2022 Volume 69 Issue 7 Pages 863-875
2022 Volume 69 Issue 7 Pages 863-875
Polycystic ovary syndrome (PCOS) is a common gynecological disease accompanied by a variety of clinical features, including anovulation, hyperandrogenism, and ovarian abnormalities, resulting in infertility. PCOS affects approximately 6%–15% of all reproductive-age women worldwide. Metformin, a popular drug used to treat PCOS in patients, has beneficial effects in reducing hyperandrogenism and inducing ovulation; however, the mechanisms by which metformin ameliorates PCOS are not clear. Hence, we aimed to explore the mechanisms of metformin in treating PCOS. In the present study, we first treated a letrozole-induced PCOS rat model with metformin, detected the pathological recovery of PCOS, and then assessed the effects of metformin on H2O2-induced autophagy in ovarian granulosa cells (GCs) by detecting the level of oxidative stress and the expression of autophagy-associated proteins and key proteins in the PI3K/AKT/mTOR pathway. We demonstrated that metformin ameliorated PCOS in a rat model by downregulating autophagy in GCs, and metformin decreased the levels of oxidative stress and autophagy in H2O2-induced GCs and affected the PI3K/AKT/mTOR signaling pathway. Taken together, our results indicate that metformin ameliorates PCOS in a rat model by decreasing excessive autophagy in GCs via the PI3K/AKT/mTOR pathway, and this study provides evidence for targeted reduction of excessive autophagy of ovarian granulosa cells and improvement of PCOS.
POLYCYSTIC OVARY SYNDROME (PCOS) is a common gynecological disease associated with both endocrine and metabolic disorders, and it affects approximately 6%–15% of all reproductive-age women worldwide [1]. PCOS is characterized by anovulation, hyperandrogenism, and ovarian abnormalities, and it is a complex and intractable disorder associated with menstrual disorders, obesity, infertility, hirsutism, metabolic syndrome, hyperinsulinemia, insulin resistance, impaired glucose tolerance, type 2 diabetes, dyslipidemia, cardiovascular syndrome, etc. [2]. In addition, PCOS is accompanied by some psychological symptoms, such as increased anxiety and depression and reduced quality of life [3]. PCOS is an endocrine disorder that is considered to be the main cause of infertility in reproductive-age women, and it presents long-term health risks throughout life. Although numerous studies have shown that environmental, genetic, and hormonal factors are important in PCOS development, the etiology of PCOS is still unclear due to its clinically heterogeneous characteristics [4]. Clinically, the aim of PCOS treatment is to decrease hyperandrogenism and improve insulin sensitivity; however, overcoming these symptoms remains challenging because of the complex etiology and variable responses among PCOS patients [5]. Recently, clinical studies have shown that obvious autophagy occurs in ovarian granulosa cells (GCs) of patients with PCOS [6, 7], and reviews have reported that autophagy in GCs is an important cause of PCOS [8, 9]. In addition, studies have shown that imbalanced oxidative stress occurs in ovarian GCs of PCOS patients [10-12]. Therefore, these investigations suggested that autophagy of GCs induced by oxidative stress may be an important inducer of the occurrence and development of PCOS pathology.
Metformin, one of the most widely used insulin-sensitizing drugs for PCOS treatment, can increase the insulin sensitivity of ovaries to enhance glucose uptake and improve steroidogenesis and the menstrual cycle [13]. Furthermore, metformin can ultimately recover ovulatory function, enabling embryo implantation and improving pregnancy rates in women with PCOS [14]. Metformin has become a popular drug for treating PCOS patients with symptoms of insulin resistance, as it has beneficial effects on reducing hyperandrogenism and inducing ovulation [15]. In addition, metformin can reduce endogenous mitochondrial reactive oxygen species (ROS) involved in maintaining cell survival [16-18]. Although numerous studies have confirmed that metformin exerts its anticancer function by enhancing autophagy in cancer cells, it has been recently reported that metformin can also protect cells by reducing autophagy rates [19-21]. However, the mechanisms of metformin on GCs in the therapy of PCOS patients remain unclear. Furthermore, metformin can protect against oxidative stress-induced autophagy through the PI3K/AKT/mTOR pathway [19-23]. These results indicate that metformin may play an important role in reducing autophagy in GCs induced by oxidative stress through the PI3K/AKT/mTOR pathway.
