Abstract
To evaluate the clinical efficacy of a new enzyme-linked immunosorbent assay (ELISA) system for simultaneously detecting three islet cell autoantibodies against glutamic acid decarboxylase (GADA), insulinoma-associated antigen-2 (IA-2A), and zinc transporter 8 (ZnT8A) (3 Screen ICA ELISA) in Japanese patients with acute-onset type 1 diabetes (T1D). In addition, clinical factors affecting the 3 Screen ICA ELISA index were investigated. We compared the positivity values of 3 Screen ICA ELISA with that of each autoantibody alone in 97 patients with acute-onset T1D (mean age 48.7 years, 49% male) and 100 non-diabetic subjects (mean age 47.0 years, 50% male). Serum thyroid stimulating hormone receptor antibody, thyroid peroxidase antibody (TPOAb) and thyroglobulin autoantibody levels were also evaluated. The cut-off value of the 3 Screen ICA ELISA was determined based on the 97th percentile of 100 non-diabetic controls (threshold for positivity, ≥14 index). The mean age of disease onset and duration of diabetes were 34.2 years and 14.5 years, respectively. Among all T1D patients, the positivity of 3 Screen ICA ELISA was 71.1%, while that of GADA, IA-2A, and ZnT8A were 59.8%, 25.8%, and 25.8%, respectively. The median 3 Screen ICA index was 121.9 (8.7–468.2) and was associated with titers of each autoantibody, most so with GADA, and was significantly higher in TPOAb-positive patients than in TPOAb-negative patients. Our findings suggests that the 3 Screen ICA ELISA may be a time-saving diagnostic tool for evaluating islet autoantibodies in acute-onset T1D patients.
TYPE 1 DIABETES (T1D) results from the destruction of the insulin-producing beta cells caused when islet autoimmunity is subjected to various environmental factors, such as viral infections [1, 2]. The presence of islet autoantibodies serves as an indicator of an autoimmune assault against pancreatic beta cells [3-5]. Therefore, autoantibodies against islet antigens, such as glutamic acid decarboxylase (GADA), insulinoma-associated antigen-2 (IA-2A), and zinc transporter 8 (ZnT8A) are currently being used as reliable biomarkers for the diagnosis of T1D [3]. The prevalence of GADA, IA-2A, and ZnT8A in patients with recent-onset of T1D is reported to be 80–90% [4, 5], 49–78% [4, 5], and 60–80% [3], respectively. Islet autoantibodies could be exploited as biomarkers for identifying at-risk individuals from first-degree relatives of patients with T1D [6]. Furthermore, individuals with multiple types of islet autoantibodies are at a higher risk of developing T1D than those without autoantibodies or those with a single type of islet autoantibodies [7]. However, checking various islet autoantibodies serially is a labor-intensive and costly process; therefore, development of a novel tool that enables the examination of multiple islet autoantibodies simultaneously is highly desirable for both diagnosing and evaluating the future risk of T1D.
Recently, an enzyme-linked immunosorbent assay (ELISA) system for simultaneously detecting three islet cell autoantibodies against glutamic acid decarboxylase (GADA), insulinoma-associated antigen-2 (IA-2A), and zinc transporter 8 (ZnT8A) (3 Screen ICA ELISA) was developed (Elisa RSRTM 3 Screen ICATM, RSR limited, UK) [8, 9]. The sensitivity of this assay in detecting new-onset T1D was 92% with a cut-off index of 30, based on the 97th percentile of 1,200 Caucasian healthy adults and blood donor sera [8, 9]. However, the optimal cut-off values and prevalence of the 3 Screen ICA ELISA in Japanese individuals with acute-onset T1D, including those with longstanding disease duration, remain unknown.
We compared the positivity values of the novel 3 Screen ICA ELISA with those of the currently used individual GADA, IA-2A, and ZnT8A ELISA using serum samples collected from Japanese patients with acute-onset T1D and non-diabetic controls. In addition, we investigated the clinical factors that could affect the 3 Screen ICA ELISA index.
