2023 Volume 70 Issue 10 Pages 987-998
2023 Volume 70 Issue 10 Pages 987-998
Immune checkpoint inhibitors (ICIs) are used for various malignancies, although they frequently cause immune-related adverse events involving the thyroid gland (thyroid irAEs). We conducted a retrospective cohort study to elucidate thyroid function outcomes. Fifty of 639 patients who received PD-1 blockade therapy met criteria and were divided into the following groups: thyrotoxicosis with subsequent hypothyroidism (Toxic-Hypo, n = 21); thyrotoxicosis without subsequent hypothyroidism (Toxic, n = 9); and hypothyroidism without prior thyrotoxicosis (Hypo, n = 20). The Toxic-Hypo group developed thyroid irAEs earlier than the Toxic group (26 vs. 91 days; p < 0.001), and had higher serum free T4 levels (3.210 vs. 1.880 ng/dL; p = 0.011). In addition, positive anti-thyroglobulin antibodies (TgAbs) at thyroid irAE onset were more common in the Toxic-Hypo group (93.3%) than in the Toxic group (0.0%; p = 0.005) and Hypo group (44.4%; p = 0.007). The Toxic-Hypo group developed severe hypothyroidism and required larger levothyroxine (LT4) doses than the Hypo group (75 vs. 25 μg/day; p = 0.007). We predicted that patients with positive TgAbs who developed severe thyrotoxicosis within 4 weeks after the first ICI administration would develop subsequent hypothyroidism. We treated 4 such patients with prompt LT4 replacement, characterized by LT4 initiation after thyrotoxicosis improvement and quick dose titration. Their euthyroid state was successfully maintained, in contrast with patients receiving conventional replacement. In conclusion, rapid-onset severe thyrotoxicosis in patients with TgAbs correlated with a high likelihood of subsequent hypothyroidism. Accordingly, prompt LT4 replacement is suggested to prevent a severely hypothyroid state.
IMMUNE CHECKPOINT INHIBITORS (ICIs) are used for various malignancy types and stages, although associated immune-related adverse events (irAEs) remain an issue. Among ICIs, antibodies against programmed cell death-1 (PD-1) and programmed cell death ligand-1 (PD-L1) frequently lead to irAEs involving the thyroid gland (thyroid irAEs). Several recent cohort studies clarified the clinical features of thyroid irAEs [1-5]. These studies varied in their definitions of thyroid irAEs, but they distinguished overt thyroid irAEs and found that they had an incidence of 11.9–22.2% in clinical settings. Predictive factors for thyroid irAE development were also reported: positive anti-thyroglobulin antibody (TgAb) and/or anti-thyroperoxidase antibody (TPOAb) [6], thyroid uptake on FDG-PET [1], pretreatment with tyrosine kinase inhibitors [7], the presence of an irregular thyroid echo pattern [8], high serum levels of IL-1β, IL-2, and GM-CSF [9], and high body mass index (BMI) [4].
Meanwhile, the typical clinical course of thyroid irAEs is informative for management: transient thyrotoxicosis develops within 2 to 6 weeks after the first ICI administration, with hypothyroidism occurring after 12 weeks [1]. Thyrotoxicosis is generally mild, with clinical trials reporting only rare cases of grade 3 or higher according to the Common Terminology Criteria for Adverse Events v5.0 [10]. On the other hand, hypothyroidism can become severe, and serum TSH levels often rise above 100 μIU/mL [1, 11, 12]. Therefore, treatment with levothyroxine (LT4) replacement is important. A recent study proposed 1.45 μg/kg/day as an appropriate LT4 dosage for hypothyroidism following thyroid irAEs [13]. However, the optimal timing for initiating LT4 replacement has not been determined.
In this retrospective cohort study, we aimed to elucidate best practices regarding thyroid function outcome after thyroid irAEs. Our analysis of patients who developed thyroid irAEs identified several factors that predicted severe subsequent hypothyroidism. Four patients with thyrotoxicosis were considered highly likely to develop subsequent hypothyroidism, so we treated them promptly with LT4 replacement.
The retrospective cohort analysis in this study was performed using the medical records of patients who started nivolumab, pembrolizumab, or atezolizumab as monotherapy at Kyoto University Hospital between September 1, 2014, and December 31, 2020. Clinical data on some patients who received nivolumab therapy were previously reported [1, 14]. The patients who received pembrolizumab or atezolizumab therapy were the same as those in our previous report [15]. Instead of obtaining informed consent, we provided each patient with the opportunity to opt out of the study using our website. We excluded patients according to the following criteria: prior ICI therapy, subsequent ICI rechallenge within 6 months, and ICI use as part of a clinical trial or patient-proposed healthcare services. The institutional review board and ethics committee of the Kyoto University Graduate School of Medicine approved this study (approval number, R1400). For the case series involving prompt LT4 replacement, patients were treated at Kyoto University Hospital after January 1, 2022, and the institutional review board and ethics committee approved the retrospective analysis of their clinical data (approval number, R3075). We conducted these studies in accordance with the principles of the Declaration of Helsinki.