In this study, to test our hypothesis, we investigated the therapeutic effect of metformin and found that metformin decreased the autophagy level in the GCs of PCOS rats. Subsequently, we used a cell model of ovarian granulosa cell autophagy induced by H2O2 to study the mechanism by which metformin reduces autophagy.
Twenty-four six-week-old specific pathogen-free (SPF) inbred female Sprague–Dawley (SD) rats (mean body weight of 180 ± 20 g at the onset of the experiments) were obtained from the Experimental Animal Center of Ningxia Medical University. All treatments and animal care procedures were performed in accordance with the National Institute of Health guidelines and were approved by the Medical Ethical Committee of Ningxia Medical University, China (NXMU-2021-009). All animals were fed a free-access commercial diet and sterile water at a controlled temperature of 22 ± 2°C in a 12-h light/dark cycle environment.
Before the experiment, all rats were confirmed to have normal estrous cycles by examination of vaginal smears for two sequential cycles. The rats were randomly assigned to two groups: the control group (Control, n = 8) and the PCOS group (PCOS, n = 16). The rats in the PCOS group received a gavage of letrozole (Hengrui Pharmaceutical Co., Ltd., Jiangsu, China) suspension with 0.5% carboxymethylcellulose at a dose of 1 mg/kg once daily for 21 consecutive days, and the control group rats received an isopycnic placebo [24]. Then, the subsequent letrozole treatment was terminated for the PCOS group, which was randomly divided into two groups as follows: the PCOS control group (PCOS, n = 8) and the metformin-treated group (PCOS + MET, n = 8). The rats in the PCOS group were treated with 500 mg/kg/day metformin (suspension with 0.5% carboxymethylcellulose) orally once daily for 21 consecutive days, and the control group rats received an isopycnic placebo [25]. After 21 days of metformin treatment, oral glucose tolerance tests (OGTTs) were performed before all the rats were sacrificed. Briefly, the fasting blood glucose of all the rats was measured after fasting for 12 h, after 30% glucose (0.5 mL) was given by gavage, and at time points of 0.5, 1, and 2 h [26], the blood glucose level of each rat was detected with a blood glucose monitor (Yuyue710, Jiangsu, China) at the corresponding time points. After 21 days of metformin treatment, cardiac blood and ovarian tissue samples were obtained for subsequent experiments. The ovarian organ coefficient was evaluated by the ratio of the two ovary weights to body weight.
Measurement of hormone levelsBlood samples (n = 8 each group) were used to measure hormone levels, including estradiol and testosterone, with commercially available enzyme-linked immunosorbent assay (ELISA) kits (USCN Life Science, Wuhan, China) according to the manufacturer’s instructions. The intra- and interassay coefficients of variation for estradiol and testosterone were less than 8% and less than 10%, respectively.
Histology and immunostainingOvary tissues were fixed in 4% paraformaldehyde for 24 h, embedded in paraffin, sectioned at a thickness of 5 μm, stained with hematoxylin-eosin (H&E) according to standard procedures, examined under a microscope, and photographed using a Micro Image (Olympus, Japan). The follicle count was performed in the ovary through the interval of 5 ovarian sections. The ovarian tissue sections were deparaffinized in xylene and rehydrated with gradient concentrations of ethanol, endogenous peroxidase was blocked with 3% hydrogen peroxide, and nonspecific binding was blocked with 2% BSA for 20 minutes. Subsequently, the sections were incubated with rabbit polyclonal anti-LC3II antibody (Beyond Biotech, Jiangsu, China) at a concentration of 1:100. After washing, the sections were incubated with anti-rabbit secondary antibody conjugated with horseradish peroxidase for 30 minutes and washed with PBS, and then, the protein expression was visualized with diaminobenzidine and hematoxylin staining. After mounting, the sections were photographed with a photographic system (Nikon) under a light microscope, and the quantification of LC3-II and Beclin-1 expression in immunohistochemical images was performed using ImageJ software (National Institutes of Health, USA).
