2022 Volume 69 Issue 2 Pages 179-188
2022 Volume 69 Issue 2 Pages 179-188
Resistance to thyroid hormone beta (RTHβ) caused by germline mutations in genes encoding thyroid hormone receptor beta (TRβ) is a rare disorder. Little information is available regarding the clinical experience of this syndrome in Japan. We retrospectively reviewed the records of 34 patients with RTHβ (21 adult females and 13 adult males) with positive TRβ mutations identified at our division between 2000 and 2020. Of the 24 patients with available clinical history, 10 (41.7%) received inappropriate treatments such as antithyroid drugs, thyroidectomy, or radioactive iodine. Diagnostic delay and inappropriate management of RTHβ are still present in Japan. Every patient except one demonstrated thyroid hormone profiles indicative of syndrome of inappropriate secretion of thyrotropin (SITSH), characterized by a hormonal profile of hyperthyroxinemia with a non-suppressed TSH concentration. Since the most common forms of hyperthyroidism including Graves’ disease feature elevated thyroid hormone levels with suppressed TSH concentrations, early diagnosis of SITSH is critical for preventing inappropriate management. One patient positive for anti-thyroglobulin antibody (Tg-Ab) and anti-thyroperoxidase antibody (TPO-Ab) showed remarkably elevated TSH (>200 μIU/mL) despite thyroid hormone concentrations within the reference ranges. At least one thyroid autoantibody (Tg-Ab, TPO-Ab, or thyrotropin receptor antibodies) was identified in 37.9% (11/29) of the patients tested. One patient developed overt Graves’ disease nine years after RTHβ diagnosis. These findings suggest that RTHβ is frequently comorbid with additional autoimmune thyroid disorders. Further research is required to identify the most appropriate treatments for RTHβ patients who develop a second thyroid disorder.
RESISTANCE TO THYROID HORMONE (RTH) is a syndrome characterized by a reduced responsiveness of target tissues to thyroid hormones [1]. Thyroid hormones regulate various processes, including growth, development, and metabolism [2]. The effects of thyroid hormones are mainly mediated by the transcriptional activity of thyroid hormone receptors encoded by the thyroid hormone receptor alpha (THRA) and thyroid hormone receptor beta (THRB) genes. RTH syndromes that occur due to THRA and THRB gene mutations are termed RTHα and RTHβ, respectively.
The incidence of RTHβ is estimated to be approximately 1 case per 40,000 live births [3]. The frequency was reportedly similar in Japan, as Tajima et al. analyzed 83,232 newborns in a T4-based neonatal screening program and detected two patients with RTHβ [4]. The Japanese cases of RTHβ began to be reported by Nakamura and other investigators in the early 1980s, with more than 100 case reports in Japan (Japan Medical Abstract Society database [5]); there have been no reported cases of RTHα in Japan. RTHβ is characterized by the syndrome of inappropriate secretion of thyrotropin (SITSH), which presents as a non-suppressed thyrotropin (TSH) concentration despite hyperthyroxinemia [6]. The main differential diagnosis of RTHβ is TSH-secreting pituitary adenoma (TSHoma), another cause of SITSH [7].
Little information is available regarding the clinical experience of RTHβ diagnosis in Japan. Genetic testing of THRB has not been routinely performed outside the academic setting until the approval of the Japanese Health Care Insurance System (April 2020); we have provided a mutation testing service free of charge for more than 20 years. In the present study, we share our experience reviewing the clinical outcomes of 34 Japanese RTHβ patients with positive TRβ mutation.
We retrospectively reviewed the records of 75 patients with suspected RTHβ who underwent mutational analysis at the Second Division, Department of Internal Medicine, Hamamatsu University School of Medicine, between 2000 and 2020. Among these, we identified 34 patients with RTHβ. Regarding 28 patients, blood samples for genetic testing of THRB and medical information were sent from many hospitals in various prefectures throughout Japan where patients visited first and asked for our consultation.
The following medical information was retrieved manually: age at diagnosis; sex; body mass index; the results of genetic testing of THRB; pituitary MRI findings; treatment before referral (past treatment); chief complaint; pulse rate; goiter on physical examination; thyroid weight; serum TSH, free T4 (T4), and free T3 (FT3) concentrations; anti-thyroglobulin antibody (Tg-Ab) and anti-thyroperoxidase antibody (TPO-Ab) status; the presence of thyrotropin receptor antibody (TRAb); TSH response after administration of 500 μg TRH; and the location of the hospital where the blood samples for genetic testing and the medical information were obtained. As the reference range of the hormones differed based on the measurement kits, serum TSH, FT4, and FT3 concentrations were adjusted according to the upper limit of the reference range in each case. Thyroid weight was estimated based on ultrasonographic findings. The estimated thyroid weight (g) was calculated using the following formula: [long diameter (cm)] × [short diameter (cm)] × [thickness (cm)] × 0.7, as previously described [8]. A positive TRH test finding was defined as a greater than two-fold increase in peak TSH levels compared to baseline levels during stimulation with 500 μg TRH [9].
