2022 Volume 69 Issue 10 Pages 1159-1172
2022 Volume 69 Issue 10 Pages 1159-1172
Findings of preclinical studies and recent phase I/II clinical trials have shown that mesenchymal stem cells (MSCs) play a significant role in the development of diabetic kidney disease (DKD). Thus, MSCs have attracted increasing attention as a novel regenerative therapy for kidney diseases. This review summarizes recent literature on the roles and potential mechanisms, including hyperglycemia regulation, anti-inflammation, anti-fibrosis, pro-angiogenesis, and renal function protection, of MSC-based treatment methods for DKD. This review provides novel insights into understanding the pathogenesis of DKD and guiding the development of biological therapies.
Diabetes mellitus (DM) still commonly leads to progressive chronic kidney disease (CKD) despite the availability of advanced medical practices. The pathogenesis of diabetic kidney disease (DKD) involves a complex network of primary and secondary mechanisms with intrarenal and systemic components [1, 2]. Apart from inhibiting the renin–angiotensin–aldosterone system, sodium–glucose cotransporter-2 inhibitors also attenuate DKD progression, according to the results of a DAPA trial [3]. The natural course of DKD includes glomerular hyperfiltration, progressive albuminuria, declining glomerular filtration rate, and end-stage renal disease (ESRD) [4, 5]. Consequent to this global pandemic, the incidence of macro- and microvascular complications associated with DM, particularly DKD and ESRD, has increased [6]. DKD is now understood to occur in the setting of a pro-inflammatory milieu driven by metabolic dysregulation and mediated by humoral factors that cause pathogenic structural and functional alterations in the kidney. The main clinical manifestation is the presence of albuminuria, which can range from mildly increased albuminuria (albumin–creatinine ratio (ACR) <30 mg/g) to moderate (ACR 30–300 mg/g) or severe (ACR >300 mg/g) albuminuria [7]. Nevertheless, the absence of albuminuria does not exclude the presence of DKD [8].
Mesenchymal stem cells (MSC), the most widely studied multipotent stem cell, lack specific and unique markers when cultured in vitro. Human MSCs do not express the hematopoietic markers CD45, CD34, and CD14 or the costimulatory molecules CD80, CD86, and CD40 but express variable levels of CD105 (also known as endoglin), CD73 (ecto-5-nucleotidase), CD44, CD90 (THY1), CD71 (transferrin receptor), ganglioside GD2, and CD271 (low-affinity nerve growth factor receptor), which are recognized by the monoclonal antibody STRO-1 [9, 10]. Furthermore, MSCs are more pluripotent than normal somatic cells and not only differentiate into adipocytes, osteoblasts, chondrocytes but also into nonmesodermal cells, such as pancreatic beta cells, both in vitro and in vivo [11]. MSCs are distributed in many tissues and organs, including the bone marrow (BM), adipose tissue, lung, kidney, liver, brain, and umbilical cord (UC) blood [12]. These cells can be implanted into the kidney, improve renal function, and regenerate glomerular structure, effectively treating DKD [13].
Among stem cells, MSCs have several advantages for therapeutic use, such as exerting a wide range of immune regulatory effects and correcting the imbalance of cytokines by regulating the proportion of lymphocyte subsets, particularly by increasing the proportion of regulatory T lymphocytes, reducing the release of pro-inflammatory cytokines, and increasing the release of anti-inflammatory cytokines [14]. In addition, MSCs can directly regulate the balance of cytokines by upregulating the expression of p21WAF1, p16INK4A, and p27Kip1 and downregulating cyclin D2 [15]. Subsequently, MSCs inhibit the proliferation of T cells by arresting their cell cycle at the G0/G1 phase. Moreover, MSC-based treatment of rats with DKD downregulates the mRNA levels of interleukin (IL)-1, IL-6, tumor necrosis factor-alpha (TNF-α), and monocyte chemotactic protein 1 (MCP-1) and reduces the infiltration of CD8 T-cells in the kidney. MSCs significantly reduce the expression of transcription factors (basic leucine zipper transcription factor ATF-like 3, Batf3, and DNA-binding protein inhibitor ID-2 and Id2) and FMS-like tyrosine kinase-3, both of which are required for the development of CD103+ dendritic cells (DCs) [16]. The protective effect of MSCs may be related to their immunosuppressive effects on CD8+ T cell proliferation and activation mediated by CD103+ DCs in DKD rat kidneys [16]. MSCs can influence T cell activation and differentiation. Several lines of evidence have shown that MSCs suppress the interferon-γ and IL-17 secretion but promote the IL-10 production of T cells by inhibiting the differentiation of Th1 and Th17 cells, thereby inducing the generation of regulatory T cells (Tregs) [17, 18]. Similarly, MSCs inhibit B-cell function by reducing IL-4-stimulated B-cell proliferation and interfering with the production and chemotaxis of IgM, IgG, and IgA antibodies [10]. Stem or progenitor cell therapies, which promote pro-regenerative mechanisms, provide an alternative strategy for modulating complex disease processes. For example, inflammatory signals stimulate MSCs to produce various growth factors that aid tissue repair by promoting angiogenesis, extracellular matrix remodeling, and differentiation of tissue progenitor cells [14].
