2B, Western blot data showed that HG/HF promoted the expressions of ER stress markers, including C/EBP homologous protein (CHOP), glucose-regulated protein 78 (GRP78), phosphorylated-protein kinase RNA-like endoplasmic reticulum kinase (p-PERK) and phosphorylated-inositol requiring enzyme-1α (p-IRE1α), and also promoted cleaved caspase-3 expression, as compared with the Control group (p < 0.001). Honokiol partially counteracted the effect of HG/HF on promoting the expressions of ER stress markers and cleaved caspase-3 in the cells (p < 0.05). In addition, the results of Western blot and RT-PCR experiments indicated that HG/HF inhibited the expression of SIRT1 in comparison with the Control group (p < 0.001), whereas honokiol partially reversed the inhibitory effect of HG/HF on SIRT1expression in the HUVECs, as compared with the HG/HF group (Fig. 2C–E, p < 0.05). As shown in Fig. 2F, relative luciferase activity of SIRT1 promoter was decreased as honokiol concentration increased from 5 to 80 μmol/L, which indicated that honokiol activated SIRT1 promoter in HUVECs.
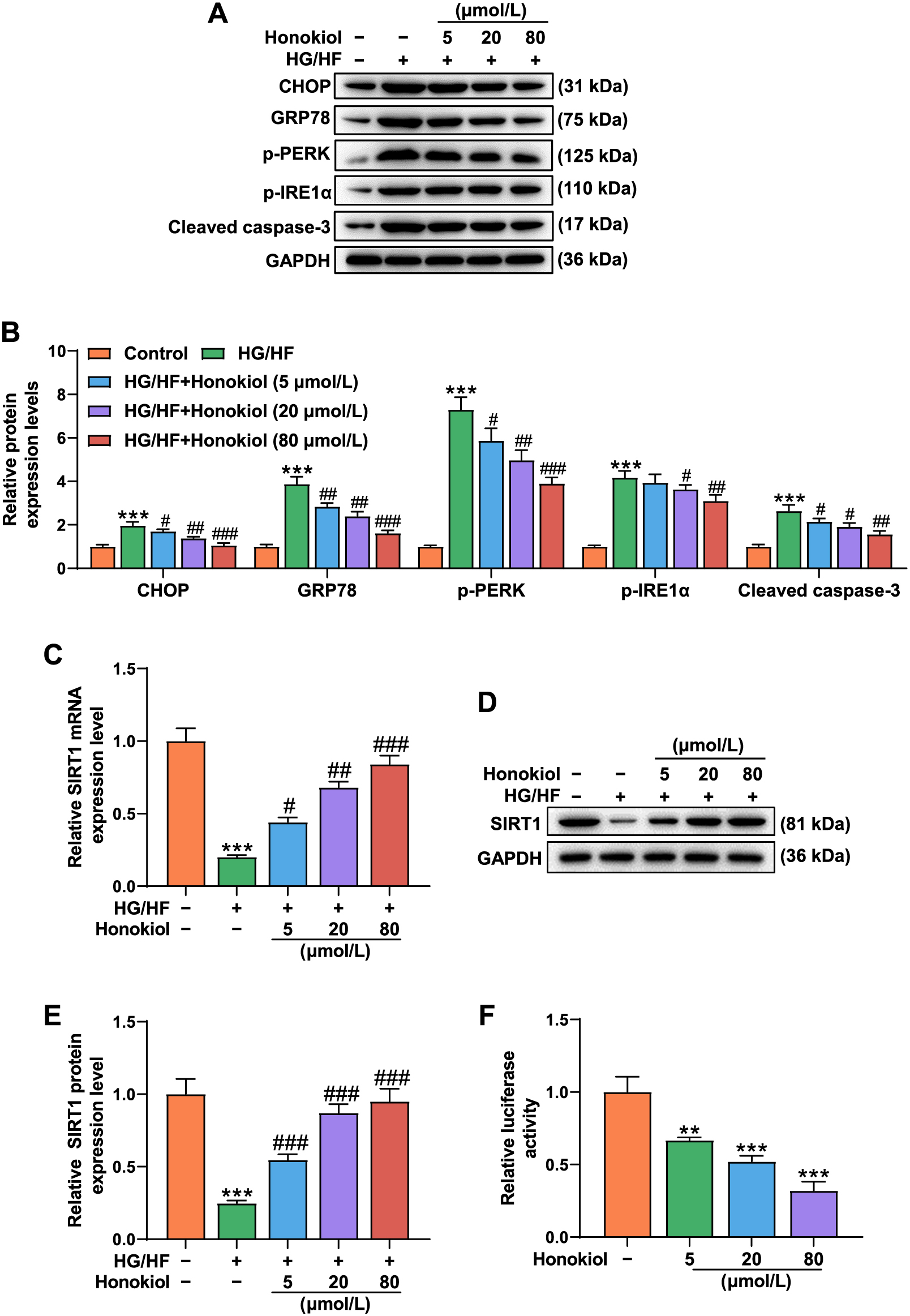
Honokiol reversed the effect of HG/HF on promoting the expression of ER stress markers and cleaved caspase-3 as well as the inhibitory effect on SIRT1 expression in HG/HF-induced HUVECs
(A–B) The expressions of CHOP, GRP78, p-PERK and p-IRE1α as well as cleaved caspase-3 in the treated cells were examined by Western blot, and GAPDH was used as an internal loading control. (C) The expression of SIRT1 in treated cells was examined by RT-qPCR, and GAPDH was used as a reference gene. (D–E) The expression of SIRT1 in treated cells was examined by Western blot, and GAPDH was used as an internal loading control. (F) The relative luciferase activity of SIRT1 promoter in HUVECs treated with honokiol was detected by dual luciferase reporter analysis. *** p < 0.001 vs. Control; # p < 0.05, ## p < 0.01, ### p < 0.001 vs. HG/HF; ** p < 0.01,*** p < 0.001 vs. Honokiol (–) (0 μmol/L of Honokiol). (HG/HF, high glucose/high fat; HUVECs, human umbilical vein endothelial cells; ER stress, endoplasmic reticulum stress; CHOP, C/EBP homologous protein; GRP78, glucose-regulated protein 78; p-PERK, phosphorylated-protein kinase RNA-like endoplasmic reticulum kinase; p-IRE1α, phosphorylated-inositol requiring enzyme-1α; RT-qPCR, quantitative reverse transcription PCR)
