2021 Volume 68 Issue 8 Pages 933-941
2021 Volume 68 Issue 8 Pages 933-941
The brain mechanism responsible for the pulsatile secretion of gonadotropin-releasing hormone (GnRH) is important for maintaining reproductive function in mammals. Accumulating evidence suggests that kisspeptin/neurokinin B/dynorphin A (KNDy) neurons in the hypothalamic arcuate nucleus (ARC) play a critical role in the regulation of pulsatile GnRH and subsequent gonadotropin secretion. Dynorphin A (Dyn) and its receptor, kappa-opioid receptor (KOR, encoded by Oprk1), have been shown to be involved in the suppression of pulsatile GnRH/luteinizing hormone (LH) release. On the other hand, it is still unclear whether the inhibitory Dyn signaling affects KNDy neurons or KOR-expressing non-KNDy cells in the ARC or other brain regions. We therefore aimed to clarify the role of ARC-specific Dyn-KOR signaling in the regulation of pulsatile GnRH/LH release by the ARC specific cell deletion of KOR-expressing cells using Dyn-conjugated-saporin (Dyn-SAP). Estrogen-primed ovariectomized female rats were administered Dyn-SAP to the ARC. In situ hybridization of Oprk1 showed that ARC Dyn-SAP administration significantly decreased the number of Oprk1-expressing cells in the ARC, but not in the ventromedial hypothalamic nucleus and paraventricular nucleus. The frequency of LH pulses significantly increased in animals bearing the ARC Dyn-SAP administration. The number of Kiss1-expressing cells in the ARC was not affected by ARC Dyn-SAP treatment. Dyn-KOR signaling within the ARC seems to mediate the suppression of the frequency of pulsatile GnRH/LH release, and ARC non-KNDy KOR neurons may be involved in the mechanism modulating GnRH/LH pulse generation.
THE BRAIN MECHANISM responsible for the pulsatile secretion of gonadotropin-releasing hormone (GnRH), which stimulates gonadotropin release and consequent follicular development/steroidogenesis, is of deep significance because GnRH induces gonadotropin release only when GnRH is secreted in a pulsatile manner at a physiological frequency in mammals [1, 2]. The mechanism generating pulsatile GnRH secretion is called the GnRH pulse generator, and its activity is suggested to be modulated by steroid feedback actions and various environmental factors, such as nutritional status, stress, and suckling stimulus. Kisspeptin (first named metastin) was identified as an endogenous ligand for GPR54 [3], and accumulating evidence suggests that kisspeptin-GPR54 signaling controls reproductive function via the stimulation of GnRH/gonadotropin release in several mammalian species, such as rodents [4-7], ruminants [8-12], and primates [13-15]. Loss of function studies of kisspeptin or GPR54 by genetic mutation or conventional knockout showed hypogonadotropic hypogonadism with low to undetectable luteinizing hormone (LH) levels, which clearly demonstrates that kisspeptin-GPR54 signaling is a fundamental factor for stimulating GnRH/LH secretion in rodents and humans [16-18]. Kisspeptin neurons located in the hypothalamic arcuate nucleus (ARC) co-express neurokinin B (NKB) and dynorphin A (Dyn) in rats [19-21], mice [22], guinea pigs [23], sheep [24], and goats [25], and are therefore referred to as KNDy neurons [9], and suggested to be the GnRH/LH pulse generator [25-31].
Dyn and its receptor, kappa-opioid receptor (KOR, encoded by Oprk1), have been suggested to be involved in the suppression of frequency and/or baseline of pulsatile GnRH/LH release because the frequency of LH pulses was reduced by intracerebroventricular Dyn administration and increased by central administration of nor-binaltorphimine (nor-BNI), a KOR antagonist, in ovariectomized goats [25] and nor-BNI administration increased the baseline level of LH pulses without affecting LH pulse frequencies in estrogen-primed female rats [32]. Dyn neurons are abundantly located in the hypothalamus, including the paraventricular nucleus (PVN) as well as the ARC [33, 34]. Our recent study suggested that PVN Dyn neurons mediate glucoprivic GnRH/LH pulse suppression, since central administration of nor-BNI blocked LH pulse suppression induced by the peripheral or central administration of 2-deoxy-D-glucose (2DG), a glucose utilization inhibitor, and the 2DG administration activated PVN Dyn neurons [35]. These studies demonstrate that Dyn-KOR signaling negatively controls pulsatile GnRH/LH secretion under normal or glucoprivic conditions. On the other hand, it is still unclear whether inhibitory Dyn signaling affects KOR-expressing cells in the ARC and/or other brain regions to control the frequency and/or baseline of GnRH/LH pulses because KOR is widely distributed in the brain, including the hypothalamus, telencephalon (amygdala, claustrum, endopiriform nucleus, etc.), and mesencephalon (periaqueductal gray, substantia nigra, etc.) [36, 37].