MSC-based therapy has broad application prospects in DKD treatment. MSC engraftment can significantly ameliorate proteinuria, serum creatinine/urea, and improve renal pathological changes, including glomerular basement membrane thickening, glomerular sclerosis, tubule dilatation, mesangial proliferation, podocyte foot process effacement, and interstitial fibrosis [12, 19]. In a study by Chen et al., diabetic rats were divided into three groups: group 1 (control group), group 2 (diabetic nephropathy (DN) group), and group 3 (UC-MSC-treated DN group) (12). The rats in the UC-MSC-treated DN group showed significantly lower (p < 0.05) serum creatinine levels and urinary albumin excretion rate after 8 weeks compared with those in the DN group [12].
Although almost all in vivo studies investigating MSCs for DKD using animal models have been performed on mice or rats, other animals can also be used as DN models [20]. Tree shrews, which are more closely related to primates than rodents, are being studied as alternatives to primates [21]. The symptoms of spontaneous diabetes in tree shrews are similar to those in humans [22]. Pan et al. reported that a new DKD model in tree shrews can be used to evaluate the effect of BM-derived MSCs (BM-MSCs) [22]. They found that BM-MSC transplantation can decrease the levels of glucose, triglycerides, total cholesterol, 24 h urine volume levels of creatinine and urea nitrogen, and 24 h proteinuria. An et al. developed a rhesus macaque model of DKD and found that MSCs reduce blood glucose levels and daily insulin requirement [20]. The two species mentioned above have greater genome homology with Homo sapiens than with rodents. In addition, they can be used to study DKD [20].
In addition, researchers have attempted to promote MSC function [23, 24]. MSCs pretreated with melatonin (MT) ameliorate kidney function in a rat model of DN. Furthermore, the metabolic environment exerts an important influence on MSC performance [25-27]. Crigna et al. found that exosome and cytokine release may be more relevant for therapeutic actions than MSCs [27]. The above-mentioned studies indicate that MSCs may act as a new target in the management of diabetes and may be used as a new therapy for DKD.
MSCs migrate to the site of injury, release soluble factors and extracellular vesicles, interact with other cells, and signal for stem cell support to exert renal trophic and immunomodulatory effects [28, 29]. MSCs differentiate into insulin-secreting cells, mesangial cells, tubular epithelial cells, endothelial cells, and podocytes [30]. In a previous study, insulin-producing cells were produced from human BM-MSCs through genetic engineering [31]. The use of renal magnetic resonance imaging techniques is expected to facilitate the early detection of DKD, break new ground in the microstructural evaluation of the kidney, provide an improved understanding of the pathophysiology of DKD at different phases, inform decision-making and monitoring for therapies, and pave the way for personalized medical care [32]. This method shows changes in the microstructure of DKD, such as tubulointerstitial and glomerular fibroses [33]. Therefore, specific and sensitive biomarkers are required for DKD diagnosis. To date, therapeutic strategies for DKD rely on comprehensive control, including amelioration of albuminuria, hyperglycemia, and hypertension and administration of renin–angiotensin–aldosterone system inhibitors and new reno-protective drugs [1, 34, 35], such as sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide-1 (GLP-1), and exosomes from MSCs. Moreover, stem cells ameliorate DKD by promoting paracrine, endocrine, or autocrine mechanisms, such as neovascularization, anti-inflammatory, cytoprotective, and immune regulatory activities [29]. MSC autocrine activity is induced by secreted factors that act on stem cells [36]. Endocrine-like effects occur when an endocrine organ secretes hormones and factors that act on other distant tissues [36]. The major benefits of MSC-based therapy are widely accepted to be derived from the effects of secreted factors acting on neighboring cells via a paracrine phenomenon [36].
MSC treatment and DKDIn addition to the higher risk of DKD, multiple interventional studies have shown a recovery of renal function after MSC therapy. According to the official database of the U.S. National Institutes of Health (Table 1). A multicenter, randomized, double-blind, dose-escalating, sequential, placebo-controlled trial registered at ClinicalTrials.gov (NCT03840343) evaluated the safety, tolerability, and efficacy of autologous adipose tissue-derived MSCs in 30 patients with progressive DKD. The patients received a single intravenous (IV) infusion (2.5 × 105 cells/kg or 5.0 × 105 cells/kg), and the trial duration was 3 months. This clinical study demonstrated that a single IV infusion in patients with DKD may be considered safe because no acute adverse events (AEs) associated with infusion, treatment-related AEs, or serious AEs were deemed treatment related by the investigators. Another randomized, placebo-controlled clinical trial (NCT01843387) assessed a single IV infusion of allogeneic mesenchymal precursor cells, 150 × 106 (n = 10), 300 × 106 (n = 10), or placebo (n = 10), in adults with DN for 60 weeks. Most of the clinical trials are aimed at exploring the safety and therapeutic effects of cell-based therapy, indicating that there is still a long way to go before such cell therapy can be used in clinical practice [37].