Western blottingThe protein expression levels of PI3K, p-AKT, p-mTOR, LC3, and Beclin-1 were detected by western blotting. For tissue sample procurement, ovaries were added to 400 μL of lysis buffer and homogenized with a homogenizer. The samples were centrifuged at 4°C for 10 minutes at 12,000 rpm, and the supernatant was collected in a new centrifuge tube for the protein concentration assay. To obtain cell proteins, 200 μL of lysis buffer supplemented with protease inhibitors and phosphatase inhibitors was added on ice for 10 minutes and then centrifuged at 4°C for 10 minutes at 12,000 rpm, and the supernatant was collected in a new centrifuge tube for the protein concentration assay. After boiling for 5 minutes, 20 μg of protein was loaded in each lane of a 12.5% sodium dodecyl sulfate polyacrylamide gel, electrophoresis was performed, and the proteins were blotted onto a polyvinylidene difluoride (PVDF) membrane. Subsequently, the PVDF membrane was preincubated in blocking buffer containing 5% nonfat milk and 0.05% Tween 20 and incubated with the appropriate primary antibodies and secondary antibodies. The expression of the proteins was visualized with an ECL western blotting detection kit (Amersham Biosciences) on a ChemiDoc MP imaging system (Bio–Rad). Band densitometry was performed using ImageJ software. Assays were independently performed in triplicate for each sample. Antibodies against p-PI3K (p100α), PI3K, p-AKT (Ser473), AKT, p-mTOR (Ser2448), mTOR, LC3, LC3-II, and Beclin-1 were purchased from Abcam Co., and antibodies against α-tubulin, β-actin, and GAPDH were purchased from Beyond Biotech Co.
Isolation, culture, and treatment of GCsFemale SD rats (21–29 days old) were obtained from the Experimental Animal Center of Ningxia Medical University. After 1 week of acclimation, the rats were injected intraperitoneally with 40 IU of pregnant mare serum gonadotropin in saline. Forty-eight hours after the injection, the ovaries were separated from the anesthetized rats under sterile conditions. After washing twice with PBS, the GCs were released after puncturing the follicles with a needle under a stereoscopic microscope. The GCs were centrifuged at 500 g for 5 minutes and suspended in Dulbecco’s modified Eagle’s medium (DMEM)/F-12 (Gibco BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, Gibco) and 1% penicillin/streptomycin (Gibco). Then, the GCs were cultured in fresh medium in an incubator with a humidified atmosphere of 5% CO2 at 37°C. For drug administration, the GCs were incubated with medium containing 200 μM H2O2 and the corresponding drugs, including LY294002 (20 μM), 3-MA (5 mM), and rapamycin (10 μM), for 24 h [27].
Monodansylcadaverine (MDC) staining of autophagic vacuolesThe GCs were divided into a series of groups, including control, H2O2, and H2O2 + MET groups, and these cells adhered to and were grown on glass coverslips for 24 h. After washing with PBS, autophagic vacuoles were labeled with 0.05 mmol/L MDC staining at 37°C for 10 minutes. Then, the cells were washed with PBS and observed under a fluorescence microscope (Nikon, Japan), and the fluorescence intensity of the MDC-stained autophagic vacuoles was measured at an excitation wavelength of 380 nm and an emission wavelength of 530 nm.
Statistical analysisAll of the data were analyzed with GraphPad Prism 6.0 software. The data were presented as the means ± SD. Student’s t test and one-way ANOVA were used to determine the significant differences, and a p value less than 0.05 was considered to indicate a significant difference. Each experiment was repeated 3 times.
After letrozole gavage for 21 days, the PCOS model rats were evaluated, and the models were confirmed to have been successfully established (data not shown). Subsequently, the PCOS model rats were orally treated with metformin (MET) for 21 consecutive days. After treatment, we found that the body weight of the PCOS + MET group was reduced from Day 14 compared with that of the PCOS group, and the body weight of the PCOS group was significantly higher than that of the control group (Fig. 1A). An OGTT was performed, and the results showed that glucose tolerance was recovered in the PCOS + MET group compared with the PCOS group, and the value of PCOS + MET was close to that of the control group (Fig. 1B). The estradiol level in the PCOS model group was lower than that in the control and PCOS + MET groups, and the testosterone level in the PCOS model group was higher than that in the control and PCOS + MET groups (Fig. 1C and D). The ovaries of the three groups were collected, and the organ coefficient of each ovary was determined. The results showed that the ovarian organ coefficient in the PCOS group was lower than that in the control and PCOS + MET groups (Fig. 1E). In the ovarian histopathology, we found subcapsular cyst formation, a capsular thickness increase, corpus luteum reduction, and atretic and cystic follicle increases in the PCOS group compared with the control, and these phenotypes were improved after metformin treatment (Fig. 1F–J).Collectively, these data indicate that the body weight, glucose tolerance, serum hormone level, ovarian organ coefficient, and histopathological characteristics were affected in the PCOS group, while metformin treatment alleviated these alterations, with the assay results nearly reaching the levels of the control group. In addition, the oestrous cycle, insulin, FSH and LH were evaluated and the results showed the phenotypes of PCOS were alleviated by metformin (As shown in Fig. S1 of supplementary materials).