Methods
Study participants
Serum samples of 97 individuals with acute-onset T1D (men, n = 48; women, n = 49) and 100 non-diabetic individuals (men, n = 50; women, n = 50) were obtained from Showa University Hospital (Tokyo, Japan) and Eiju General Hospital (Tokyo, Japan). Diagnosis of T1D was confirmed according to the criteria provided by Committee of the Japan Diabetes Society [10]. Serum samples of non-diabetic individuals with fasting blood glucose levels <110 mg/dL and a value of HbA1c <6.5% were used as non-diabetic controls. The study protocol was approved by the Ethics Committee of the Showa University School of Medicine (Permit Number: 3163; Tokyo, Japan), and written informed consent was obtained from all participants.
Islet autoantibody assays
GADA, IA-2A, and ZnT8A in the serum samples were measured using ELISA kits (RSR Ltd., Cardiff, United Kingdom) with cut-off values of 5.0 U/mL (range, 5.0–2,000 U/mL), 0.6 U/mL (range, 0.6–30 U/mL), and 15 U/mL (range, 15–2,000 U/mL), respectively, according to the manufacturer’s instructions [11-13]. When the titers of GADA (n = 6) and IA-2A (n = 5) exceeded the measurement limit value, a fill value of 2,000 IU/mL and 30 IU/mL were used for GADA and IA-2A, respectively.
A commercially available 3 Screen ICA ELISA kit (Elisa RSRTM 3 Screen ICATM) was used [8, 9].
For the 3 Screen ICA ELISA assay, 25 μL of serum was added to wells pre-coated with a cocktail of highly purified recombinant GAD 65, IA-2, and ZnT8 (325 W/R dimer molecule), incubated for 16–20 h at 2–8°C, aspirated, rinsed with wash solution, and then incubated with 100 μL of 3-screen-biotin (a combination GAD 65-biotin, IA-2-biotin, and ZnT8–biotin) for 1 h at 2–8°C. After incubation, the biotin solution was aspirated, the wells rinsed with wash solution, incubated with 100 μL of streptavidin peroxidase conjugate for 20 min at room temperature, and washed with the wash solution, followed by a final incubation with 100 μL of substrate solution containing tetramethylbenzidine for 20 min. The reaction was stopped with 100 μL of 0.25 mol/L H2SO4. The absorbance at 450 nm was measured using an ELISA plate reader (Molecular Devices Inc.). The index value was determined as follows: (absorbance of the test sample/absorbance of the reference sample) × 100. The coefficient of variation for the reproducibility of measurement and the reproducibility between measurements were 2.5–4.4% (n = 25) and 2.5–3.6% (n = 20), respectively [9].
Thyroid autoantibody assay
Serum thyroid stimulating hormone (TSH) receptor antibody (TRAb) levels were determined by radioreceptor assay using I125-labeled TSH and porcine TSH receptor (TRAb III kit; Cosmic Corporation Ltd., Tokyo, Japan). Thyroid peroxidase antibody (TPOAb) and thyroglobulin autoantibody (TgAb) levels were measured with electrochemiluminescence immunoassay (ECLIA) using ECLusys Anti-TPO and Anti-Tg, respectively (Roche Diagnostic GmbH, Mannheim, Germany). The cut-off values for TRAb, TPOAb, and TgAb were <10%, <16.0 U/mL, and <28.0 U/mL, respectively. Participants who were positive for either TRAb or TPOAb, or TgAb were classified as those with autoimmune thyroid disease (AITD).
C-peptide and hemoglobin A1c (HbA1c) assay
Random non-fasting C-peptide immunoreactivity (CPR) was measured by ECLIA with a detection limit of 0.02 ng/mL (E-test TOSOH II; Tosoh, Tokyo, Japan). When random CPR levels fell below the lower limit of detection (i.e., <0.02), a fill value of 0.001 was used (n = 55). Plasma glucose levels were measured using the glucose oxidase method. Glycated hemoglobin (HbA1c) levels were evaluated using high-performance liquid chromatography (HA-8180; Arkray, Tokyo, Japan) [14].
Statistical Analysis
Data are expressed as mean ± standard deviation, medians (interquartile range), or number (percentage). The Mann-Whitney U test was used to compare differences between groups. Non-parametric correlations were identified using the Spearman’s rank correlation coefficient. A two-tailed p-value <0.05 was considered statistically significant. All statistical analyses were performed using the JMP Pro 16.0 software (SAS Institute Japan Ltd., Tokyo, Japan).