Genomic DNA was extracted from peripheral blood leukocytes. PCR amplification of exons 7–10 of THRB (primer sequences available on request) was performed using AmpliTaq Gold DNA Polymerase (Thermo Fisher Scientific, Waltham, MA, USA). Amplification was performed using a three-step touchdown protocol, as previously described [10]. The amplified fragments were gel purified and sequenced in the forward and reverse directions using a BigDyeTM Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific) and an Applied Biosystems 3130 Sequencer (Applied Biosystems, Foster City, CA, USA). The resulting electropherograms were compared with the reference sequence of the THRB. The in silico analyses of novel THRB variants were performed using the Polymorphism Phenotyping ver. 2 (PolyPhen-2) [11]. In the PolyPhen-2 program, the investigated mutation is categorized as benign (probability score less than or equal to 0.15), possibly damaging (probability score between 0.16 and 0.85), or probably damaging (probability score greater than 0.85).
Genetic analysis of THRB was approved by the institutional review board of the Hamamatsu University School of Medicine. Written informed consent was obtained from all patients or their relatives.
Statistical analyses were performed using GraphPad PRISM v7.0 (GraphPad Software, Inc., San Diego, USA). The Mann–Whitney test or Fisher’s exact test was used for comparison between the two groups, and one-way analysis of variance was used in more than three groups.
The clinical characteristics of the 34 patients with RTHβ (21 women and 13 men) are summarized in Tables 1–3. The median age at diagnosis was 34.9 years (Table 1, Fig. 1A). Of the 18 patients with available information about chief complaints, eight (44.4%) presented with palpitations or tachycardia (Fig. 1B). Of the 24 patients with available information about neck palpation, 16 (66.7%) presented with a palpable goiter. The remaining eight patients had non-palpable goiters, however three patients were infants and three were adults who had undergone previous treatments such as antithyroid drugs (patients #12, #16, and #28), thyroidectomy (#12), or radioactive iodine (#16).
| No. | Subject ID | Age (year) | Sex | BMI | Chief complaints | Palpable goiter | Thyroid weight (g) |
|---|---|---|---|---|---|---|---|
| 1 | 1-proband | 29 | F | n/a | Goiter | Yes | n/a |
| 2 | 2-proband | 8 | F | n/a | Goiter | Yes | n/a |
| 3 | 3-proband | 0 | F | n/a | None | No | n/a |
| 4 | 4-proband | 33 | F | n/a | Palpitation, hyperhidrosis | Yes | n/a |
| 5 | 5-proband | 40 | F | n/a | n/a | Yes | n/a |
| 6 | 5-daughter | 9 | F | n/a | n/a | Yes | n/a |
| 7 | 5-sister | 33 | F | n/a | n/a | Yes | n/a |
| 8 | 5-father | 72 | M | n/a | n/a | n/a | n/a |
| 9 | 6-proband | 40 | M | 16.3 | Palpitation | Yes | 16.9 |
| 10 | 6-mother | 70 | F | 16.9 | Palpitation | Yes | 21.2 |
| 11 | 7-proband | 30 | F | 21.5 | Palpitation | Yes | 15.6 |
| 12 | 7-mother | 57 | F | 23.3 | None | No | n/a |
| 13* | 7-daughter | 18 | F | n/a | Developmental disorder | Yes | 30.4 |
| 14 | 8-proband | 31 | F | 27.5 | Goiter | Yes | 55.7 |
| 15 | 9-proband | n/a | F | n/a | n/a | n/a | n/a |
| 16 | 10-proband | 45 | M | 24.3 | Dizziness | No | 14.8 |
| 17 | 11-proband | 25 | F | 17.5 | Palpitation, hyperhidrosis | Yes | n/a |
| 18 | 12-proband | 31 | F | 19.5 | n/a | Yes | n/a |
| 19 | 12-sister | 35 | F | n/a | n/a | n/a | n/a |
| 20 | 12-father | 64 | M | n/a | n/a | n/a | n/a |
| 21 | 13-proband | 0 | M | n/a | Tachycardia | No | n/a |
| 22 | 13-father | n/a | M | n/a | n/a | n/a | n/a |
| 23 | 14-proband | 43 | M | n/a | n/a | n/a | n/a |
| 24 | 14-daughter | 1 | F | n/a | n/a | n/a | n/a |
| 25 | 14-daughter | 4 | F | n/a | n/a | n/a | n/a |
| 26 | 14-brother | 37 | M | n/a | n/a | n/a | n/a |
| 27 | 15-proband | 42 | M | n/a | Weight reduction | Yes | 39 |
| 28 | 16-proband | 53 | F | 21.5 | General malaise | No | n/a |
| 29 | 17-proband | 64 | F | 22.5 | None | Yes | n/a |
| 30 | 18-proband | 50 | M | 23.8 | Palpitation | Yes | 40 |
| 31 | 19-proband | 47 | M | 21.3 | Palpitation | No | 16.5 |
| 32 | 20-proband | 50 | M | 11.6 | None | No | 12.8 |
| 33 | 21-proband | 0 | F | n/a | None | No | n/a |
| 34 | 22-proband | 34 | M | 18.2 | None | n/a | 21.2 |
* indicates that the patient had been diagnosed with RTHβ 14 years after the proband was diagnosed.