Laboratory dataSerum levels of free T3 (fT3), free T4 (fT4), and TSH were measured with electrochemiluminescence immunoassays. During the period analyzed in the retrospective study, Elecsys kits were used (Elecsys FT3 II kit, Elecsys FT4 kit, and Elecsys TSH kit; Roche Diagnostics, Mannheim, Germany), and the reference ranges were 2.33–4.00 pg/mL, 0.880–1.620 ng/dL, and 0.500–5.000 μIU/mL, respectively. In the case series, AIA kits were used (AIA-PACK CL FT3 kit, AIA-PACK CL FT4 kit, and AIA-PACK CL TSH kit; Tosoh, Tokyo, Japan), and the reference ranges were 2.06–3.17 pg/mL, 0.880–1.620 ng/dL, and 0.500–5.000 μIU/mL, respectively. TgAb, TPOAb, and anti-TSH receptor antibody (TRAb) were measured using Elecsys kits (Roche Diagnostics), with reference ranges of <28 IU/L, <16 IU/L, and <2.0 IU/L, respectively. Measurement of thyroid stimulating antibody (TSAb) titers was performed using a TSAb enzyme immunoassay kit (Yamasa Corp., Chiba, Japan), with a cut-off level of 120%.
Assessments of irAEsAccording to our previously reported criteria, thyroid irAEs were determined based on serum fT4 and TSH levels within 6 months after the first administration of ICIs [1]. In the present study, we focused exclusively on overt thyroid irAEs, defined by abnormal serum levels of both fT4 and TSH. Pituitary irAEs were diagnosed by physicians according to clinical symptoms and low levels of ACTH and cortisol, as in our previous study [14]. The irAE of insulin-dependent diabetes was defined according to a previous report as hyperglycemia with low serum C-peptide levels, the absence of other causes, and the requirement for continuous insulin treatment [16]. Non-endocrine related irAEs were characterized as adverse events other than thyroid irAEs, pituitary irAEs, and insulin-dependent diabetes, that required intravenous or oral glucocorticoid therapy for resolution.
Statistical analysisContinuous variables are expressed as medians (interquartile range). Comparisons of parameters between two groups were performed with the Mann-Whitney U test or Pearson’s chi-square test, as appropriate. Comparisons between three groups were performed with the Steel-Dwass test or Pearson’s chi-square test. A p value of <0.05 was considered statistically significant. JMP Pro®, version 16.1.0 (SAS Institute Inc., Cary, NC, USA), was used to perform the statistical analyses.
According to the criteria described in the Materials and Methods section, we included 639 patients who received PD-1 blockade therapy with nivolumab, pembrolizumab, or atezolizumab (Fig. 1). Overt thyroid irAEs were observed in 70 patients (11.0%). We excluded 7 patients who had been treated for hypothyroidism, 1 who had been treated for hyperthyroidism, 3 who were diagnosed with overt hypothyroidism at examinations before ICI use, and 9 who were censored within 6 months of follow-up. Consequently, 50 patients were included and were divided into 3 groups: thyrotoxicosis with subsequent hypothyroidism (Toxic-Hypo group; 21 patients, 42.0%), thyrotoxicosis without subsequent hypothyroidism (Toxic group; 9 patients, 18.0%), and hypothyroidism without prior thyrotoxicosis (Hypo group; 20 patients, 40.0%).

Flowchart of retrospective patient recruitment. “n” represents number of patients; irAE, immune-related adverse event; ICI, immune checkpoint inhibitor
Patient characteristics are presented in Table 1. The groups did not differ significantly in terms of age, malignancy types, ICIs used, numbers or durations of ICI administrations, or the incidence of irAEs other than thyroid irAEs. Interestingly, the Toxic-Hypo group included more females than the Toxic group (p = 0.046) and had a higher BMI than the Hypo group (p = 0.026). Longitudinal changes in thyroid function are shown in Fig. 2. The Toxic-Hypo group developed transient thyrotoxicosis and then hypothyroidism (Fig. 2A–2C), while the Toxic group did not develop hypothyroidism (Fig. 2D–2F). The Hypo group did not develop thyrotoxicosis (Fig. 2G–2I), although 2 patients had slightly elevated serum fT4 levels due to LT4 replacement.