Results
Demographic characteristics
In non-diabetic controls, mean age, fasting glucose, and HbA1c were 47 ± 16 years, 90 ± 7.1 mg/dL, and 5.8 ± 0.3%, respectively. Clinical and laboratory characteristics of patients with T1D are summarized in Table 1. The mean age of the patients, age of disease onset, duration of diabetes, and BMI were 48.7 ± 15.9 years, 34.2 ± 17.6 years, 14.5 ± 11.2 years, and 23.6 ± 4.5 kg/m2, respectively. The median random CPR was 0.001 (0.001–0.195) ng/mL. The median 3 Screen ICA index was 121.9 (8.7–468.2). The median GADA, IA-2A, and ZnT8A titers were 69.3 (16.3–367.5) U/mL, 3.4 (1.6–20.2) U/mL, and 58.5 (20.5–195.1) U/mL, respectively. The daily insulin dosage was 0.66 ± 0.27 units/kg. The prevalence of AITD among individuals with T1D was 22.7% (22/97).
Table 1
Patient characteristics
| Number |
97 |
| Age (years) |
48.7 ± 15.9 |
| Age of disease onset (years) |
34.2 ± 17.6 |
| Duration of disease (years) |
14.5 ± 11.2 |
| Body mass index (kg/m2) |
23.6 ± 4.5 |
| Glycated hemoglobin (%) |
7.5 ± 1.1 |
| Random CPR (ng/mL) (n = 96)† |
0.001 (0.001–0.195) |
| 3-screen ICA (index) |
121.9 (8.7–468.2) |
| GADA titers (IU/mL) (n = 58)‡ |
69.3 (16.3–367.5) |
| IA-2A titers (IU/mL) (n = 25)§ |
3.4 (1.6–20.2) |
| ZnT-8A titers (IU/mL) (n = 25)¶ |
58.5 (20.5–195.1) |
| Total insulin dose/ body weight (units/kg) |
0.66 ± 0.27 |
| Thyroid disease, % (n) |
22.7 (22) |
Data are expressed as mean ± standard deviation, medians (interquartile range), or percentage (number). Abbreviations: CPR, C-peptide immunoreactivity; ICA, islet cell autoantibodies; GADA, autoantibodies against glutamic acid decarboxylase; IA-2A, autoantibodies against insulinoma-associated protein 2; ZnT-8A, autoantibodies against zinc transporter-8. † Data missing from one patient. When random CPR levels fell below the lower limit of detection (i.e., <0.02), a fill value of 0.001 was used. When the titers of GADA (n = 6) and IA-2A (n = 5) exceeded the measurement limit value, a fill value of 2,000 IU/mL and 30 IU/mL were used for GADA and IA-2A, respectively. ‡, §, ¶ Number of GADA, IA-2A, and ZnT-8A positivity, respectively.
The cut-off value of the 3 Screen ICA ELISA was determined based on the 97th percentile of 100 non-diabetic controls (threshold for positivity, ≥14 index) because the index in the controls did not follow a normal distribution. Based on this cut-off value, the calculated sensitivity and specificity of the 3 Screen ICA ELISA for T1D were 71.1% (69/97) and 97.0%, respectively.