Abbreviations: BMI, body mass index; F, female; ID, identification; M, male; n/a, not available
| No. | Past treatment | TSH (μIU/mL) | FT4 (ng/dL) | FT3 (pg/mL) | Tg-Ab | TPO-Ab | TRAb |
|---|---|---|---|---|---|---|---|
| 1 | ATD | 8.41 [200.2] | 2.1 [122] | 6.0 [148] | Pos | Pos | n/a |
| 2 | None | 5.72 [n/a] | 3.1 [n/a] | 14.6 [n/a] | Pos | Pos | Neg |
| 3 | None | 5.30 [140.2] | 2.9 [171] | 6.0 [149] | Neg | Neg | Neg |
| 4 | None | 2.40 [64.9] | 2.5 [147] | 4.3 [105] | Neg | Neg | Neg |
| 5 | ATD→LT3 | >200 [>5,000] | 0.7 [63] | n/a | Pos | Pos | n/a |
| 6 | n/a | 2.71 [54] | 2.4 [153] | 6.9 [187] | n/a | n/a | n/a |
| 7 | n/a | 2.91 [65] | 2.3 [128] | 6.9 [154] | Pos | n/a | n/a |
| 8 | n/a | 2.39 [48] | 2.4 [148] | 4.4 [120] | n/a | n/a | n/a |
| 9 | None | 0.56 [11] | 3.4 [213] | 6.9 [173] | Neg | Neg | Neg |
| 10 | PT | 7.28 [146] | 2.8 [175] | 5.8 [145] | Neg | Neg | Neg |
| 11 | None | 3.62 [72] | 2.4 [141] | 4.4 [110] | Pos | Pos | n/a |
| 12 | ATD→TT | 3.52 [70] | 3.2 [200] | 5.0 [125] | Neg | Neg | n/a |
| 13 | None | 2.82 [57] | 1.8 [122] | 5.1 [161] | Neg | Neg | Neg |
| 14 | ATD | 2.44 [49] | 4.4 [275] | 10.8 [270] | n/a | n/a | n/a |
| 15 | ATD→None | 1.27 [25] | 2.2 [127] | 5.9 [148] | n/a | n/a | n/a |
| 16 | ATD→RIT | 2.53 [51] | 2.1 [131] | 7.4 [185] | Neg | Neg | Neg |
| 17 | None | 2.24 [45] | 4.0 [236] | 9.7 [224] | Neg | Neg | Neg |
| 18 | None | 3.84 [78] | 2.8 [186] | n/a | Pos | Pos | Neg |
| 19 | n/a | 1.30 [26] | 2.6 [178] | 6.6 [177] | n/a | n/a | Pos |
| 20 | n/a | 7.91 [160] | 3.0 [202] | n/a | Neg | Neg | Neg |
| 21 | ATD | 5.38 [140] | 4.8 [305] | 6.7 [191] | Neg | Neg | Neg |
| 22 | n/a | 0.82 [21] | 3.5 [224] | 8.3 [237] | n/a | n/a | n/a |
| 23 | n/a | 0.80 [21] | 2.5 [159] | 7.4 [211] | Pos | Neg | n/a |
| 24 | n/a | 3.12 [81] | 2.4 [154] | 8.7 [249] | Neg | Neg | n/a |
| 25 | n/a | 1.66 [43] | 2.2 [143] | 5.8 [166] | Neg | Neg | n/a |
| 26 | n/a | 0.88 [23] | 2.4 [153] | 5.6 [160] | Neg | Neg | n/a |
| 27 | None | 0.95 [18] | 2.6 [163] | 6.2 [155] | Neg | Neg | Neg→Pos* |
| 28 | ATD→None | 1.07 [25] | 3.9 [215] | 3.5 [81] | Pos | Neg | Neg |
| 29 | None | 4.54 [91] | 2.1 [124] | 4.1 [103] | Pos | Pos | Neg |
| 30 | ATD | 0.68 [14] | 2.2 [149] | 4.5 [122] | Neg | Neg | Neg |
| 31 | None | 3.57 [71] | 3.5 [208] | 4.8 [121] | Neg | Neg | Neg |
| 32 | None | 5.23 [105] | 2.4 [141] | 2.5 [63] | Neg | Neg | Neg |
| 33 | None | 4.95 [100] | 2.7 [180] | 6.6 [177] | Neg | Neg | n/a |
| 34 | None | 2.45 [49] | 4.3 [252] | 11.8 [296] | Neg | Neg | Neg |