| ClinicalTrials.gov identifier | Cell type | Subject number | Cell dosage | Route | Trial status |
|---|---|---|---|---|---|
| NCT01843387 | BM-MPC | 30 | 1 dose: 150, 300 × 106 cells | IV | Completed |
| NCT04562025 | UC-MSCs | 38 | 3 doses: 1 × 106 cells | IV | |
| NCT02585622 | BM-MSCs | 48 | 1 dose: 80, 160, 240 × 106 cells | IV | |
| NCT04216849 | UC-MSCs | 54 | 5 doses: 1.5 × 106 cells/kg | IV | Phase 2 |
| NCT03288571 | UC-MSCs | 20 | 3 doses: 1 mL cell suspension | Renal parenchyma | Phase 2 |
| NCT03840343 | AD-MSCs | 30 | 2 doses: 2.5, 5 × 106 cells/kg | Intra-arterial delivery | Phase 1 |
| NCT04125329 | UC-MSCs | 15 | 3 doses: 1 × 106 cells/kg | IV | Early phase 1 |
The recruitment and differentiation of MSCs can replace damaged cells to ameliorate the inflammatory microenvironment [38] (Table 2). In rats with type 1 diabetes mellitus (T1DM) induced by a single intraperitoneal injection of streptozotocin (STZ), Li et al. demonstrated that early intervention with MSCs prevents renal injury via immune regulation, which restores homeostasis of the immune microenvironment, contributing to the prevention of kidney dysfunction and glomerulosclerosis [39]. Furthermore, MCP-1 is a specific chemokine that recruits and activates monocytes from the circulation to the inflammatory site and contributes to the infiltration of macrophages into both mesangial and tubulointerstitial lesions in DKD [40]. Importantly, the expression of MCP-1 and number of infiltrated macrophages in the kidney are effectively suppressed by MSC treatment [41]. In addition, MSC treatment ameliorates DKD by inhibiting MCP-1 expression via hepatocyte growth factor secretion, thus reducing macrophage infiltration in diabetic kidneys and downregulating IL-1β, IL-6, and TNFα expression in renal tissue [41].
| MSC types | Injection methods | Frequency and Dose | Experimental models | Outcomes | Reference |
|---|---|---|---|---|---|
| Rat BM-MSCs |
Tail vein injection | Four doses (2, 4, 5, and 7 weeks after the onset) 5 × 106 |
STZ-induced type I Sprague Dawley rats | Upregulate anti-inflammatory cytokines IL-10 and EGF Downregulate inflammatory cytokines such as IL-6, MCP-1, TNF-α, and IL-1β |
[39] |
| Rat BM-MSCs |
Tail vein injection | Once a week for twice 2 × 106 |
STZ-induced type I female Wistar rats | Suppress the expression of MCP-1 and the number of infiltrated macrophages Downregulate the expressions of IL-1β, IL-6, and TNF-α Upregulate the expression of HGF |
[41] |
| Rat BM-MSCs |
Tail vein injection | Weekly for 6 weeks 1 × 107 |
STZ-induced type I Sprague Dawley rats | Recover kidney function and diminish renal injury, fibrosis Suppress CD103+ DCs-mediated CD8+ T cell responses |
[16] |
| Rat BM-MSCs |
Tail vein injection | Once a week for 2 weeks 5 × 106 |
STZ-induced type I Sprague Dawley rats | Downregulate serum TNF-a, IL-6, IL-8, and IFN-r Inhibit TGF-β/Smad signaling Upregulate LXA4 |
[48] |
| Mice UC-MSCs |
Tail vein injection | Weekly for 4 weeks 1 × 104 |
STZ-induced BALB/C mice | Decrease the deposition of fibronectin and collagen I by inhibiting TGF-β1-triggered MFT Elevate the levels of MMP2 and MMP9 Inhibit PI3K/Akt and MAPK signaling pathways |
[47] |
| Human UC-MSCs |
Tail vein injection | Three doses (5, 9, and 13 weeks after STZ injection) 5 × 106 |
STZ-induced type I CD1 mice | Reduce M1 macrophage polarization Increase arginase-1 (Arg1) expression in macrophages |
[45] |
| Human UC-MSCs |
Tail vein injection | One dose 2 × 106 |
STZ-induced type I Sprague Dawley rats | Downregulate the expressions of IL-1β, IL-6, and TNF-α Reduce the level of pro-fibrotic factor (TGF-β) Suppress the recruitment of F4/80-labeled macrophages and CD3-labeled lymphocytes |
[44] |
In the immune microenvironment, MSCs reduce renal CD68+ macrophage infiltration and inflammatory cytokine expression in the kidneys of diabetic rats [39]. Recent studies have shown that MSC administration reduces macrophage infiltration into renal tissue [42] and switches macrophages from an M1 to M2 phenotype, promoting tissue homeostasis [38]. MSCs increase arginase-1 expression in macrophages to reduce M1 macrophage polarization, and these effects may halt the vicious cycle of inflammation and mitochondrial dysfunction in tubular epithelial cells (TECs) [43]. In addition, UC-MSC treatment inhibits the recruitment of F4/80-labeled macrophages and CD3-labeled lymphocytes in kidney tissues and reduces the inflammatory response [44]. The expression of transcription factor EB (TFEB) is also related to macrophage polarization. According to one study, MSCs induce macrophage transformation into the M2 phenotype via a TFEB-dependent mechanism in STZ-induced type 1 BALB/c mice [45]. The restoration of lysosomes and autophagy, as well as mitochondrial bioenergetics in macrophages, is activated by TFEB transcription, which inhibits pro-inflammatory reactions [45]. These results suggest that MSCs inhibit inflammation by affecting macrophage function. Similarly, in the kidneys of diabetic mice, MSCs have been associated with macrophage recruitment via the expression of markers, such as C-C motif chemokine ligand 2, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1 [41].