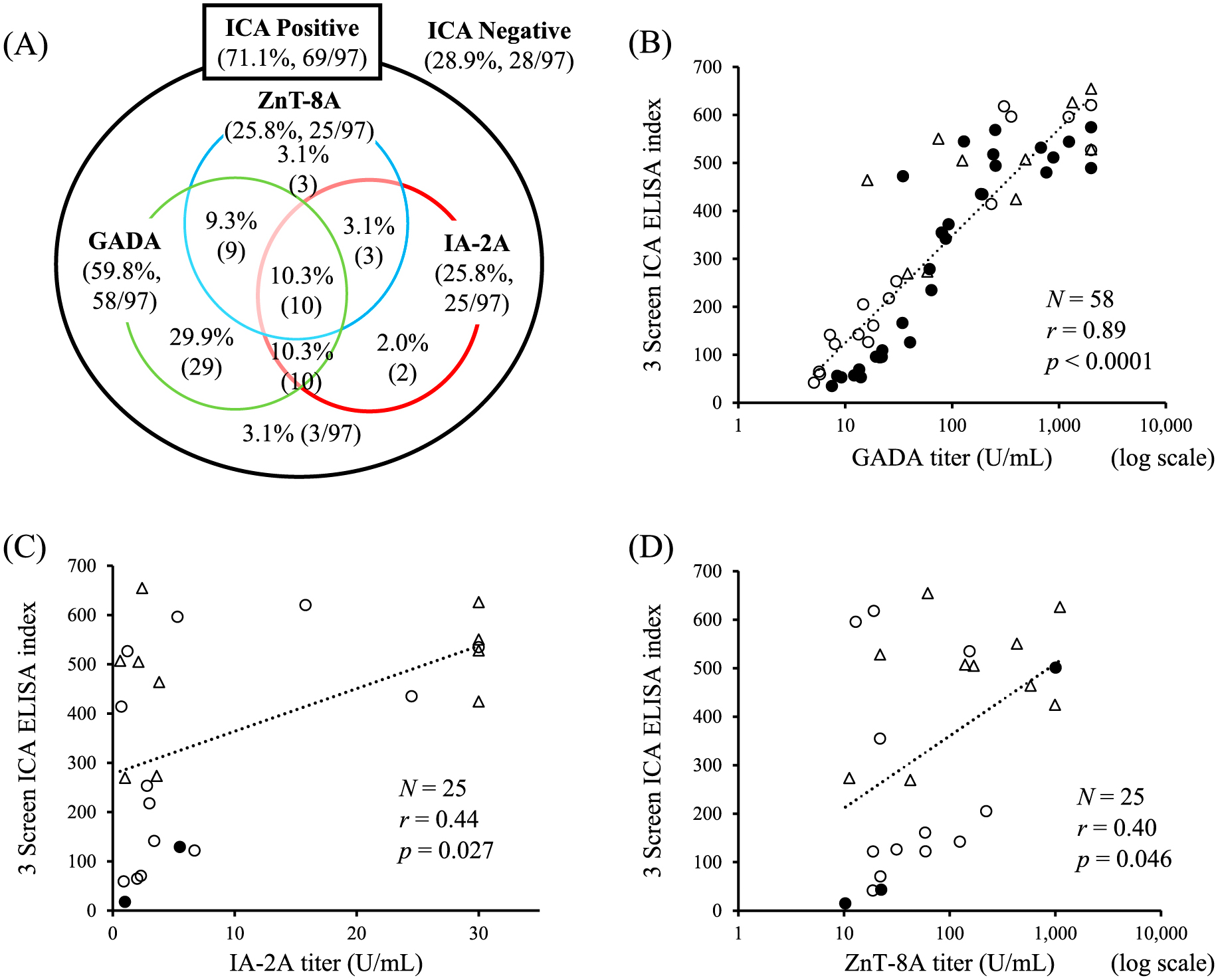
Evaluation of Venn diagram data revealed the prevalence of 3 Screen ICA ELISA positivity was 71.1% (69/97), while that of GADA, IA-2A, and Zn-T8 were 59.8% (58/97), 25.8% (25/97), and 25.8% (25/97), respectively (Fig. 1A). Among 69 patients who were ICA-positive, the prevalence of GADA, IA-2A, and ZnT8A was 84.1% (58/69), 36.2% (25/69), and 36.2% (25/69), respectively; 95.7% (66/69) were positive for at least one islet autoantibody, while 4.3% (3/69) were negative for all of the three islet autoantibodies. The patients with T1D who were ICA-negative (28.9%, 28/97) were also negative for each of the islet autoantibodies. The association between the 3 Screen ICA ELISA index and GADA, IA-2A, and ZnT8A titers are represented in Fig. 1B–D. There was a significant correlation between the two variables. The 3 Screen ICA ELISA index was significantly correlated with GADA titers among 29 positive subjects for single GADA (closed circle in Fig. 1B; r = 0.92, p < 0.0001). The 3 Screen ICA ELISA index was significantly higher in subjects positive for all three autoantibodies (480.4 ± 129.5, n = 10) than in those positive for two (291.9 ± 210.6, n = 22, p = 0.019) or one (278.1 ± 208.6, n = 34, p = 0.012) (data not shown). Three non-diabetic controls were positive for the 3 Screen ICA ELISA index (506.6, 541.8, and 14.0), two for GADA (14.9 IU/mL and >2,000 IU/mL), three for IA-2A (49.4 U/mL, 2.0 U/mL, and 1.4 U/mL), and one for ZnT8A (36 U/mL). In addition, one non-diabetic control was negative for the 3 Screen ICA ELISA, but positive for IA-2A (1.0 U/mL).