KOR-expressing cells are reported to be distributed in the hypothalamus, including the ARC, PVN, and ventromedial hypothalamic nucleus (VMH) [37, 38]. An in vitro study by De Croft et al. [39] demonstrated that Dyn treatment reduced the firing rate of ARC kisspeptin neurons via KOR in mouse brain slices. Oprk1-expressing cells were widely observed in the ARC, and some of those cells were KNDy neurons in mice [22, 40] and rats [35]. These findings suggest that Dyn-KOR signaling in the ARC is involved in GnRH/LH pulse generation by directly acting on KNDy neurons. On the other hand, another study showed that KOR immunoreactivities were also found in Kiss1-negative cells in the ARC as well as GnRH neurons in the preoptic area (POA) in female rats [38], suggesting that Dyn may affect ARC non-KNDy KOR-expressing cells and/or GnRH neurons in the rat. To further understand the role of Dyn-KOR signaling in the regulation of GnRH/LH pulse generation, it is worth clarifying the role of ARC-specific Dyn-KOR signaling in the regulation of pulsatile GnRH/LH release and examining whether the KOR-expressing cells involved in pulse generation are KNDy neurons and/or non-KNDy neurons.
The present study aimed to clarify the role of ARC Dyn-KOR signaling in GnRH/LH pulse generation. For this purpose, we administered Dyn-conjugated-saporin (Dyn-SAP), which specifically causes cell death in KOR-expressing cells, into the ARC of adult female rats. The effect of the administration on the number of Oprk1-expressing cells in the ARC, PVN, and VMH was examined to confirm whether Dyn-SAP causes ARC-specific attenuation of KOR-expressing cells. Animals administered with Dyn-SAP into the ARC were subjected to frequent blood sampling to detect LH pulses as a marker of GnRH pulses to examine the effects of ARC-specific attenuation of KOR-expressing cells on the frequency and baseline of pulsatile LH secretion. Further, the effect of intra-ARC Dyn-SAP treatment on the number of kisspeptin gene (Kiss1)-expressing cells in the ARC of female rats was examined to determine if KOR-expressing cells deleted by the current Dyn-SAP treatment were KNDy or non-KNDy neurons.
Adult Wistar-Imamichi strain female rats (8-weeks old at the time of brain surgery, 11-weeks old, 200–250 g at the time of blood sampling; Institute for Animal Reproduction, Kasumigaura, Japan) were individually housed in a temperature (22 ± 2°C) and humidity (40%–60%)-controlled room in The University of Tokyo with a 14-h light and 10-h dark condition (lights on at 0500 h) and ad libitum access to water and food (CE-2; Clea Japan Inc., Tokyo, Japan). Animals showing at least two normal estrous cycles confirmed by observation of their vaginal smear were subjected to brain surgeries under isoflurane anesthesia. All protocols were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals, Graduate School of Agricultural and Life Sciences, The University of Tokyo.