Data in square brackets are expressed as the percentage to the upper limit of the reference range in each institute. * indicates that the diagnosis of Graves’ disease was made based on positive thyrotropin receptor antibody (TRAb) findings with undetectable TSH levels nine years after the diagnosis of RTHβ.
Abbreviations: ATD, antithyroid drug; FT3, free T3; FT4, free T4; LT3, liothyronine; n/a, not available; Neg, negative; Pos, positive; PT, partial thyroidectomy; RIT, radioactive iodine therapy; Tg-Ab, anti-thyroglobulin antibody; TPO-Ab, anti-thyroperoxidase antibody; TT, total thyroidectomy
| No. | Mutation | Pituitary MRI | Fold change of TRH testa) | Prefecture IDb) | Year at diagnosis |
|---|---|---|---|---|---|
| 1 | V264A***,† | NN | 20.2 | H1 | 2000 |
| 2 | D265G***,† | NN | 5.3 | A | 2000 |
| 3 | R320C** | NN | 7.5 | S1 | 2001 |
| 4 | F451L* | NN | n/a | K | 2001 |
| 5 | K342I** | n/a | n/a | H1 | 2002 |
| 6 | K342I** | n/a | n/a | H1 | 2002 |
| 7 | K342I** | NN | n/a | H1 | 2002 |
| 8 | K342I** | n/a | n/a | H1 | 2002 |
| 9 | P453R* | NN | 9 | S2§ | 2004 |
| 10 | P453R* | NN | n/a | S2§ | 2004 |
| 11 | P453T* | NN | n/a | S2§ | 2005 |
| 12 | P453T* | NN | n/a | S2§ | 2005 |
| 13 | P453T* | NN | 11.2 | S2 | 2019 |
| 14 | L330S** | n/a | n/a | T | 2005 |
| 15 | R243W*** | NN | n/a | T | 2006 |
| 16 | T329S**,† | NN | n/a | H2 | 2006 |
| 17 | G332E** | NN | 7.3 | S2 | 2006 |
| 18 | K443E* | n/a | n/a | S2 | 2007 |
| 19 | K443E* | n/a | n/a | S2 | 2007 |
| 20 | K443E* | n/a | n/a | S2 | 2007 |
| 21 | R243W*** | NN | n/a | F | 2007 |
| 22 | R243W*** | n/a | n/a | F | 2007 |
| 23 | I250F*** | n/a | n/a | F | 2007 |
| 24 | I250F*** | n/a | n/a | F | 2007 |
| 25 | I250F*** | n/a | n/a | F | 2007 |
| 26 | I250F*** | n/a | n/a | F | 2007 |
| 27 | Q340H** | NN | n/a | S2§ | 2009 |
| 28 | R243W*** | Microadenoma | 8.7 | N | 2011 |
| 29 | F439L* | NN | n/a | A | 2017 |
| 30 | R282G*** | NN | n/a | S2 | 2018 |
| 31 | P453S* | NN | 5.2 | S2 | 2018 |
| 32 | L246V***,† | NN | 6 | S2§ | 2018 |
| 33 | R320C** | NN | n/a | K | 2019 |
| 34 | R338W** | NN | n/a | S2 | 2019 |
a) indicates TSH fold change calculated as the ratio between the TSH peak and baseline levels. b) represents the location of the hospital where the blood samples for genetic testing and the medical information were obtained. *, **, *** indicate that mutations are located in the first, second, and third cluster of thyroid hormone receptor beta mutations, respectively. † indicates a novel mutation. § represents that the patient visited the Hamamatsu University School of Medicine for the diagnosis of RTHβ.