The application of MET alleviated the symptoms in the PCOS model. (A) The body weight change in the control, PCOS, and metformin-treated PCOS (PCOS + MET) groups during metformin treatment. (B) Oral glucose tolerance tests (OGTTs) in the control, PCOS, and PCOS + MET groups. The serum hormone levels, including (C) estradiol and (D) testosterone, in the control, PCOS, and PCOS + MET groups. (E) The ovarian organ coefficient in the control, PCOS, and PCOS + MET groups. (F) The ovarian histopathology characteristics in the control, PCOS, and PCOS + MET groups; P, primordial follicles; CF, cystic follicles; CL, corpus luteum; AF, atretic follicles. * p < 0.05 and ** p < 0.01. Scale bars in (F), up: 500 μm; down: 200 μm.
Autophagy plays an important role in PCOS occurrence. To determine whether metformin alleviates PCOS by improving autophagy, we detected the ovarian total expression of autophagic markers LC3 and Beclin-1 in the control, PCOS, and PCOS + MET groups. The results showed that the expression of LC3-II/LC3-I was statistically higher in the PCOS than in the control and PCOS + MET groups, and the expression of Beclin-1 was also significantly higher in the PCOS than the control and PCOS + MET groups; on the other hand, the expression levels of LC3-II/LC3-I (Fig. 2A and C) and Beclin-1 (Fig. 2B and D) were deceased after metformin treatment compared to those in the PCOS group. These results suggested that metformin can improve ovarian autophagy in the PCOS model.

Metformin improved ovarian autophagy in the PCOS model. (A) The expression of LC3 in the control, PCOS, and PCOS + MET groups. (B) The expression of Beclin-1 in the control, PCOS, and PCOS + MET groups. (C) Quantification of LC3-I/LC3-II in (A). (D) Quantification of Beclin-1 in (B). Data represent mean ± SD, n = 3, * p < 0.05, ** p < 0.01.
To investigate whether metformin improves the autophagy of ovarian cells in the PCOS model, we detected the expression of LC3-II and Beclin-1 by immunohistochemistry. The results showed that LC3-II and Beclin-1 were predominantly expressed in GCs and were higher in the PCOS model than in the control group, while their expression was obviously decreased in the PCOS + MET group compared to the PCOS model (Fig. 3). Therefore, it is likely that the downstream targets of metformin regulation are involved in decreasing autophagy in the GCs of the ovary.

(A) The immunostaining of LC3-II in the control, PCOS, and PCOS + MET groups. (B) Immunostaining of LC3-II in the control, PCOS, and PCOS + MET groups. (C) Quantification of the immunostaining for LC3-II in (A). (D) Quantification of the immunostaining for Beclin-1 in (B). Scale bars in (A) and (B): the upper panel was 500 μm, and the lower panel was 200 μm.
To determine the causes of ovarian autophagy and the mechanisms by which metformin improves autophagy in the GCs of the PCOS model, we detected the MDA level in ovarian tissue from the different groups. We found that the MDA level in the PCOS group was higher than that in the control and PCOS + MET groups (Fig. 4A). This result suggested that the oxidative stress level of the PCOS model was higher than that of the control and that metformin can attenuate the oxidative stress of the PCOS model. Additionally, studies have shown that the PI3K/AKT/mTOR pathway plays an important role in metformin functions and pathogenesis; therefore, the related proteins of PI3K/AKT/mTOR were detected, and the results showed that metformin ameliorated PCOS via the PI3K/AKT/mTOR signaling pathway (Fig. 4B–E). Combining the immunostaining results, we hypothesized that metformin reduces GC autophagy caused by oxidative stress in the PCOS model via the PI3K/AKT/mTOR signaling pathway.