Stereotaxic injection of Dyn-SAP into the ARC and frequent blood samplingWe used Dyn-SAP (Advanced Targeting Systems, San Diego, CA, USA) to determine the effect of deletion of KOR-expressing cells in the ARC on GnRH/LH pulse. Saporin (SAP) is a ribosome-inactivating protein that can induce cell death by preventing protein synthesis [41]. SAP itself is a non-toxic molecule when it exists extracellularly, while a composite of SAP conjugated to a specific ligand can enter cells via specific receptors for the ligand, and consequently induce cell death. Female rats were stereotaxically injected with Dyn-SAP (n = 7) or SAP (n = 6) as a control bilaterally into the anterior and posterior ARC (totally 4 injection sites) according to the rat brain atlas [42] as follows: 2.5 mm posterior and ±0.5 mm lateral to the bregma and 9.8 mm ventral to skull surface for the anterior ARC; 3.6 mm posterior and ±0.5 mm lateral to the bregma and 10.1 mm ventral to skull surface for the posterior ARC. Dyn-SAP (20 ng/200 nL) or SAP (18.6 ng/200 nL) dissolved in PBS was slowly infused at a rate of 100 nL/min into each injection site. After infusion, the injector was kept in place for an additional 5 min for adequate reagent diffusion. The amounts and the position of Dyn-SAP injection were determined according to a previous report using another ligand-conjugated saporin targeting the ARC [43].
Two weeks after the brain surgery for Dyn-SAP/SAP injection, rats were bilaterally ovariectomized (OVX) and implanted with a silicone tubing (i.d., 1.57 mm; o.d., 3.18 mm; 25 mm in length; Dow Corning, Midland, MI, USA) filled with estradiol (E2; Sigma-Aldrich, St. Louis, MO) dissolved in peanut oil (Sigma-Aldrich) at 20 μg/mL to serve as diestrus model [44]. Six days after the OVX, a silicone tubing (i.d., 0.5 mm; o.d., 1.0 mm; Shin-Etsu Polymer Co., Tokyo, Japan) for blood sampling was inserted into the right atrium of rats through the jugular vein. On the next day, three weeks after brain surgery, 100 μL of blood was collected every 6 min for 3 h from 1300 h from free-moving conscious rats to detect pulsatile LH release. An equivalent volume of rat red blood cells prepared with heparinized saline was replaced through the same atrial cannula after each blood collection to keep the hematocrit level constant. Plasma samples were separated by centrifugation (15,300 g, 20 min, 4°C) and stored at –20°C until LH assay.
Histological analysisRats were transcardially perfused with 4% paraformaldehyde under deep anesthesia with sodium pentobarbital for brain sampling on the same day after frequent blood sampling. Brains were collected and post-fixed in the same fixative overnight at 4°C, and then immersed in 30% sucrose in phosphate buffer for 48 h at 4°C before sectioning. Serial coronal brain sections of 50 μm thickness were obtained using a cryostat. Every fourth section was stained with thionin solution to verify the injection placement in the ARC by microscopic inspection (DM 2500, Leica Microsystems, Wetzlar, Germany).
Another series of every fourth brain section was subjected to free-floating in situ hybridization (ISH) of Oprk1 or Kiss1. Oprk1 mRNA was detected by ISH using the digoxigenin (DIG)-labeled antisense cRNA probe, synthesized by in vitro transcription from rat hypothalamic complementary DNA using a DIG RNA labeling kit (Roche Diagnostics, Basel, Switzerland) as described in a previous study [45], aiming at position 264–1404 of NM_017167. After hybridized with the cRNA probe for 16 h at 60°C, sections were incubated with anti-DIG antibody conjugated to alkaline phosphatase (1:1,000, Roche Diagnostics) for another 16 h at room temperature. The signal was visualized using 4-nitroblue tetrazolium chloride/5-bromo-4-chloro-3-indoyl-phosphate (NBT/BCIP, Roche Diagnostics). The sections were sealed with Entellan New Rapid Mounting Medium (Merck, Darmstadt, Germany). For Kiss1 ISH, a fluorescein isothiocyanate (FITC)-labeled Kiss1 probe (33–348 of NM_181692), synthesized by a FITC-labeling kit (Roche Diagnostics), was hybridized for 16 h at 60°C. Sections were incubated with anti-FITC antibody conjugated to horseradish peroxidase (1:500, PerkinElmer, Boston, USA) for 1.5 h at room temperature. A tyramide-biotin amplification system (PerkinElmer) was applied, and then sections were incubated with Alexa 488-conjugated streptavidin (Invitrogen, Eugene, OR, USA) to visualize Kiss1 signals.