As shown in Fig. 3A–C, the expression of SIRT1 in the treated cells was detected by Western blot and RT-PCR. The data showed that HG/HF obviously inhibited the expression of SIRT1 compared with the siNC group (p < 0.001), but significantly promoted the expression of SIRT1 compared with the HG/HF + siNC group (p < 0.001) siSIRT1 sharply inhibited the expression of SIRT1 compared with the HG/HF + honokiol + siNC group (p < 0.001). By examining cell viability, we found that HG/HF significantly inhibited cell viability in comparison with the siNC group (p < 0.001), while honokiol reversed the inhibitory effect of HG/HF on cell viability in comparison with the HG/HF + siNC group (p < 0.01). siSIRT1 further overturned the effect of honokiol on cell viability when compared with the HG/HF + honokiol + siNC group (Fig. 3D, p < 0.05). Additionally, the apoptosis experiment results showed that HG/HF evidently promoted apoptosis as compared with the siNC group (p < 0.001), however, honokiol counteracted the effect of HG/HF on promoting apoptosis as compared with the HG/HF + siNC group (p < 0.001). Moreover, siSIRT1 further reversed the effect of honokiol on cell apoptosis as compared with the HG/HF + honokiol + siNC group (Fig. 3E–F, p < 0.001).

siSIRT1 further overturned the effect of honokiol on SIRT1 expression, cell viability and apoptosis of HG/HF-induced HUVECs
(A) The expression of SIRT1 in honokiol treatment cells after transfection was examined by RT-qPCR, and GAPDH was used as a reference gene. (B–C) The expression of SIRT1 in honokiol treatment cells after transfection was examined by Western blot, and GAPDH was used as an internal loading control. (D) The viability of honokiol treatment cells after transfection was examined by MTT. (E–F) The apoptosis of honokiol treatment cells after transfection was examined by flow cytometry. *** p < 0.001 vs. siNC; ## p < 0.01, ### p < 0.001 vs. HG/HF + siNC; & p < 0.05, &&& p < 0.001 vs. HG/HF + honokiol + siNC. (HG/HF, high glucose/high fat; HUVECs, human umbilical vein endothelial cells; RT-qPCR, quantitative reverse transcription PCR; MMT, Methyl tetrazolium)
We found that HG/HF greatly increased the contents of ROS and MDA as compared with the siNC group (p < 0.001), however, honokiol inhibited the effect of HG/HF on promoting the contents of ROS and MDA in the cells compared with the HG/HF + siNC group (p < 0.01), but siSIRT1 further reversed the effect of honokiol as compared with the HG/HF + honokiol + siNC group (Fig. 4A–B, p < 0.05). As shown in Figure 4C, the content of SOD was significantly reduced in HG/HF + siNC group compared with the siNC group (p < 0.001), but was increased by honokiol compared with the HG/HF + siNC group (p < 0.001). However, siSIRT1 inhibited the content of SOD compared with the HG/HF + honokiol + siNC group (Fig. 4C, p < 0.01).