Abbreviations: ID, identification; MRI, magnetic resonance imaging; n/a, not available; NN, no nodules

Clinical characteristics in 34 patients with resistance to thyroid hormone beta (RTHβ). (A) Bar graph depicting the number of RTHβ patients in different age groups. (B) Bar graph depicting the number of chief complaints in the 18 RTHβ patients with available information.
The thyroid hormone profiles demonstrated SITSH levels in 97.1% (33/34) of patients (Table 2, Fig. 2A). Only one patient with positive Tg-Ab and TPO-Ab showed a remarkably high level of serum TSH (>200 μIU/mL), although FT4 concentrations were within the reference range (patient #5). At least one thyroid autoantibody (Tg-Ab, TPO-Ab, or TRAb) was positive in 37.9% (11/29) of the patients tested. Tg-Ab and/or TPO-Ab were positive in 32.1% (9/28) of the patients tested (Fig. 2B). The positivity of thyroid autoantibodies in women was 47.1% (8/17), which was significantly higher than that in men (9.1% [1/11]; p = 0.049). The FT4 concentrations in the positive group were lower than those in the negative group, although the difference did not reach a statistically significant level (Fig. 3). There was no difference in the serum TSH concentrations between the two groups.

Laboratory findings in 34 patients with resistance to thyroid hormone beta (RTHβ). (A) Bar graph depicting the frequency of thyroid hormone profiles. (B) Bar graph depicting the positivity of thyroid autoantibodies (anti-thyroglobulin antibody [Tg-Ab] or anti-thyroperoxidase antibody [TPO-Ab]) in the 28 RTHβ patients with available information.

Free T4 and TSH concentrations in patients with negative results for both anti-thyroglobulin antibody (Tg-Ab) and anti-thyroperoxidase antibody (TPO-Ab) and patients with at least one positive result for TPO-Ab or Tg-Ab. Serum free T4 and TSH concentrations were adjusted according to the upper limit of the reference range (ULRR) in each case. Dotted horizontal bars represent median values. Arrows indicate a patient with a remarkably elevated TSH despite thyroid hormone concentrations being within reference ranges (patient #5). The p values were derived using the Mann–Whitney test.
Among the 24 patients with available information about past treatments, ten (41.7%) were previously treated with antithyroid drugs, radioactive iodine therapy, or surgery (Tables 3 and 4). The first edition of Japan Thyroid Association (JTA) guideline (GL) for the diagnosis of RTHβ was published in 2014. The percentage of inappropriate treatments was as high as 52.9% (9/17) during the first 15 years of the study period (2000–2014) and tended to decrease to 14.3% (1/7) during the last 6 years (2015–2020) after the JTA-GL publication, although the difference was insignificant (p = 0.172). We also conducted similar analyses after excluding the family member patients of RTHβ, since those patients may tend to avoid inappropriate therapy. Among the 22 probands with available information about past treatments, eight (36.4%) were previously treated with inappropriate treatment. The percentage was 43.8% (7/16) during the first 15 years of the study period (2000–2014) and tended to decrease, but not significantly, to 16.7% (1/6) during the last 6 years (2015–2020; p = 0.351).
We summarized the ten patients who had inappropriate past treatments (Table 4). The median duration of inappropriate treatments was ten years. All ten patients were initially diagnosed with hyperthyroidism, and five were further diagnosed with Graves’ disease. In at least two patients, antithyroid drugs were initiated despite the presence of SITSH (patients #21 and #30).
| No. | Subject ID | Past treatment | Durationa) | Age (year)b) | Chief complaintsb) | Diagnosisb) | SITSHb) |
|---|---|---|---|---|---|---|---|
| 1 | 1-proband | ATD | 10 | 19 | Goiter | Hyperthyroidism | n/a |
| 5 | 5-proband | ATD→LT3 | 30 | 11 | Goiter, palpitation | GD | n/a |
| 10 | 6-mother | PT | 47 | 23 | n/a | GD | n/a |
| 12 | 7-mother | ATD→TT | 15 | 42 | n/a | Hyperthyroidism | n/a |
| 14 | 8-proband | ATD | 6 | 25 | Palpitation | GD | n/a |
| 15 | 9-proband | ATD→None | n/a | n/a | n/a | GD | n/a |
| 16 | 10-proband | ATD→RIT | 12 | 33 | General malaise | GD | n/a |
| 21 | 13-proband | ATD | <1 | 0 | Tachycardia | Hyperthyroidism | Yes |
| 28 | 16-proband | ATD→None | 4 | 49 | General malaise | Hyperthyroidism | n/a |
| 30 | 18-proband | ATD | 10 | 40 | PAF | Hyperthyroidism | Yes |
a) indicates the duration of the inappropriate treatment. b) indicates information at the initial diagnosis.