The number of Oprk1- or Kiss1-expressing cells was counted for every fourth ARC section (totally 12–13 sections/animal). Oprk1 mRNA-expressing cells were identified using a bright-field microscope (Olympus BX51, Tokyo, Japan), and images of Kiss1 mRNA-expressing cells were captured using an LSM 700 laser scanning microscope (Carl Zeiss, Inc. Thornwood, USA). The number of Kiss1-expressing cells was counted using ImageJ software (https://imagej.nih.gov/ij/).
Radioimmunoassay (RIA) for LHPlasma LH concentration was determined by a double-antibody radioimmunoassay (RIA) with a rat LH RIA kit provided by the National Hormone and Peptide Program (Baltimore, MD, USA). LH concentrations were expressed in terms of the NIDDK rat LH-RP-3. The least detectable level of the LH assay was 0.16 ng/mL for 50-μL plasma. The intra-assay coefficient of variation was 6.81% at 3.29 ng/mL. All samples were measured in one assay, so there was no inter-assay variation.
Statistical analysisLH pulses were identified using the PULSAR computer program, as previously described [46]. Statistical differences in LH pulse parameters and the number of Oprk1- or Kiss1-expressing cells between SAP and Dyn-SAP groups were evaluated by Student’s t-test using Microsoft Excel software. P values <0.05 were considered statistically significant.
The correct placement of intra-ARC injection of Dyn-SAP or SAP was confirmed using Nissl-stained brain sections. The injection sites in all animals were relatively dorsal in the ARC and similar to the sites in a representative animal, whose injection sites in the anterior and posterior parts of the ARC are indicated by arrowheads (Fig. 1). No destruction of brain parenchyma other than the cannula tracts was observed.

Verification of injection sites of Dyn-SAP or SAP into the ARC
Schematic illustration indicating the injection site at the anterior (bregma –2.5 mm) and posterior (bregma –3.6 mm) ARC according to the rat brain atlas [42] (top panels). Photomicrographs of Nissl-stained brain sections of the anterior and posterior ARC (bottom panels) in a representative animal. Arrowheads show the trace made by inserting the cannula for intra-ARC injection of Dyn-SAP, and arrows point to the tip of the cannula. Dotted lines indicate the areas of the ARC. Scale bars, 200 μm.
3V, third ventricle; ARC, arcuate nucleus; Dyn-SAP, dynorphin-saporin; SAP, saporin
A number of Oprk1-expressing cells were detected in the ARC, PVN, and VMH of the SAP-injected control group (Fig. 2A, 2B, and 2C). In the Dyn-SAP-injected group, Oprk1-expressing cells were scattered in the ARC (Fig. 2D), whereas the distribution of Oprk1-expressing cells in the PVN and VMH was similar to that of the SAP-injected control group (Fig. 2E and 2F). Statistical analysis revealed that the number of Oprk1-expressing cells in the ARC of Dyn-SAP-treated group was significantly lower than in the SAP-injected control group (p < 0.05, Fig. 2G). On the other hand, in the PVN and VMH, the numbers of Oprk1-expressing cells were comparable between Dyn-SAP-injected and SAP-injected control groups (Fig. 2G).

Effect of intra-ARC Dyn-SAP treatment on Oprk1 expression in the hypothalamus
(A–F) Representative photomicrographs of Oprk1 (kappa-opioid receptor gene)-expressing cells in the ARC (A, D), PVN (B, E), and VMH (C, F) of Dyn-SAP- or SAP-injected animals. Insets: Oprk1-expressing cells at a higher magnification. Scale bars, 100 μm. (G) Number of Oprk1-expressing cells in the ARC, PVN, and VMH of Dyn-SAP (DS)- or SAP-injected groups. Values are means ± SEM. The numbers in each column represent the number of animals in each group. * p < 0.05, Student’s t-test.