By contrast, MSCs acquire an anti-inflammatory phenotype and suppress the activation and effector functions of inflammatory DCs, T lymphocytes when engrafted in tissues with high levels of inflammatory cytokines, which occurs during the late phase of inflammation [46]. Zhang et al. found that BM-MSCs could reduce the population and maturation of CD103+ DCs; alleviate CD103+ DC-mediated CD8 T cell responses; decrease expression levels of IL-1β, IL-6, TNF-α, and MCP-1; ameliorate local inflammation; and exhibit a protective effect on the diseased kidney of rats with DKD [16]. Moreover, MSC-conditioned media indirectly impacted the proliferation and activation of CD8 T cells by reducing the number and suppressing the maturation of CD103+ DCs in vitro [16].
UC-MSCs can alleviate renal fibrosis in a DKD cell model by inhibiting myofibroblast transdifferentiation caused by TGF-β, blocking mesangial cell proliferation induced by PI3K/Akt and MAPK signaling pathways, and elevating the levels of MMP2 and MMP9 [47]. Fibrosis and epithelial–mesenchymal transformation are typical pathological changes in DKD, resulting in serious glomerular sclerosis and impaired filtration function. Interestingly, fibrosis and inflammation share several common pathways, and treatment with MSCs ameliorates both conditions. Aside from decreasing the expression of inflammatory factors, MSC-based treatment also decreases the expression of collagen type I, fibronectin, laminin β1, and TGF-β in the kidneys of rats with DKD, suggesting that MSCs can inhibit fibrosis as well [44]. Lipoxin A4 derived from MSCs reduces TGF-β and Smad2/Smad3 expression, two critical factors attributed to extracellular matrix dysfunction, thereby reversing fibrosis in rats with DKD (Fig. 1) [48].
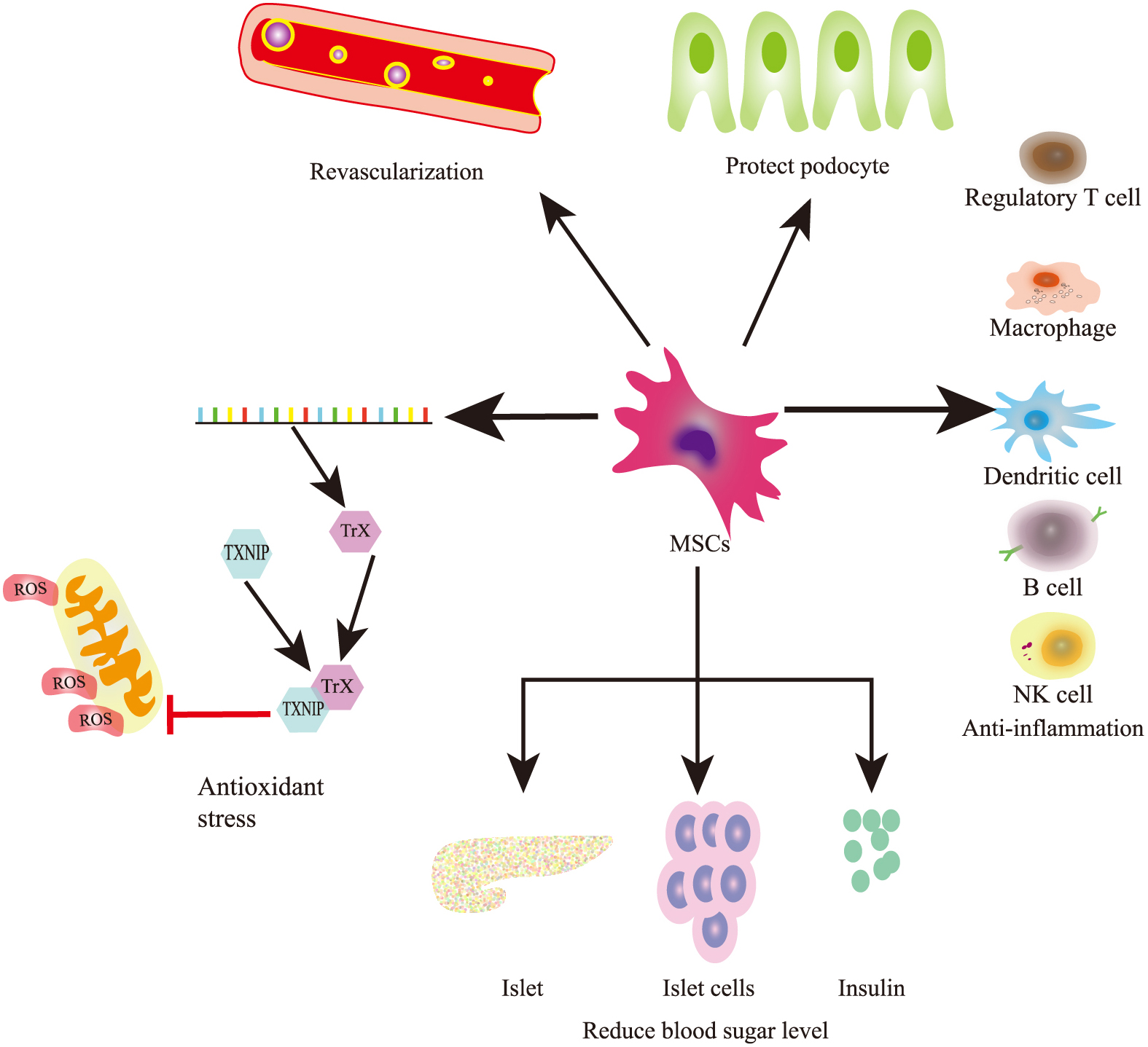
The therapeutic mechanisms of MSCs in DKD
Oxidative stress increases the vulnerability of renal cells. Adipose-derived mesenchymal stem cell (AD-MSC) transplantation significantly inhibits the increase in malondialdehyde and carbonyl protein, markers of oxidative stress, compared with vehicle treatment (Table 3). The reduced generation of reactive oxygen species (ROS) after MSC treatment is mainly attributed to the decline in blood glucose levels and cellular glucose uptake mediated by GLUT1 [49]. The reduction of blood glucose levels by MSC transplantation promotes the inhibition of oxidative stress in kidneys with diabetes [49].