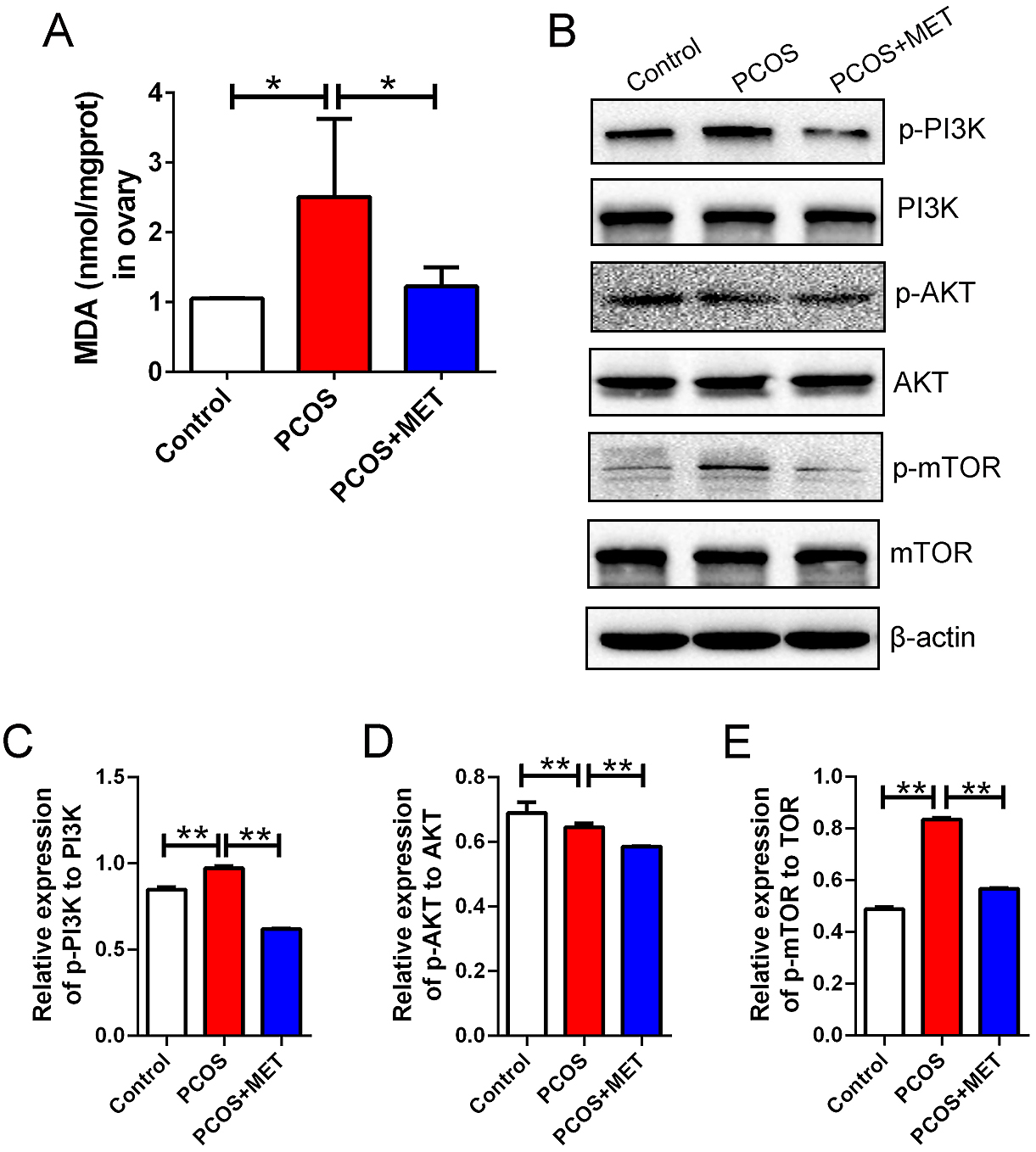
Metformin alleviate PCOS model by oxidative stress and PI3K/AKT/mTOR pathway. (A) The MDA level (nmol/mg protein) in the control, PCOS and PCOS + MET groups. (B) p-PI3K, p-AKT and p-mTOR expression in ovary in the control, PCOS and PCOS + MET groups. (C) Quantification of the immunoblot for p-PI3K in (B). (D) Quantification of the immunoblot for p-AKT in (B). (E) Quantification of the immunoblot for p-mTOR in (B). MDA, malondialdehyd. n = 3, * p < 0.05, ** p < 0.01.