Abbreviations: ATD, antithyroid drug; GD, Graves’ disease; LT3, liothyronine; n/a, not available; PAF, paroxysmal atrial fibrillation; PT, partial thyroidectomy; RIT, radioactive iodine therapy; TT, total thyroidectomy
THRB point mutations cluster in the three major hot spots in exons coding for the ligand-binding domain. The number of patients with point mutations in THRB for clusters #1 (429–460), #2 (310–353), and #3 (234–283) were 11, 11, and 11, respectively. There were no significant differences in the distribution of serum TSH or FT4 concentrations among the three clusters (Fig. 4). Of the 19 different mutations identified (Table 3), four (V264A, D265G, T329S, and L246V) were found to be novel after a literature review [5]. These four mutations were predicted to be pathogenic by the PolyPhen-2. Their categories (scores) are probable (1.000), possible (0.838), probable (0.999), and possible (0.620), respectively.
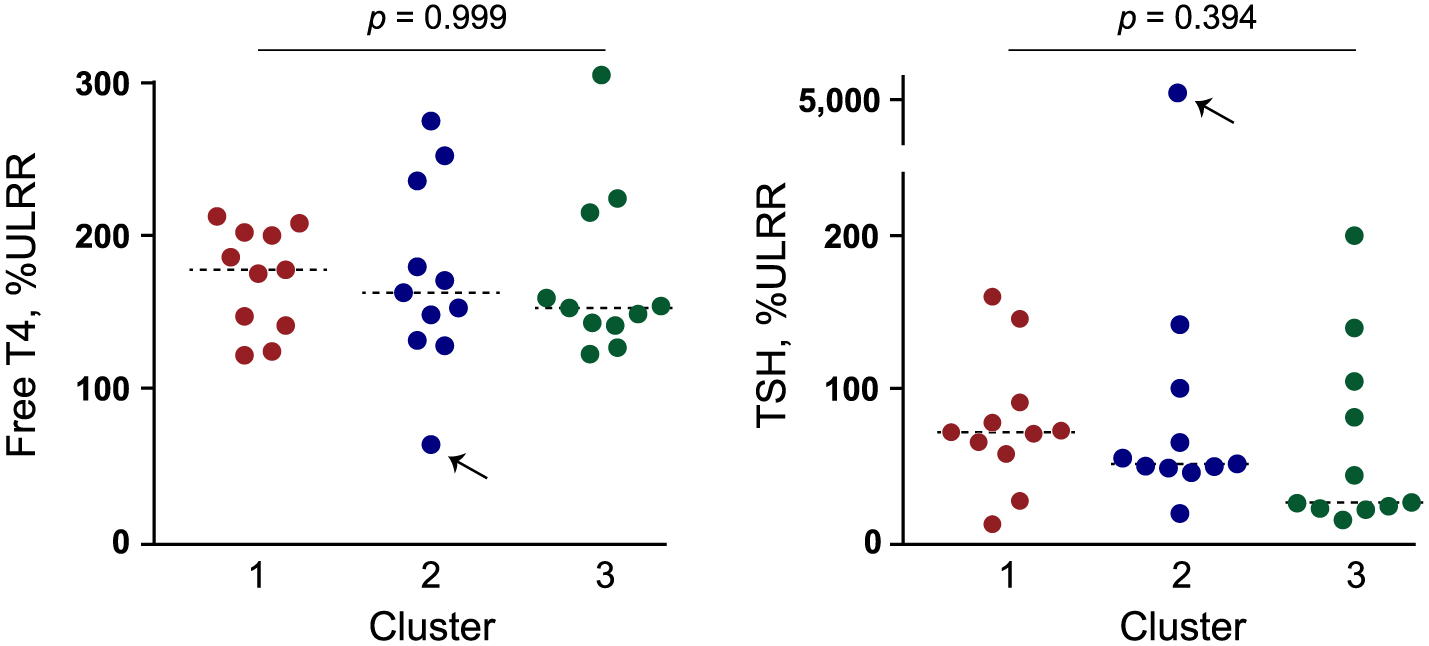
Free T4 and TSH concentrations among the patients with mutations in different mutation hotspots in the exons coding for the ligand binding domain of thyroid hormone receptor beta. Serum free T4 and TSH concentrations were adjusted according to the upper limit of the reference range (ULRR) in each case. Dotted horizontal bars represent median values. Arrows indicate a patient with a remarkably elevated TSH despite thyroid hormone concentrations being within reference ranges (patient #5). The p values were derived using one-way analysis of variance.