siSIRT1 reversed the effect of honokiol on the contents of ROS, MDA and SOD, and the expressions of ER stress markers and cleaved caspase-3 as well as the p-Akt (A) The relative fluorescence intensity of cells after transfection was examined. (B) The MDA content of cells after transfection was examined. (C) The SOD content of cells after transfection was examined. (D–E) The expressions of CHOP, GRP78, p-PERK and p-IRE1α as well as cleaved caspase-3 in transfected cells were examined by Western blot, and GAPDH was used as an internal loading control. (F–G) The expressions of p-Akt and Akt in transfected cells were examined by Western blot, and GAPDH was used as an internal loading control. (H) The ratio of p-Akt to Akt in transfected cells was analyzed. ** p < 0.01, *** p < 0.001 vs. siNC; # p < 0.05, ## p < 0.01, ### p < 0.001 vs. HG/HF + siNC; & p < 0.05, && p < 0.01, &&& p < 0.001 vs. HG/HF + Honokiol + siNC. (ROS, reactive oxygen species; MDA, malondialdehyde; SOD, superoxide dismutase; ER stress, endoplasmic reticulum stress; CHOP, C/EBP homologous protein; GRP78, glucose-regulated protein 78; p-PERK, phosphorylated-protein kinase RNA-like endoplasmic reticulum kinase; p-IRE1α, phosphorylated-inositol requiring enzyme-1α; p-Akt, phosphorylated-Akt)
Western blot results showed that HG/HF promoted the expressions of CHOP, GRP78, p-PERK, p-IRE1α and cleaved caspase-3 compared with the siNC group (p < 0.001), while honokiol partially counteracted the effect of HG/HF on promoting the expression levels of ER markers and cleaved caspase-3 compared with the HG/HF + siNC group (p < 0.01). siSIRT1 further overturned the effect of honokiol on the cells when compared with the HG/HF + honokiol + siNC group (Fig. 4D–E, p < 0.05). In addition, the results of Western blot also demonstrated that HG/HF inhibited the expression of p-Akt compared with the siNC group (p < 0.01), while honokiol reversed the inhibitory effect of HG/HF on the expression of p-Akt compared with the HG/HF + siNC group (p < 0.05). However, siSIRT1 inhibited the promoting effect of Honokiol on the expression of p-Akt compared with the HG/HF + honokiol + siNC group (Fig. 4F–G, p < 0.001). However, Akt expression did not differ among groups. Therefore, the p-Akt/Akt ratio was sharply decreased after HG/HF intervention as compared with the siNC group (p < 0.001), however, honokiol promoted the p-Akt/Akt ratio as compared with the HG/HF + siNC group (p < 0.01). siSIRT1 partially reversed the effect of honokiol on the p-Akt/Akt ratio in HUVECs as compared with the HG/HF + honokiol + siNC group (Fig. 4H, p < 0.001).
SIRT1 directly modulated activity of Akt in HUVECsFurthermore, the direct interrelation between SIRT1 and Akt was detected with Co-IP assay in HUVECs (Fig. 5A). By the same way, we observed that the acetylation of Akt was increased in HUVECs when SIRT1 was knocked down (Fig. 5B).

SIRT1 directly modulated activity of Akt in HUVECs
(A) Co-IP technique was performed to investigate the interrelation between SIRT1 and Akt in HUVECs. (B) Deacetylation of Akt by SIRT1 was detected by Co-IP assay after overexpressing Akt and knocking down SIRT1 in HUVECs. Co-IP, Co-immunoprecipitation; L-Ac, lysine acetylation.
Oxidative stress injury and changes in blood glucose and blood lipids are closely related to the development of diabetes, and honokiol has positive effects on anti-oxidation and regulation of blood glucose and a high safety [20-23]. Exposure of HUVECs to HG/HF could mimic diabetic vascular endothelial cells in a physiological condition [29]. To explore the effect of honokiol on HG/HF-induced diabetic vascular endothelial cells, HUVECs was induced by HG/HF following a previous report [27, 28]. The results of related experiments showed that high-glucose treatment inhibited the viability of the HUVECs and promoted apoptosis [30, 31]. In this experiment, we also found that HG/HF significantly inhibited viability and promoted apoptosis of HUVECs, but the effect of HG/HF on HUVECs should be further validated.