To confirm the abovementioned hypothesis, we used H2O2 to treat rat GCs to establish a cell model of oxidative stress. In the H2O2-treated group, the fluorescence intensity of MDC-stained autophagic vacuoles was higher than that in the control and H2O2 + MET groups (Fig. 5A). Subsequently, markers of oxidative stress, including MDA, GSH, and SOD, were detected, and the results showed that the MDA level was higher than that in the control and H2O2 + MET groups (Fig. 5B) and that the GSH level was lower than that in the control and H2O2 + MET groups (Fig. 5C). SOD activity was also lower than that in the control and H2O2 + MET groups (Fig. 5D). These results suggested that metformin can decrease excessive autophagy caused by oxidative stress.

Metformin attenuated H2O2-induced oxidative stress in GCs. (A) Autophagic vacuoles were stained in the control, H2O2 and H2O2 + MET groups, scale bars: 200 μm. (B) The MDA level (nmol/mg protein) in the control, H2O2 and H2O2 + MET groups. (C) GSH level (μmol/g protein) in the control, H2O2 and H2O2 + MET groups. (D) SOD activity (U/mg protein) in the control, H2O2 and H2O2 + MET groups. H2O2, hydrogen peroxide; GSH, reduced glutathione; SOD, superoxide dismutase; n = 6, * p < 0.05, ** p < 0.01.
Numerous studies have reported that the PI3K/AKT/mTORC1 signaling pathway plays a central role in autophagy [28, 29]. Therefore, the present study investigated whether metformin affects oxidative stress-induced excessive autophagy through the PI3K/AKT/mTOR pathway. First, the autophagy marker proteins LC3 and Beclin-1, phosphorylation of PI3K, phosphorylation of AKT, and phosphorylation of mTOR were detected after metformin treatment. As shown in Fig. 6, LC3-II, Beclin-1, and p-PI3K were significantly lower in the H2O2 + MET group than in the H2O2 group. In the metformin-treated H2O2-exposed GCs, the levels of AKT phosphorylation (p-AKT) and mTOR phosphorylation (p-mTOR) were lower in the PI3K/AKT/mTOR pathway interference groups (H2O2 + 3-MA, H2O2 + LY294002, and H2O2 + Rapa) than in the metformin-treated H2O2-exposed groups. In addition, Beclin-1 and LC3II/I were increased after adding PI3K and mTOR inhibitors. These results suggested that metformin alleviated H2O2-induced autophagy of GCs through PI3K/AKT/mTOR. Furthermore, in contrast to the H2O2-exposed autophagy group, the levels of p-PI3K, p-AKT, and p-mTOR were significantly affected by metformin treatment. These results indicated that metformin decreased H2O2-induced excessive autophagy of rat GCs through the PI3K/AKT/mTOR pathway.

Metformin decreased oxidative stress-induced excessive autophagy via the PI3K/AKT/mTOR pathway in GCs. (A) Immunoblotting analysis of GCs treated with H2O2 and intervention in the PI3K/AKT/mTOR pathway. (B) Quantification of the immunoblot for p-PI3K to PI3K in (A). (C) Quantification of the immunoblot for p-AKT to AKT in (A). (D) Quantification of the immunoblot for p-mTOR to mTOR in (A). (E) Quantification of the immunoblot for Beclin-1 to α-tubulin in (A). (F) Quantification of the immunoblot for LC3II/I in (A). n = 3, * p < 0.05, ** p < 0.01.
PCOS is a common and complex gynecological disease in reproductive-age women and is characterized by hyperandrogenemia, hyperinsulinemia, and abnormal ovulation [4]. Although metformin has been widely used for PCOS patients with hyperglycemia and insulin resistance and is beneficial for ovarian function in fertility clinics, the mechanisms of metformin in ameliorating PCOS and improving ovarian function are still unclear. In the present study, we used metformin-treated letrozole-induced PCOS model rats in vivo and metformin added to H2O2-induced GC model rats in vitro to investigate the mechanism by which metformin improves PCOS. The results indicated that metformin ameliorates PCOS by decreasing oxidative stress-induced autophagy through the PI3K/AKT/mTOR pathway. We demonstrated for the first time the regulation of GC autophagy by metformin in vivo and in vitro.