| MSC types | Injection methods | Frequency and Dose | Experimental models | Outcomes | Reference |
|---|---|---|---|---|---|
| Rat BM-MSCs |
Tail vein injection | Once a week for twice 2 ×106 |
STZ-induced type I female Wistar rats | Suppress the expression of TGF-β and membrane localization of GLUT1 | [49] |
| Human UC-MSCs |
Tail vein injection | Once a week for twice 2 × 106 |
STZ-induced type I Sprague Dawley rats | Inhibit TXNIP upregulation Reduce 24-hour urinary total protein, urinary albumin to creatinine ratio, serum creatinine, and blood urea nitrogen |
[12] |
Apoptosis signal-regulating kinase 1 (ASK1), a redox-regulated apoptosis signal kinase, is usually bound to thioredoxin 1 (TRX1) under basal conditions [50]. TRX1 is a small redox protein that controls ROS levels and limits apoptosis due to oxidative stress, whereas thioredoxin-interacting protein (TXNIP) inhibits the antioxidant function of TRX. Once TXNIP shuttles into the cytosol from the nucleus under high-glucose conditions, TXNIP binds to TRX1 and the ASK1–TRX1 complex is disrupted. The TXNIP–TRX1 complex inhibits TRX1 in response to excessive ROS, resulting in oxidative stress and apoptosis [50]. As a redox-sensitive protein, HMGB1 translocates from the nucleus to the cytosol under oxidative stress stimulation, which further damages high-glucose-induced endothelial cells [51]. However, in rats with STZ-induced T1DM, BM-MSCs downregulated the expression of TXNIP induced by hyperglycemia and reduced the oxidative stress reaction in endothelial cells, significantly alleviating nephrocyte injury and improving renal function [12].
Mitochondrial dysfunction and excessive mitochondrial ROS levels are significant contributors to diabetic endothelial injury [52]. When human umbilical vein endothelial cells are cultured in medium, MSCs induce the recovery of mitophagy-related genes PINK1 and PARKIN, in addition to inducing the expression of TFAM, a gene related to the biogenesis process. TFAM contributes to removing dysfunctional mitochondrial debris that can renew mitochondrial networks, in association with the sustained formation of novel mitochondrial structure through biogenesis [52].
MSCs protect podocytes and proximal tubular cellsPodocytes play a major role in maintaining the integrity and permeability of the glomerular filtration barrier [19] (Table 4). In DKD, the effacement of podocyte foot processes and loss of slit diaphragm proteins result in leakage of albumin and proteinuria [53]. MSC treatment reduces podocyte injury and protein excretion via BMP-7 secretion, which can suppress albuminuria and renal tubule injury in vitro and in vivo [54]. In addition, MSC-derived BMP-7 improves glomerular fibrosis and podocyte injury by inhibiting the TGF-β/Smad signaling pathway in vivo and in vitro [55]. LipoxinA4 derived from MSCs could reduce TGF-β and Smad2/Smad3 expression, two critical factors attributed to extracellular matrix dysfunction, reversing the fibrosis process in DKD rats [48].
| MSC types | Injection methods | Frequency and Dose | Experimental models | Outcomes | Reference |
|---|---|---|---|---|---|
| Rat BM-MSCs |
Tail vein injection | Once a week for twice 2 × 106 |
STZ-induced type I female Wistar rats | Secrete a significant amount of BMP7 Inhibit the TGF-β/Smad signaling pathway |
[55] |
| Rat BM-MSCs |
Left renal artery injection | One dose 2 × 106 |
STZ-induced type I Sprague Dawley rats | Inhibit the Loss of podocytes Upregulate the express of BMP-7 |
[56] |
| Rat BM-MSCs |
Tail vein injection | Single-dose (4 weeks after the onset) 3 × 106 |
STZ-induced type I Sprague Dawley rats | Suppress Notch signalling pathway MSCs combined with miR-124a enhance proliferation and inhibit apoptosis of podocytes |
[59] |
Simultaneously, MSC treatment reduces podocyte loss, effacement of foot processes, widening of foot processes, thickening of glomerular basement membrane, and loss of glomerular nephrin and podocin [4, 56-58]. Sun et al. reported that stem cells from the BM relieve high glucose-induced podocyte apoptosis in combination with miR-124a by inhibiting the Notch signaling pathway [59].
In addition to podocytes, proximal tubular cells (PTCs) play a crucial role in the pathogenesis of DKD and can be used as therapeutic targets. Exfoliation and apoptosis of TECs eventually result in renal fibrosis and accelerate the progression of DKD to ESRD. Therefore, MSCs are a promising cell source for protecting PTCs [60]. Islam et al. found that HU-MSCs modulate high glucose-induced inflammatory responses in renal PTC monolayers [61]. Konari et al. demonstrated that BM-MSCs also transfer their mitochondria to impaired PTCs when cocultured in vitro, which suppress the apoptosis of impaired PTCs [62].