Of the 22 patients with available information about pituitary MRI, one (4.5%) had a small pituitary nodule with the largest diameter of 8 mm (patient #28). All nine patients who underwent the TRH stimulation test showed a positive TSH reaction to TRH.
We retrospectively analyzed the records of 34 patients with RTHβ and found several important findings. RTHβ is a rare autosomal dominant genetic disease and misdiagnosis can occur when clinicians are unaware of this disease. A recent study reported from Italy revealed that diagnostic delay and inappropriate treatments are prevalent in cases with thyroid hormone profiles suggestive of RTHβ [12]. We identified inappropriate management in Japan also, including thyroidectomy, radioactive iodine treatment, and antithyroid drug administration in as high as 41.7% (10/24) of RTHβ patients who had available information about the past treatment (Tables 3 and 4). The percentage of the inappropriate treatments tended to decrease in the last six years of the study period after the JAT-GL publication, compared with that in the first 15 years (before the JAT-GL; 14.3% vs. 52.9%). This reduction tendency may be due to the wide acceptance of information about RTHβ and guidelines including JTA-GL for its diagnosis and management in recent years.
The signs and symptoms of RTHβ are heterogeneous because the degree of compensation for tissue hyposensitivity to elevated thyroid hormone concentrations varies among individuals as well as in different tissues [1]. Similar to the current study (Table 1), previous reports from outside Japan [13, 14] have demonstrated goiter and palpitations as common clinical features of RTHβ. With regard to TSHoma, a single-center study of 90 consecutive cases in Japan identified goiter and palpitations as the two most common symptoms [9]. These two manifestations are common in Graves’ hyperthyroidism [15], therefore medical interviews and physical examinations are important for the differential diagnosis of hyperthyroidism, especially between RTHβ and TSHoma. The presence of other family members with SITSH strongly supports RTHβ, while symptoms associated with pressure from a pituitary adenoma or concomitant hyper- or hyposecretion of other pituitary hormones supports the probability of TSHoma [14]. In our study, every patient except one presented SITSH (Fig. 2A), the most important indicator of RTHβ (Fig. 2A). Early recognition and appropriate diagnosis of SITSH are mandatory to prevent inappropriate treatments that may be harmful.
The coexistence of primary thyroid disorders and RTHβ is relatively frequent [16] and may cause additional uncertainty in management. Concurrent primary hypothyroidism or primary thyrotoxicosis has been reported in patients with RTHβ [17-19]. Regarding autoimmune thyroid disease, Barkoff et al. demonstrated that at least one thyroid autoantibody (Tg-Ab or TPO-Ab) was identified in 23.3% (77/330) of patients with RTHβ in their cohort [16]. The frequency of thyroid autoantibody positivity is more than two-fold higher than that in the general population, as the National Health and Nutrition Examination Survey III (NHANES III) reported Tg-Ab and TPO-Ab in 10.4% and 11.3% of participants without known thyroid disorders, respectively [20]. Campi et al. have reported that concurrent autoimmune thyroiditis is diagnosed in 34.4% (21/61) of patients with RTHβ [12]. In agreement with their results, our retrospective study demonstrated a high frequency of autoimmune thyroiditis (positive Tg-Ab and/or TPO-Ab) of 32.1% (9/28; Table 3). Note that the positivity of thyroid autoantibodies in women was as high as 47.1% (8/17; Fig. 2A). The frequency of thyroid autoantibody positivity is much higher than that in the general population, as the NHANES III reported Tg-Ab and TPO-Ab in 13.8% and 14.6%, respectively, of female participants without known thyroid disorders [20]. The frequency of positivity in our male patients (9.1% [1/11]) was similar to that in the general population, as the NHANES III study reported positive Tg-Ab in 6.9% and positive TPO-Ab in 8.0% of male participants [20].