3V, third ventricle; ARC, arcuate nucleus; Dyn-SAP, dynorphin-saporin; PVN, paraventricular hypothalamic nucleus; SAP, saporin; VMH, ventromedial nucleus of the hypothalamus
Plasma LH profiles in representative rats treated with Dyn-SAP or SAP into the ARC are shown in Fig. 3A. The frequency of LH pulses (number of LH pulses in 3-h sampling period) in the Dyn-SAP-injected group was significantly higher than in the SAP-injected control group (p < 0.01, Fig. 3B). The inter-pulse interval was significantly shorter in the Dyn-SAP group than in the SAP group (p < 0.01, Fig. 3B). The baseline LH concentration was significantly higher in the Dyn-SAP group than in the SAP group (p < 0.05, Fig. 3B). The mean plasma LH level in the Dyn-SAP group tended to be higher than in the SAP group (p = 0.056, Fig. 3B), and the mean amplitude of LH pulses in the Dyn-SAP group tended to be lower than in the SAP group (p = 0.069, Fig. 3B).
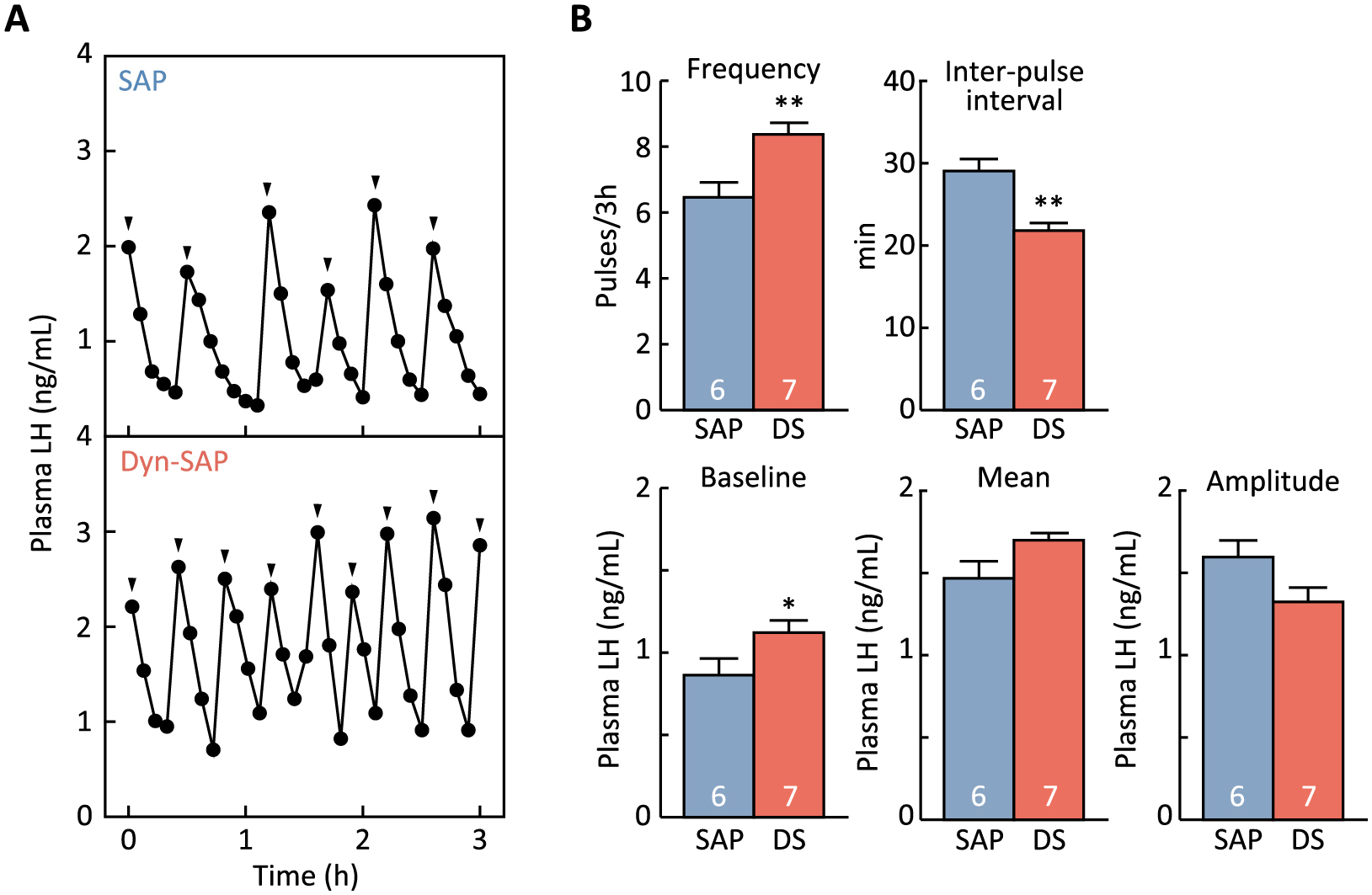
Effect of intra-ARC Dyn-SAP treatment on pulsatile LH secretion in estrogen-primed ovariectomized rats
(A) Profiles of plasma LH concentration in representative rats injected with Dyn-SAP or SAP into the ARC. Arrowheads indicate the peaks of LH pulses identified by the PULSAR computer program. (B) Number of LH pulses (Frequency), mean inter-pulse interval (Inter-pulse interval), mean baseline LH concentration (Baseline), mean LH concentration (Mean), and mean amplitude of LH pulses (Amplitude) during a 3 h sampling period in rats treated with Dyn-SAP (DS) or SAP into the ARC. Values are means ± SEM. The numbers in each column represent the number of animals used in each group. * p < 0.05, ** p < 0.01, Student’s t-test.
ARC, arcuate nucleus; Dyn-SAP, dynorphin-saporin; LH, luteinizing hormone; SAP, saporin
A number of Kiss1-expressing cells were found in the ARC of both intra-ARC Dyn-SAP- and SAP-injected animals (Fig. 4A). No significant difference was found in the number of Kiss1-expressing cells in the ARC between the Dyn-SAP and SAP groups (Fig. 4B).