MSCs in the maintenance of blood glucose homeostasisUC-MSCs may serve as a novel cell source for the treatment of DKD. MSCs can differentiate into beta cells that express beta cell-specific genes (Pdx/1, Ngn/3, and NeuroD1) [63] (Table 5). UC-MSCs were collected for genome-wide methylation analysis after being cultured in a high-glucose medium. Pan et al. discovered that the Notch signaling pathway of MSCs is highly inhibited by high glucose treatment via the methylation of Notch-related genes (NOTCHI and KAT2A), implying that MSCs could be directionally differentiated into functional β-cells [22]. In addition, HUMSCs in Wharton’s jelly (WJ) of the UC have the potential to be excellent candidates in β-cell replacement therapy for diabetes. Gao et al. found that infusion of apelin-transduced WJ-MSCs into rats with type 2 diabetic mellitus (T2DM) not only significantly improves insulin sensitivity and glucose disposal but also promotes the proliferation of endogenous pancreatic β cells and increases the plasma levels of insulin and C-peptide [64]. In addition, WJ-MSCs can develop into pancreatic lineage cells in vitro and operate both in vitro and in vivo as insulin-producing cells [63, 65]. Conversely, WJ-MSCs, which contribute to the regeneration of β cells, repair the destroyed islets by reducing the severity of insulitis in DM mice [66]. UC-MSC transplantation may be a novel therapeutic approach for the treatment of T2DM. Preculturing islets with MSCs using a direct contact configuration maintains functional β-cell mass in vitro and the capacity of scultured islets to reverse hyperglycemia in STZ induced DM mice. In addition, researchers have demonstrated that MSC infusion during the early phase significantly ameliorates the destruction of pancreatic islets and produces a morphology similar to that of normal islets in insulitis of NOD mice [66].
| MSC types | Injection methods | Frequency and Dose | Experimental models | Outcomes | Reference |
|---|---|---|---|---|---|
| Rat AD-MSCs |
Tail vein injection | Four doses (Once every 2 weeks) 2 × 106 |
HFD-induced type 2 Sprague Dawley rats | Inhibit skeletal muscle MG53 elevation Alleviate insulin resistance Decrease IRS-1 and phosphorylated-AKT levels |
[68] |
| Mice AD-MSCs |
Tail vein injection | One dose 3 × 106 |
HFD-induced type 2 Sprague Dawley rats | Downregulate inflammatory cytokines such as IL-6 Reduce blood glucose levels |
[69] |
| Human WJ-MSCs |
Tail vein injection | One week 2 × 106 |
HFD-induced type 2 Sprague Dawley rats | Increase insulin and C-peptide levels in the plasma Decrease IL-6 and TNF-α |
[64] |
| Human WJ-MSCs |
Retro-orbital vein | One dose 5 × 105 |
STZ-induced type I NOD mice | Increase regulatory T cell Decrease dendritic cells Repair the destroyed islets |
[66] |
| Human BM-MSCs |
Tail vein injection | (1, 3 and 6 weeks after the onset) 2 × 106 |
HFD-induced type 2 Sprague Dawley rats | Reduce blood glucose levels Promote β-cell function Upregulate GLUT4 expression |
[67] |
| Human UC-MSCs |
Tail vein injection | One dose 3 × 106 |
HFD-induced type 2 Sprague Dawley rats | Alleviate insulin resistance Elicit M2 polarization |
[70] |
MSC infusion may restore the concentration of total GLUT4 and improve the translocation of GLUT4 to the cell membrane, thus facilitating glucose utilization to ameliorate insulin resistance [67]. Si et al. established a diabetic rat model through high-fat diet/STZ administration, performed MSC infusion during the early phase (seven days) or late phase (21 days) after STZ injection, and then evaluated the therapeutic effects of MSC infusion [67]. They found that MSC-based treatment not only increases the expression of GLUT4 on the insulin target cell membrane of rats with T2DM but also partially restores the feeding-induced phosphorylation of insulin receptor substrate-1 (IRS-1) and Akt. Mitsugumin 53 (MG53) is an E3 ligase that has recently been implicated in the aggravation of insulin resistance by promoting IRS-1 ubiquitination in the skeletal muscles. Deng et al. revealed that the infusion of AD-MSCs inhibits skeletal muscle MG53 elevation, thereby alleviating insulin resistance in rats with T2DM [68].
Macrophage polarization is another anti-inflammatory approach for diabetes that enhances insulin sensitivity. A novel mechanism involves the regulation of M2 macrophage polarization and promotion of IL-6 production in adipose tissue [69]. Another study indicated that MSCs improve hyperglycemia by regulating hepatic glucose metabolism in an AMP-activated protein kinase signaling pathway-dependent manner [70]. In rats with T2DM induced by a high-fat diet combined with STZ, human UC-MSC infusion produces significant antidiabetic effects, promotes insulin sensitivity, and directs adipose tissue macrophages into an alternatively activated phenotype (M2, anti-inflammatory). UC-MSCs control the polarization of alternatively activated macrophages (M2) and function in the resolution of inflammation and maintenance of insulin sensitivity via the IL-4R-STAT3/STAT6-PPARγ axis [71]. Montanari et al. suggested that MSC interactions via N-cadherin provide an optimal microenvironment for fine tuning insulin secretion and that MSC interactions are pivotal to support islet graft function in clinical islet transplantation in diabetic C57BL/6 mice [72].