In our study, one patient developed Graves’ disease nine years after the diagnosis of RTHβ (patient #27). We searched PubMed and identified six cases of RTHβ coexisting with Graves’ disease (Table 5). Similar to our patient, two patients developed Graves’ disease after the diagnosis of RTHβ (Table 5) [21, 22]. Three patients were suspected of having RTHβ because of sustained thyroid hormone profiles of SITSH during treatment for Graves’ disease [23-25]. Interestingly, Silvakumar et al. identified RTHβ in a patient with Graves’ disease because the patient developed hypothyroidism after radioiodine ablation therapy and required a much higher dose of levothyroxine than his weight-based estimated replacement dose to maintain normal TSH concentrations [26]. Finding Graves’ disease coexisting with RTHβ is challenging when RTHβ is not diagnosed before the onset of Graves’ disease, but if a patient with Graves’ disease demonstrates an unusual course during the treatment, the presence of RTH can be considered.
| No. | Agea) (year) |
Sex | Mutation | Time sequence of disease onset | TSH (μIU/mL)a) | FT4 (ng/dL)a) | FT3 (pg/mL)a) | Reported year | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 34 | F | P453T | RTHβ→GD | <0.003 [0.3–4.0] | >10.0 [0.8–1.8] | >30.0 [2.5–4.5] | 2010 | [21] |
| 2 | 53 | M | G347W | GD→RTHβ | 0.2 [0.3–6.7] | n/a [n/a] | n/a [n/a] | 2010 | [26] |
| 3 | 17 | M | A234T | GD→RTHβ | <0.05 [0.54–4.26] | 6.5 [0.7–1.5] | 24.5 [2.4–4.1] | 2011 | [23] |
| 4 | 33 | F | G251R | GD→RTHβ | 0.007 [n/a] | 3.0 [n/a] | 9.3 [n/a] | 2011 | [24] |
| 5 | 47 | F | P453R | RTHβ→GD | 0.45 [0.3–5.6] | 2.9 [0.8–1.7] | 8.2 [2.5–3.9] | 2016 | [22] |
| 6 | 14 | F | T415I | GD→RTHβ | 0.037 [0.27–4.2] | 3.2 [0.9–1.7] | 2.8 [2.2–4.4] | 2017 | [25] |
| 7 | 51 | M | Q340H | RTHβ→GD | <0.01 [0.50–5.00] | 5.7 [0.9–1.6] | 15.2 [2.3–4.0] | Current study (patient #27) | |
a) indicates information at the diagnosis of GD. Data in square brackets are the reference ranges in each institute. Serum free T4 (FT4) and free T3 (FT3) values were rounded to one decimal place.
Abbreviations: F, female; M, male; n/a, not available
Little is known about the thyroid hormone dose required to stabilize the disturbed hypothalamic-pituitary-thyroid axis when patients with RTHβ develop a second thyroid disorder. One patient with positive Tg-Ab and TPO-Ab demonstrated a remarkably elevated TSH concentration despite FT4 concentrations being within the reference range (patient #5). Fukata et al. previously reported a similar pattern of abnormal hormone concentrations in a patient with RTHβ coexisting with Hashimoto’s thyroiditis [17]. The treatment of these conditions is an important issue that must be addressed.
This study has some limitations. First, its retrospective design has the potential to introduce bias. Second, the sample size was small. Owing to the low frequency of RTHβ, further multicenter or nationwide studies with larger sample sizes are necessary to clarify the clinical characteristics of RTHβ in Japan. Unfortunately, since they are not available yet, the study of a single institution is important. Especially the strength of our study lies in the premise that we obtained medical information from many other hospitals in various prefectures throughout Japan.
In conclusion, we analyzed the clinical outcomes of 34 Japanese patients with RTHβ and showed that inappropriate management of RTHβ is still present in Japan. Early recognition and appropriate diagnosis of SITSH are essential to prevent inappropriate and likely harmful treatments. Our study also identified a relatively high percentage of positive thyroid autoantibodies (Tg-Ab, TPO-Ab, or TRAb). The diagnosis and treatment of patients with RTHβ who develop a second thyroid disorder are important issues that need to be addressed. Further nationwide studies are required to evaluate the clinical characteristics of RTHβ in Japan.
This work was partially supported by the Japan Society for the Promotion of Science KAKENHI Grants (19K17981 for KO, 20303547 for SS, and 08457262 for HN).
The authors have nothing to declare.
The authors would like to acknowledge all the patients and healthcare professionals who treated these patients. The author would like to thank Editage (www.editage.com) for providing English language editing.