ROS are highly reactive small molecules that can oxidize lipids and proteins, and play an important role in cell signaling and homeostasis. ROS levels will significantly increase under abnormal environments, and high glucose can induce ROS production [32, 33]. MDA is a stable product of lipid peroxidation, and the level of MDA is generally considered as a marker of oxidative stress, and high glucose can also induce an increase in MDA content [34-36]. The SOD family plays an important role in the regulation of oxidative stress, and the activity of SOD is decreased in a high-glucose environment [37, 38]. In this experiment, we found that HG/HF increased the levels of ROS and MDA and reduced the level of SOD, but honokiol reversed the effect of HG/HF on cells. Moreover, the experimental results showed that honokiol could reduce ER stress, but this should be further verified at a molecular level.
Studies have shown that CHOP is involved in ER stress-mediated apoptosis pathway, and excessive ER stress can lead to CHOP-mediated apoptosis [39, 40]. In addition, it has been found that GRP78 is a heat-shock protein and a key regulator of ER stress, similarly, both the PERK pathway and IRE1α are important regulators of ER stress response [41-43]. In the process of apoptosis, caspase is responsible for the majority of proteolysis, therefore the detection of the expression level of cleaved caspase-3 is considered as a marker of apoptosis [44]. When stimulated by external stimuli, cells activate ER stress and further activate signaling pathways, thereby inducing cell death and apoptosis [45]. Moreover, honokiol has been found to regulate cell migration and apoptosis in human lung cancer cells by regulating the expression of ER stress-induced apoptotic signaling molecules, such as CHOP, GRP78, p-PERK and Cleaved caspase 9 [46]. In this experiment, we found that HG/HF promoted the expressions of ER markers and cleaved caspase-3, however, Honokiol reversed the effects of HG/HF on ER markers and cleaved caspase-3 in cells. Studies indicated that increased ROS generation and ER stress will interact with each other [47, 48]. The above experimental results illustrated that honokiol reversed HG/HF-induced decreases of viability and apoptosis by inhibiting ER stress response. However, the mechanism of action of honokiol remained unclear.
SIRT1 belongs to the Sirtuins family, and recent studies have shown that SIRT1 plays an important role in inflammation, oxidative stress and ER stress [49-51]. SIRT1 was found to inhibit ER stress-induced apoptosis in mouse experiments [51]. Losartan inhibited ER stress of tubular epithelial cells by up-regulating SIRT1 [52]. In addition, honokiol could reduce oxidative stress and apoptosis in rats through mediating the SIRT1-Nrf2 signaling pathway [26]. Resveratrol suppresses apoptosis of rat hippocampal cells through inhibiting ER stress and activating the SIRT1/AMPK/Akt signaling pathway, and the PI3K-Akt-GSK3β signaling pathway is involved in the effect of SIRT1 on ER stress [19, 53]. We found that HG/HF inhibited the expression of SIRT1, and that siSIRT1 reversed the effect of honokiol on the expressions of ER stress markers and cleaved caspase-3 as well as the p-Akt.
Avtanski et al. [54] found that SIRT1 is involved in the beneficial effects of honokiol in antagonizing the oncogenic actions of leptin in breast cancer. Zhang et al. [54] found that honokiol relieves myocardial ischemia/reperfusion injury in Type 1 diabetic rats by inhibiting oxidative stress and apoptosis via activating the SIRT1-Nrf2 signaling pathway. These findings did not reveal whether honokiol can activate SIRT1 promoter or not, but they only demonstrated that honokiol increased SIRT1 expression or activity. In our study, we found that honokiol increased SIRT1 expression and we further confirmed that honokiol activated SIRT1 promoter. Moreover, SIRT1 directly interacted with Akt, consequently regulating Akt activity. Collectively, these data indicated that honokiol regulates SIRT1 expression thereby controlling the Akt pathway regulating ER stress of HG/HF-induced HUVECs.
This study has several limitations. Although the present study have reported protective effect and potential mechanism of honokiol on HG/HF-induced HUVECs, further experiments are needed to perform on animal model.
In conclusion, our results indicate that honokiol directly regulates SIRT1 expression to modulate ER stress through activating the Akt pathway, and this in turn promotes cell viability and inhibits apoptosis of HG/HF-induced HUVECs. Our results provide a theoretical basis for honokiol as a new drug for the treatment of patients with diabetic vascular complications.
Not applicable.
The authors declare no conflicts of interest.
Not applicable.