Some studies have consistently suggested that MSCs are competent in mediating improvements in islet function. Rackham et al. demonstrated that the co-transplantation of islets and MSCs into diabetic animals improves islet functional survival and glycemic control [73]. Simultaneously, researchers have demonstrated that MSC infusion during the early phase significantly ameliorates the destruction of pancreatic islets and produces a morphology similar to normal islets [73]. Montanari et al. demonstrated that subcapsular MSC transplantation might be an ideal route for improving the survival and function of islets in STZ-induced T2DM mice [72]. These results reveal that MSCs are beneficial for decreasing blood glucose levels by improving islet function.
MSCs induce angiogenic activitiesA study using a Transwell system showed that MSCs can increase the expression of vascular endothelial growth factor by the islets themselves compared with islets cultured alone or those cultured with MSCs [74, 75] (Table 6). Another study investigated the pro-angiogenic function of exosomes from MSCs. Urine stem cell-derived exosomes, which are involved in angiogenesis and cell survival, contain increased levels of VEGF, TGF-β, and angiogenin [76]. Li et al. demonstrated that exosomes are active components in paracrine secretion by human endothelial progenitor cells and can promote vascular repair by upregulating endothelial cell function [77]. Taken together, MSC treatment can rescue kidney dysfunction and slow down DKD progression by inducing angiogenesis.
| MSC types | Injection methods | Frequency and Dose | Experimental models | Outcomes | Reference |
|---|---|---|---|---|---|
| Rat BM-MSCs |
Conditioned media | BM-MSCs were seeded at 104/cm2 in a conditioned medium | Type 2 diabetes mellitus rats | Unique secretome with distinct angiogenic properties in the diabetic milieu | [74] |
| Urine-derived exosomes | Tail vein injection | Weekly for 12 weeks (100 μg of exosomes) | STZ-induced type I Sprague Dawley rats | The VEGF, TGF-β, and angiogenin might be related to angiogenesis | [76] |
Stem cell therapy remains challenging because of several reasons, such as poor engraftment and limited differentiation of stem cells caused by the diabetic microenvironment, differentiation into unwanted cell lineages, and malignant transformation or genetic aberrations of stem cells. At present, various studies on the therapeutic effects of stem cells in DKD have been reported, but the specific mechanism of action of stem cells remains unclear.
MSCs have emerged as potential candidates for cell-based therapies to modulate the immune response in organ transplantation and tissue repair after acute or chronic kidney injury. In vivo injection of exogenous MSCs can migrate to the injured kidney, where the MSC secretome generates an environment that limits kidney injury and promotes tissue repair and regeneration. In addition, the frequency of MSCs in primary sources, cell expansion potential, and MSC secretome vary considerably from donor to donor and depend on donor age and disease condition [78, 79]. These shortcomings, coupled with the evidence that MSCs are low-immunogenic and immune-evasive cells, have stimulated the development of allogeneic MSC products. Methods to purify the MSC population are currently being investigated with the aim of isolating a specific cell subpopulation with an efficacy similar or superior to that of the heterogeneous MSCs currently used [80]. Thus, efficacy trials of MSC-based therapy should also include long- and short-mechanistic studies, and methods should be standardized to facilitate comparison, such as quantitative RNA analysis for specific genes, flow cytometry analysis for cell surface markers, and protein-based assay of the secretome [81]. Although this approach will enable the development of a clinical-grade allogeneic homogeneous cell population for use in future clinical trials, a consensus on how best to ascertain the immune potency of MSCs remains to be established. The lack of specific cell markers for MSCs complicates isolating an effective cell subpopulation from the initial heterogeneous cell preparation. Furthermore, extensive ex vivo expansion of BM- or UC-derived MSCs is required to generate enough cells to treat a given patient cohort. This is a major challenge, because MSCs lose their immunosuppressive and trophic potencies over time during culture. The debate regarding the cell dose and whether single or repeated infusions should be used for a given outcome is far from being resolved. Therefore, well-designed dose-finding studies and appropriate cost-effectiveness analyses are needed before applying this innovative cell therapy to widespread clinical trials [80].
There have been approximately 530 clinical studies that focused on allogeneic stem cell treatment using a variety of stem cell types, including hematopoietic stem cells, MSCs, and CAR-T cells, as well as 629 trials that utilized autologous cell sources [82]. Autologous MSCs undergo culture-expansion characteristics comparable to those of individuals with normal kidney function and maintain the capacity to inhibit the anti-donor human leukocyte antigen immune response [83]. In accordance with the International Society for Cell & Gene Therapy guidelines, a recent update includes analyses that mitigate the heterogeneity of MSCs, such as assays that demonstrate the secretion of trophic factors, modulation of immune cells, and other relevant functional properties, such as angiogenesis [84]. In terms of efficacy, autologous- and allogeneic-derived MSCs effectively change the metabolic hallmarks of DM, such as C-peptide synthesis, and lower exogenous insulin requirement, fasting blood glucose, and glycated hemoglobin levels [85]. The therapeutic potential of MSCs may be affected by the source of MSCs and type of DM. MSCs isolated from patients with T1DM show preserved morphology, growth kinetics, multipotency, and proliferative, immunomodulatory, immunosuppressive, and migratory abilities [86]. By contrast, MSCs extracted from patients with T2DM exhibit increased senescence, decreased viability, increased apoptosis (increased proapoptotic gene expression, such as p53, caspase 9, and BAX, and low antiapoptotic gene expression, such as Bcl-2), reduced proliferative potential associated with increased doubling time, and decreased angiogenic potential [87].