Effect of intra-ARC Dyn-SAP injection on Kiss1 expression in the ARC of estrogen-primed ovariectomized rats
(A) Representative photomicrographs of Kiss1 (kisspeptin gene)-expressing cells in the ARC of intra-ARC Dyn-SAP- or SAP-injected animals. 3V, third ventricle; scale bars, 100 μm. (B) Number of Kiss1-expressing cells in the ARC of intra-ARC Dyn-SAP (DS) and SAP-injected animals. Values are means ± SEM. The numbers in each column represent the number of animals in each group.
3V, third ventricle; ARC, arcuate nucleus; Dyn-SAP, dynorphin-saporin; SAP, saporin
The present study demonstrated that Dyn-KOR signaling in the ARC, at least in part, plays a role in reducing the frequency of GnRH/LH pulse generation, because the ARC-specific decline of KOR-expressing cells by local Dyn-SAP administration into the ARC increased LH pulse frequency and decreased the inter-pulse interval in female rats. The number of Oprk1-expressing cells in the ARC was significantly decreased in Dyn-SAP-treated rats compared to SAP-treated control rats, suggesting that a part of KOR-expressing cells was successfully deleted by the intra-ARC Dyn-SAP injection in vivo. Importantly, the number of Oprk1-expressing cells in the PVN and VMH of Dyn-SAP-treated groups was comparable to that of the SAP-treated control group, demonstrating that deletion of KOR-expressing cells by ARC-specific Dyn-SAP injection was limited within the ARC. Taken together, the stimulation of the frequency of LH pulses by Dyn-SAP administration may be due to the specific lesion of KOR-expressing cells in the ARC. A previous study reported that GnRH neurons express KOR in rats [38], implying that Dyn neurons may directly act on GnRH neurons to regulate LH secretion in rats. However, the current study demonstrated that ARC-specific attenuation of Dyn signaling accelerated GnRH/LH pulse frequency, suggesting that KOR-expressing cells within the ARC rather than GnRH neurons or other brain regions are involved in the suppression of the firing rate of the GnRH/LH pulse generator.