Metformin is beneficial to PCOS patients and can improve glucose metabolism and fertility. Numerous studies have shown that metformin can increase the ovulation and pregnancy rates and enhance the live birth rates among PCOS patients [30, 31]. Compared with the letrozole-induced PCOS animal model, the heterogeneity of PCOS diseases determines the complexity of the inducing factors and pathogenesis in clinical outcomes. However, most studies have shown that the letrozole-induced PCOS animal model can basically simulate the characteristics of PCOS patients, including hyperandrogenemia, hyperinsulinemia, and abnormal ovulation; in addition, this PCOS model has been widely used and accepted in numerous studies [32-34]. In evaluating the therapeutic effect of metformin on a PCOS rat model, this study showed that metformin could decrease body weight, improve insulin resistance, restore sex hormones, improve ovarian weight, and reduce cystic follicle numbers, which is consistent with previous studies [35, 36]. It has been reported that metformin has a direct effect on steroidogenesis by culturing GCs and theca cells, and studies have suggested that steroidogenic enzymes such as CYP17A1 and CYP11A1 are potential targets for metformin to regulate related signaling pathways [37, 38]. However, the precise signaling pathways or sites of metformin on steroidogenic enzymes and the mechanisms of its effects remain to be determined. In addition, the phenotype caused by letrozole as an inhibitor of aromatase to inhibit estrogen synthesis is very similar to the PCOS clinical symptoms, including increased body weight, glucose resistance, and altered sex hormone levels. Therefore, these results indicated that the effect of metformin in the treatment of the PCOS model rats can better simulate the clinical outcome.
Currently, various studies have shown that autophagy of GCs is an important factor in PCOS occurrence [39, 40]. In addition, a study reported that oxidative stress participates in the pathophysiology of PCOS and is independent of weight excess [41]. Therefore, these results indicated that autophagy in GCs induced by oxidative stress may be the main factor in the occurrence of PCOS. In the present study, the letrozole-induced PCOS rat model also showed the characteristics of GC autophagy and a high level of ovarian oxidative stress, which indicated that this letrozole-induced PCOS model is valid and is consistent with the clinical pathological features of PCOS. Oxidative stress in GCs originates from endogenous and exogenous factors, and the biosynthesis of sex steroids and other accelerated metabolic rates can generate high levels of intracellular reactive oxygen species (ROS). On the other hand, exogenous factors such as environmental pollutants, ionizing radiation, and unhealthy lifestyle factors can also promote excess production of ROS in GCs and potentially induce PCOS [42, 43]. A recent study showed that pineal gland-secreted melatonin, as an endogenous mitochondrial-targeted antioxidant, can ameliorate excessive mitophagy in GCs of PCOS patients, indicating that oxidative stress is the main cause of mitochondrial autophagy in GCs of patients with PCOS [44]. In the present study, the level of oxidative stress and autophagy in ovarian GCs and the level of androgen in the PCOS model were decreased by metformin, and an earlier study showed that androgen levels play a critical role in GC autophagy in patients with PCOS [6]. Additionally, the oxidative stress level increase in GCs was considered to be the primary pathogenesis of PCOS [45], implying that androgen may be the key trigger leading to the increase in oxidative stress in ovarian GCs and autophagy, which results in the occurrence and development of PCOS; however, the detailed mechanisms need to be further studied.
While investigating the effect of oxidative stimulation on GCs, Shen et al. found that melatonin, as a robust antioxidant, could repress H2O2-induced GC autophagy by upregulating antioxidative enzymes and scavenging ROS directly or indirectly [42, 46]. H2O2, as an exogenous inducer, has been widely applied in many studies to investigate damage mechanisms, such as apoptosis and autophagy, caused by oxidative stress in GCs [47, 48]. In this study, we established a rat GC model of H2O2-induced autophagy that can be reversed by metformin, implying that metformin plays a role as an antioxidant in scavenging ROS. Subsequently, we found that the levels of oxidative stress markers, including MDA, GSH, and SOD, were restored in the metformin treatment group compared with the H2O2 group. In the in vitro results, it was found that only MDA levels showed a significant difference, which may be related to the limited number of GCs in the ovarian tissue and the loss of GCs in PCOS. Furthermore, the antioxidant system in the tissue is more complex than that at the cellular level, which requires further study.
Metformin, a widely used drug to treat type 2 diabetes and PCOS, has been found to improve endothelial function and protect against oxidative stress by increasing AMP-activated protein kinase activity and promoting antioxidant protection at the cellular level [49]. In addition, a recent study showed that metformin has a protective effect on the myocardial ischemia-reperfusion injury-dependent AMPK pathway through upregulation of antioxidant enzymes to reduce oxidative damage accumulation [50]. In a study of metformin-treated cerebral ischemia, indicators of oxidative stress, including antioxidant enzyme activities of catalase, MDA, SOD, and glutathione peroxidation enzyme, were affected by metformin treatment [51]. In the present study, we found that metformin can decrease the MDA level and increase the levels of GSH and SOD. Therefore, these results indicate that metformin has an underlying function in regulating antioxidant enzymes and sequentially plays an antioxidant role [52]. However, this potential function of metformin still needs to be further studied.