Since the coexistence of AITD is associated with a higher titer of islet autoantibodies in T1D patients, we investigated the 3 Screen ICA ELISA index among patients with T1D associated with AITD. As shown in Fig. 2A, the median 3 Screen ICA ELISA index was significantly higher in T1D patients with AITD (n = 22) than in those without (n = 75; 271.5 [58.4–550.7] vs. 70.6 [6.8–434.4], respectively, p < 0.05). The 3 Screen ICA ELISA index was significantly higher in TPOAb-positive subjects (n = 18) compared to that in TPOAb-negative subjects (n = 79) (307.7 [48.1–575.4] vs. 94.5 [7.3–424.6], respectively, p < 0.05; Fig. 2B). There was no difference in the 3 screen ICA ELISA index irrespective of the presence or absence of TRAb or TgAb (data not shown).
Relationship of the 3 Screen ICA ELISA index and random C-peptide with the duration of diabetes
A negative correlation was observed between the 3 Screen ICA ELISA index and disease duration (p = 0.09). Random C-peptide levels were significantly decreased with increased duration of diabetes (p < 0.001). There was no correlation between C-peptide levels and the 3 screen index. In addition, numbers of islet autoantibodies and GADA, IA-2A, or ZnT8A titers did not affect C-peptide levels (data not shown).
Discussion
The most salient findings of the present study are as follows: (i) the cut-off index value of the 3 screen ICA ELISA in Japanese patients with T1D was lower than that previously reported in a Caucasian population [8, 9], (ii) the 3 screen ICA ELISA had a high positive concordance ratio to the currently used individual ELISA against GAD, IA-2, and ZnT8; and (iii) the 3 screen ICA ELISA indices were significantly associated with the GADA titer and TPOAb positivity.
The present study evaluated the sensitivity of the 3 screen ICA ELISA in Japanese patients with T1D and found it to be 71% with our cut-off index of 14 and 67.0% with an index of 30, both of which were significantly lower than that reported previously, in which ELISA sensitivity was 92% for Caucasian patients with T1D [8, 9]. Since our participants were older at diabetes onset and also had a longer disease duration compared to those in the Caucasian studies [9], genetic and clinical differences may account for the variances in the observed results. In addition, we found that random C-peptide levels were significantly lowered with increasing duration of diabetes. Thus, islet autoantibodies may decline under these conditions, which could partly explain the lower prevalence of 3 screen ICA ELISA in our study. However, when the patients were divided into the shorter duration (<5 years) and longer duration group (≥5 years) based on the disease duration, the sensitivity of ELISA of the former group was 68.4% (13/19), while the latter was 71.8% (56/78). If those who are positive for any of the three autoantibodies were picked up, positive predictive value and sensitivity in patients with T1D should be 66/69 (96%) and 66/66 (100%), respectively. Therefore, this kit could successfully pick up all of the 66 islet autoantibodies-positive subjects, at least in patients with T1D.
Currently, to diagnose autoimmune T1D, the absence of an islet autoantibody in the first measurement warrants additional measurements of alternate islet autoantibodies. However, it is labor-intensive and costly to check each of the islet autoantibodies serially to obtain a definite diagnosis of T1D. In the present study, approximately 16% of ICA-positive patients were negative for GADA, the most frequently checked islet autoantibodies for suspected patients with T1D. The present findings support the clinical relevance of the 3 screen ICA ELISA for timely diagnosis of acute-onset T1D. Furthermore, among the ICA-positive patients with T1D, three patients were negative for any of three islet autoantibodies. Therefore, even when the titers of the individual islet autoantibodies, GADA, IA-2A, and ZnT8A would be below the assay limit of detection, a cut-off index value of ≥14 in the 3 screen ICA ELISA may enable the detection of very low levels of potential autoimmunity against human pancreatic beta cells; consequently, helping to identify at-risk individuals for T1D.