Interestingly, the number of ARC Kiss1-expressing cells in the Dyn-SAP group was comparable to that of the SAP-treated control group, while Dyn-SAP treatment decreased about half of the number of KOR-expressing cells in the ARC. These results raised the possibility that most KOR-expressing cells deleted by the current Dyn-SAP treatment were non-KNDy KOR neurons. Previous studies reported that 6–20% of ARC kisspeptin neurons express KOR in mice [22, 40] and around 60% of ARC Kiss1 neurons express KOR in female rats [35]. Importantly, our recent study showed that KOR-expressing cells are abundantly located in the whole ARC of female rats [35]. These studies suggest that some KNDy neurons express KOR, while a number of KOR-expressing cells exist other than KNDy neurons in the ARC. More specifically, the majority of non-kisspeptin KOR-expressing cells are located in the dorsal region of the ARC, while KNDy neurons are located in the ventral part of the nucleus [35]. In this context, the current sites where Dyn-SAP was injected were located in the dorsal region of the ARC. Thus, the present Dyn-SAP treatment may have resulted in the deletion of non-KNDy KOR neurons in the ARC, but not KNDy neurons located in the ventral ARC. The deletion of non-KNDy KOR neurons was thought to lead to the changes in LH secretion observed in the current study. This is largely consistent with a previous study by Ruka et al. [47], which showed that Dyn application reduced the firing of the majority of KNDy neurons in mouse brain slices, despite the low colocalization of KOR in KNDy neurons. Ruka’s study and our current findings imply the existence of an indirect Dyn pathway affecting the activity of KNDy neurons. The present study suggests that non-KNDy KOR-expressing cells may play a suppressive role as a modulator for the GnRH pulse generator. In other words, GnRH pulse generation may be controlled by a complicated-neuronal complex composed of KNDy neurons as well as non-KNDy neurons via KOR signaling. Further studies are required to clarify the mechanism mediating Dyn-KOR signaling affecting KNDy neurons and/or non-KNDy neurons to modulate the frequency of GnRH/LH pulse generation.
The present results are consistent with those of previous studies showing that ARC-specific agonism/antagonism of KOR inhibited/activated the frequency of GnRH/LH pulse generation: intra-ARC administration of U50488, a KOR agonist, decreased LH pulse frequency, and elongated pulse interval in OVX rats primed with negative feedback level of E2 [48]; the inter-pulse interval of LH pulses was decreased by the microimplant of nor-BNI into the ARC of OVX ewes [12]. It is likely that the agonist or antagonist administered into the ARC may widely affect KOR-expressing neurons located in the ARC, including a part of KNDy neurons as well as non-KNDy neurons in the ARC [35, 38]. Thus, the total response of ARC KOR-expressing neurons would affect the activity of KNDy neurons and subsequent GnRH/LH pulses. It should be noted that the present study had the advantage of suggesting the involvement of non-KNDy KOR expressing cells in GnRH pulse generation because this approach using Dyn-SAP enabled the irreversible deletion of KOR-expressing cells compared with the approach using KOR agonists/antagonists that only transiently cause KOR agonism/antagonism.
In conclusion, the present study demonstrated that KOR-expressing cells in the ARC have an inhibitory effect on the frequency of GnRH/LH pulse generation. Our results also suggest that non-KNDy neurons expressing KOR in the ARC would be involved in mediating the Dyn inhibitory signal to suppress the activity of KNDy neurons and subsequent GnRH/LH release. Further studies are needed to identify the non-KNDy KOR neurons in the ARC and understand the role of Dyn-KOR signaling in regulating GnRH pulse generation as a whole.
We respectfully acknowledge the contributions of our late colleague Professor Kei-ichiro Maeda, who suddenly passed away during the preparation of this manuscript. His leadership and supervision contributed significantly to this work. We thank Prof. Yoshitaka Oka and Dr. Tomomi Karigo at the Graduate School of Sciences, The University of Tokyo for designing the cRNA probe of Oprk1. We are grateful to the National Hormone and Peptide Program for the rat LH RIA kit. The 125I-labeled rat LH for RIA was kindly provided by the Laboratory of Animal Production Science at Nagoya University. This work was supported in part by the Grants-in-Aid for Scientific Research (Grant 18H03973 to H. T. and Grant 16H06206 to F. M.) from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
None of the authors have any potential conflicts of interest associated with this research.