It has been reported that the PI3K/Akt/mTOR signaling pathway is essential for autophagy, and the activation of the PI3K/Akt/mTOR pathway can inhibit autophagy [53, 54]. Akt is a principal mediator, and mTOR plays a targeted role downstream of the PI3K/Akt pathway; moreover, it has been accepted that the process of autophagy is negatively regulated through mTOR activation [55]. Rapamycin is an inhibitor of mTOR and an agonist of autophagy [56]. 3-MA can block the transition from LC3-I to LC3-II, inhibit PI3K, and destroy the antagonistic function of mTOR against autophagy [57]. LY294002 is a broad-spectrum PI3K inhibitor that activates autophagy [58]. Numerous studies have reported that the PI3K/AKT signaling pathway plays a critical role in PCOS pathogenesis, likely because PCOS is cured by activating the PI3K/AKT signaling pathway in the ovary [59-61]. Zhao and his colleagues reported that the traditional Chinese medicine Heqi San has beneficial effects on PCOS and restores serum hormone levels, recovers ovary morphological lesions, and attenuates insulin resistance, which is mediated through the PI3K/AKT pathway in ovary tissue of a PCOS rat model after dehydroepiandrosterone treatment [62]. On the other hand, another traditional Chinese medicine, Liuwei Dihuang pills, obviously improved ovarian polycystic pathogenesis and alleviated insulin resistance by acting on the PI3K/Akt signaling pathway in a letrozole-established PCOS rat model. Furthermore, the development of follicles was regained by upregulating cytochrome P450 family 19 subfamily A member 1 (CYP19A1), which plays an important role in the estrogen synthesis of ovarian GCs and whose irregular expression is involved in the development of PCOS [63, 64]. In addition, metformin was used to treat PCOS in model rats induced by insulin plus hCG, and metformin improved uterine dysfunction and inhibited insulin resistance-induced inflammation through downregulation of the PI3K/AKT pathway [61]. This outcome suggests that the PI3K/AKT pathway in GCs is a potential target for PCOS therapy. In our study, intervention in autophagy and the PI3K/AKT/mTOR pathway suggested that metformin decreased H2O2-induced autophagy in GCs through the PI3K/AKT/mTOR pathway, and these results are consistent with previous findings that PI3K/AKT/mTOR pathway activation has beneficial effects on PCOS [65-67]. Therefore, in summary, the PI3K/AKT/mTOR pathway of GCs is a possible therapeutic target in the treatment of PCOS with metformin. In the present study, we explored the detailed mechanism of metformin in the treatment of PCOS; however, there were some limitations in this study. First, the subsequent pregnancy outcomes of the PCOS rats were not observed; second, primary granulosa cells do not reflect human GCs in PCOS; therefore, these two areas should be further investigated in the future. Furthermore, whether metformin therapy can improve ovarian function in infertile patients with PCOS needs to be addressed in large clinical trials.
In conclusion, this study demonstrated a beneficial effect of metformin on PCOS pathology in a rat model, including recoveries in body weight, glucose tolerance, serum hormone levels, and ovarian morphology. Subsequently, we found that autophagy of GCs and oxidative stress levels were restored in the ovaries of PCOS model rats after metformin treatment, and then, a H2O2-induced GC model was established to investigate the mechanisms by which metformin decreases oxidative stress, resulting in excessive autophagy of GCs. We found that metformin could decease excessive autophagy as well as oxidative stress through the PI3K/AKT/mTOR signaling pathway in GCs. Therefore, we propose the novel view that metformin improves PCOS by altering the oxidative stress level and upregulating antioxidant enzymes in GCs through the PI3K pathway.
The authors declare that they are no conflicts of interest.
XF F, XY P and X D designed the study. B X, WJ D and L L performed the experiment. XF F and X D drafted the manuscript. H H and JJ Z participated in the statistical analysis. XY P assisted in the manuscript revision.
This study was supported by grants from the Key Research and Development Program of Ningxia (Grant No. 2019BFG02007), the National Natural Science Foundation of China (Grant No. 81960270, 82160290, 81760286), the Natural Science Foundation of Ningxia (2022AAC02031, 2021AAC03133).