In the present study, three out of 100 non-diabetic controls with a cut-off index value of ≥14 in the 3 screen ICA ELISA were positive for one or more islet autoantibodies. Several studies have demonstrated that the presence of multiple islet autoantibodies in the preclinical stage is associated with an increased risk of developing T1D. Discreet and longer follow-up might be needed for the 3 screen ICA ELISA-positive non-diabetic individuals because of the absence of recommended therapeutic options to prevent the progression to clinical T1D [7, 15].
Although there is no clear evidence that GAD is expressed in the thyroid gland, AITD is frequently associated with GADA-positive patients with T1D [16, 17]. There was a significant correlation between the 3 screen ICA ELISA indices and GADA titers (Fig. 1B). Given that GADA titers were significantly higher in T1D patients with AITD than in those without AITD (data not shown), this may explain the increased 3 screen ICA ELISA index observed in the T1D patients with associated with AITD. Among the thyroid autoantibodies evaluated in the present study, a significantly higher 3 Screen ICA ELISA index was observed in samples from TPOAb-positive patients, but not in those from TRAb-positive or TgAb-positive subjects. In addition, there was a significant correlation of TPOAb titers with GADA titers (r = 0.22, p < 0.05) and with the 3 screen ICA ELISA indices (r = 0.23, p < 0.05). Given the fact that TPOAb positivity is associated with future deterioration of beta cell function in GADA-positive patients with T1D [18], the presence of TPOAb might be clinically linked to GADA in T1D patients.
There are several limitations to this study. First, the current study had a relatively small sample size, and the majority of patients with T1D had long disease duration. Therefore, optimal cut-off value of the 3 screen ICA ELISA needs be re-evaluated using a larger number of non-diabetic controls and patients with newly onset T1D. Second, it seems to be better to select control subjects without any islet-associated autoantibodies, leading to the accurate analysis of the cut-off value. However, the aim of the present study was to analyze the cut-off value of the ELISA system for Japanese T1D patients and to evaluate the sensitivity and specificity of 3 Screen ICA ELISA. Therefore, we did not exclude the control subjects showing the positivity of individual islet-associated autoantibodies before analyzing the cut-off value. When we excluded two control subjects that were statistical outliers (who might be at high-risk of developing future T1D), the 97th percentile of 3 Screen ELISA was 8.2. Based on this cut-off value, the calculated sensitivity and specificity of the 3 Screen ICA ELISA for T1D were 76.3% (74/97) and 99.0% (97/98), respectively. Third, in the present study, TRAb was measured using a radioreceptor assay to detect patients with AITD. Although this assay has been traditionally used for the diagnosis of Grave’s disease, its sensitivity is lower than that of the latest assay system, such as ELISA and ECLIA [19]. Therefore, false-negative TRAb individuals might have been included in the AITD-negative group. Fourth, we did not collect the human leucocyte antigen genotypes influencing to the intensity of the immune response against islet antigens. Fifth, we did not know the exact reason why only one case was a false negative. However, since IA-2Ab specificity has been reported to be 98.0% [20], this individual may have had a false positive reaction in the IA-2Ab assay system. Sixth, we did not have any data for discussing a difference in the clinical significance of measuring the ELISA system between subjects with acute-onset T1D and those suspicious of slowly progressive insulin-dependent diabetes mellitus (SPIDDM) and fulminant T1D. In conclusion, our present study indicates that the 3 Screen ICA ELISA may be a time-saving diagnostic tool for evaluating islet autoantibodies in acute-onset T1D patients.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Disclosure
Funding
This research was supported by grants from Cosmic Co., Ltd.
Conflict of Interest
T Fukui received honoraria for lectures from Novo Nordisk Pharma, Sanofi, and Terumo Corporation and received a clinical commissioned/joint research grant from Cosmic Co., Ltd. T Kikuchi is an employee of Cosmic Co., Ltd. The other authors have no conflict of interest to declare regarding this publication.
References
- 1 Eisenbarth GS (1986) Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med 314: 1360–1368.
- 2 Coppieters KT, Dotta F, Amirian N, Campbell PD, Kay TW, et al. (2012) Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med 209: 51–60.
- 3 Wenzlau JM, Hutton JC (2013) Novel diabetes autoantibodies and prediction of type 1 diabetes. Curr Diab Rep 13: 608–615.
- 4 Kawasaki E (2014) Type 1 diabetes and autoimmunity. Clin Pediatr Endocrinol 23: 99–105.