From 2006 to 2012, the frequency of new phase 1 and 2 MSC clinical trials increased steadily, but plateaued and declined in 2018 [88]. Importantly, MSC efficiency may be affected by other factors, such as infusion number, delivery route, homing capacity, microenvironment, and condition severity [87, 89, 90]. Therefore, systemic administration of MSCs (intravenous) has been clinically effective in reducing the insulin intake of patients with T2DM, but with limited duration, which could be explained by host factors, such as chronic hyperglycemia and sustained oxidative stress and inflammation, and MSC properties, including being trapped in the lungs, decreased proliferation and migration abilities, and increased apoptosis and senescence rates in DM setting. As part of the safety evaluation of these cells for stem cell-based therapy, reliable analyses must be implemented to detect adipose-derived stem cell (ASC) populations that accumulate chromosomal abnormalities or even undergo malignant transformation after extensive in vitro multiplication [91]. Concerning the possible side effects of MSC-based therapies, one could say that the widely used immunosuppressive drugs, which have many infectious, metabolic, and cancerous side effects, would not be approved for clinical use under the current safety standards [90].
Potential of MSC therapy in DKDCell therapy is a new strategy for the treatment of complex disorders and has potentially more value than single-agent drug therapy because of the highly versatile response of cells to their environment in situ and systemically. The UC is composed of embryonic tissues, including the umbilical vein, umbilical artery, WJ, and amniotic membrane. UC blood, WJ, and amnion are sources of fetal appendage-derived MSCs, which are reportedly better for cell proliferation and expansion than BM-MSCs [92]. WJ and perivascular tissues contain various biologically active substances, including growth factors, cytokines, extracellular matrixes, and microvesicles, which are essential for providing the physiological environment to preserve MSC properties [79].
Other studies have shown that the cellular prion protein (PrPC) is a therapeutic target for protecting the mitochondria against CKD-induced endoplasmic reticulum stress [93]. One team observed that cotreatment of MSCs with MT and pioglitazone may represent a novel strategy for the development of MSC-based therapies for patients with CKD [94]. MT preserves the proliferative power of MSCs and decreases their senescence and dysfunction. MSC treatment downregulates the pro-inflammatory cytokine TNF-α and upregulated the anti-inflammatory and organ-protective cytokine IL-10 in kidney tissue. Additionally, Han et al. found that MT protects MSCs from senescence in CKD via normal PrPC-dependent enhancement of mitochondrial function [94]. Furthermore, they demonstrated that PrPC binds to PINK1 in the mitochondria of normal CKD-mouse MSCs, with reduced binding to help retain mitochondrial function via PrPC expression [95]. MT can enhance MSC viability, motility, stemness, and proliferation and inhibit senescence induced by uremic toxins accumulated in CKD, thereby inducing the proliferation of MSCs [93, 96].
In clinical practice, circulating exomiRs isolated from urine or serum have great potential as promising biomarkers in DKD [97]. However, exosomes contain typical markers, such as TSG101, Alix, integrins, and tetraspanins, such as CD63, CD9, and CD81 [98]. BM-MSC-derived exosomes in the kidney can improve the histopathological features of DKD by reducing atrophic alterations, vacuolation, and degeneration and decreasing inflammatory cell infiltration of proximal tubule epithelial cells [25, 99, 100]. Tsai et al. demonstrated that T2DM patients with microalbuminuria have higher urinary levels of exo-miR-15b-5p than normoalbuminuric patients and healthy individuals. Furthermore, miR-15b-5p participates in high-glucose-mediated kidney injury by inducing mesangial cell apoptosis via targeting BCL-2 [101]. Several studies have demonstrated that MSCs can improve their functionality under hypoxic conditions [102]. Carmona et al. found that only the group that received ASCs showed a reduction in glucose and creatinine levels, although this difference was not statistically significant [103]. Hypoxia preconditioning enhances the ability of healthy rat-derived ASCs to improve kidney injury in a rat model of DKD.
Based on successful preclinical experiments in the treatment of DN in animals with stem cells, further clinical trials should be conducted to validate the efficacy of stem cell therapy in the treatment of DKD. We hope that future experiments will provide mechanistic insights and develop new curative strategies. Additional in vitro and in vivo studies are warranted to describe the mechanisms of MSC-mediated cell therapy in detail, and challenges remain in terms of engraftment, persistence, tissue targeting, and cell manufacture. Regardless of the stem cell source, certain challenges remain to be addressed, such as how to solve the low induction rate of somatic cells and how to distribute large number of stem cells more effectively in damaged kidneys. Therefore, managing the survival of stem cells in the large environment of diabetes and maximizing their regenerative and reparative effects may be promising prospects for treating DKD. MSC-based therapies may provide substantial benefits for patients with DKD.
LXL: manuscript writing. XN: study conduct, data analysis, and manuscript writing. LJ: data analysis. All authors contributed to the article and approved the submitted version.
None.
This work was supported by the Clinical Research Project Program of Affiliated Hospital of Weifang Medical University (2021wyfylcyj03).
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.