- 5 Gabbay MA, Sato MN, Duarte AJ, Dib SA (2012) Serum titres of anti-glutamic acid decarboxylase-65 and anti-IA-2 autoantibodies are associated with different immunoregulatory milieu in newly diagnosed type 1 diabetes patients. Clin Exp Immunol 168: 60–67.
- 6 Redondo MJ, Geyer S, Steck AK, Sharp S, Wentworth JM, et al. (2018) A type 1 diabetes genetic risk score predicts progression of islet autoimmunity and development of type 1 diabetes in individuals at risk. Diabetes Care 41: 1887–1894.
- 7 Ziegler AG, Kick K, Bonifacio E, Haupt F, Hippich M, et al. (2020) Yield of a public health screening of children for islet autoantibodies in Bavaria, Germany. JAMA 323: 339–351.
- 8 Amoroso M, Achenbach P, Powell M, Coles R, Chlebowska M, et al. (2016) 3 screen islet cell autoantibody ELISA: a sensitive and specific ELISA for the combined measurement of autoantibodies to GAD65, to IA-2 and to ZnT8. Clin Chim Acta 462: 60–64.
- 9 Ziegler AG, Haupt F, Scholz M, Weininger K, Wittich S, et al. (2016) 3 screen ELISA for high-throughput detection of beta cell autoantibodies in capillary blood. Diabetes Technol Ther 18: 687–693.
- 10 Kawasaki E, Maruyama T, Imagawa A, Awata T, Ikegami H, et al. (2014) Diagnostic criteria for acute-onset type 1 diabetes mellitus (2012): Report of the Committee of Japan Diabetes Society on the Research of Fulminant and Acute-onset Type 1 Diabetes Mellitus. J Diabetes Investig 5: 115–118.
- 11 Brooking H, Ananieva-Jordanova R, Arnold C, Amoroso M, Powell M, et al. (2003) A sensitive non-isotopic assay for GAD65 autoantibodies. Clin Chim Acta 331: 55–59.
- 12 Chen S, Willis J, Maclean C, Ananieva-Jordanova R, Amoroso MA, et al. (2005) Sensitive non-isotopic assays for autoantibodies to IA-2 and to a combination of both IA-2 and GAD65. Clin Chim Acta 357: 74–83.
- 13 Dunseath G, Ananieva-Jordanova R, Coles R, Powell M, Amoroso M, et al. (2015) Bridging-type enzyme-linked immunoassay for zinc transporter 8 autoantibody measurements in adult patients with diabetes mellitus. Clin Chim Acta 447: 90–95.
- 14 Kashiwagi A, Kasuga M, Araki E, Oka Y, Hanafusa T, et al. (2012) International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig 3: 39–40.
- 15 Saydah SH, Shrestha SS, Zhang P, Zhou X, Imperatore G (2019) Medical costs among youth younger than 20 years of age with and without diabetic ketoacidosis at the time of diabetes diagnosis. Diabetes Care 42: 2256–2261.
- 16 Jonsdottir B, Larsson C, Carlsson A, Forsander G, Ivarsson SA, et al. (2017) Thyroid and islet autoantibodies predict autoimmune thyroid disease at type 1 diabetes diagnosis. J Clin Endocrinol Metab 102: 1277–1285.
- 17 Kordonouri O, Charpentier N, Hartmann R (2011) GADA positivity at onset of type 1 diabetes is a risk factor for the development of autoimmune thyroiditis. Pediatr Diabetes 12: 31–33.
- 18 Murao S, Kondo S, Ohashi J, Fujii Y, Shimizu I, et al. (2008) Anti-thyroid peroxidase antibody, IA2 antibody, and fasting C-peptide levels predict beta cell failure in patients with latent autoimmune diabetes in adults (LADA)—a 5-year follow-up of the Ehime study. Diabetes Res Clin Pract 80: 114–121.
- 19 Kamijo K (2007) TSH-receptor antibodies determined by the first, second and third generation assays and thyroid-stimulating antibody in pregnant patients with Graves’ disease. Endocr J 54: 619–624.
- 20 Törn C, Vaziri-Sani F, Ramelius A, Elding Larsson H, Ivarsson SA, et al. (2022) Evaluation of the RSR 3 screen ICATM and 2 screen ICATM as screening assays for type 1 diabetes in Sweden. Acta Diabetol 59: 773–781.