Characteristics of patients who developed thyroid irAEs
| Toxic-Hypo (n = 21) | Toxic (n = 9) | p | Hypo (n = 20) | p | |
|---|---|---|---|---|---|
| Age (years) | 62 (53–73) | 73 (62–75) | 0.339 | 71 (66–78) | 0.360 |
| Gender, n (%) | |||||
| Male | 8 (38.1) | 7 (77.8) | 0.046 | 11 (55.0) | 0.220 |
| Female | 13 (61.9) | 2 (22.2) | 9 (45.0) | ||
| BMI | 23.5 (21.2–27.2) | 21.1 (18.9–25.0) | 0.665 | 19.7 (17.7–24.4) | 0.026 |
| Malignancy type, n (%) | |||||
| Non-small cell lung cancer | 6 (28.6) | 5 (55.6) | 0.370 | 5 (25.0) | 0.507 |
| Malignant melanoma | 7 (33.3) | 2 (22.2) | 4 (20.0) | ||
| Others | 8 (38.1) | 2 (22.2) | 11 (55.0) | ||
| ICI, n (%) | |||||
| Nivolumab | 17 (81.0) | 5 (55.6) | 0.258 | 15 (75.0) | 0.885 |
| Pembrolizumab | 3 (14.3) | 2 (22.2) | 4 (20.0) | ||
| Atezolizumab | 1 (4.7) | 2 (22.2) | 1 (5.0) | ||
| ICI administration data | |||||
| Number of administrations | 9 (3–11) | 8 (6–11) | 0.780 | 9 (7–11) | 0.972 |
| Duration of administration (days) | 168 (60–180) | 127 (88–173) | 0.790 | 163 (110–179) | 0.935 |
| Other irAEs, n (%) | |||||
| Pituitary irAEs | 1 (4.6) | 1 (11.1) | — | — | — |
| Insulin-dependent diabetes | 1 (4.6) | — | — | — | — |
| Non-endocrine-related irAEs | 4 (19.1) | 1 (11.1) | 0.849 | 1 (5.0) | 0.317 |
Age, BMI, and ICI administration data are expressed as medians (interquartile range). “n” represents number of patients.
irAE: immune-related adverse event, BMI: body mass index, ICI: immune checkpoint inhibitor. Statistical analyses compared the Toxic and Hypo groups with the Toxic-Hypo group. The Steel-Dwass test was used for age, BMI, and ICI administration data. Pearson’s chi-square test was used for other parameters. Statistical analyses of pituitary irAEs and insulin-dependent diabetes were not performed due to an insufficient number of patients. Bold type represents statistical significance.

Clinical data on thyroid irAEs in each group are shown in Table 2. First, we compared the Toxic-Hypo group with the Toxic group. Thyroid function and thyroid uptake on FDG-PET before the first ICI administration were not significantly different between the 2 groups. Of note, the Toxic-Hypo group developed thyroid irAEs earlier than the Toxic group (26 vs. 91 days after the first ICI administration; p < 0.001). Thyrotoxicosis was more severe in the Toxic-Hypo group than in the Toxic group: the Toxic-Hypo group had higher levels of both fT3 (6.47 vs. 3.51 pg/mL; p = 0.001) and fT4 (3.210 vs. 1.880 ng/dL; p = 0.011) at thyrotoxicosis onset. The 2 groups also differed regarding the positivity of thyroid autoantibodies: most patients in the Toxic-Hypo group were positive for TgAbs (14 of 15 tested patients, 93.3%), compared to none in the Toxic group (0 of 3 tested patients, 0.0%) (p = 0.005). In the Toxic-Hypo group, TPOAb positivity was less common (8 of 15 tested patients, 53.3%) than TgAb positivity, while none of the patients in the Toxic group were positive for TPOAbs (0 of 3 tested patients, 0.0%) (p = 0.090).
Data on clinical course, thyroid function, and thyroid autoimmunity in patients who developed thyroid irAEs
| Toxic-Hypo (n = 21) | Toxic (n = 9) | p | Hypo (n = 20) | p | |
|---|---|---|---|---|---|
| Before first ICI administration | |||||
| fT3 (pg/mL) | 2.75 (2.54–3.03) | 2.65 (2.39–2.95) | 0.641 | 2.59 (2.50–2.70) | 0.051 |
| fT4 (ng/dL) | 1.170 (1.012–1.235) | 1.310 (1.165–1.440) | 0.148 | 1.095 (0.978–1.198) | 0.390 |
| TSH (μIU/mL) | 2.820 (1.565–4.425) | 1.840 (1.550–2.105) | 0.187 | 4.560 (3.095–7.988) | 0.015 |
| Thyroid uptake on FDG-PET (n) | 6 (tested in 13) (46.2%) | 4 (tested in 7) (57.1%) | 0.639 | 6 (tested in 13) (46.2%) | 1.000 |
| At onset of thyrotoxicosis | |||||
| Period of onset (days) | 26 (21–42) | 91 (46–106) | <0.001 | — | — |
| fT3 (pg/mL) | 6.47 (4.68–10.81) | 3.51 (2.75–4.37) | 0.001 | — | — |
| fT4 (ng/dL) | 3.210 (1.985–4.265) | 1.880 (1.725–2.120) | 0.011 | — | — |
| TSH (μIU/mL) | 0.031 (0.013–0.078) | 0.078 (0.025–0.154) | 0.160 | — | — |
| Positive TgAb (n) | 14 (tested in 15) (93.3%) | 0 (tested in 3) (0.0%) | <0.001 | — | — |
| Positive TPOAb (n) | 8 (tested in 15) (53.3%) | 0 (tested in 3) (0.0%) | 0.090 | — | — |
| After onset of hypothyroidism | |||||
| Period of onset (days) | 70 (60–84) | — | — | 94 (67–127) | 0.058 |
| Nadir fT3 (pg/mL) | 1.15 (0.90–1.93) | — | — | 2.17 (1.64–2.44) | 0.011 |
| Nadir fT4 (ng/dL) | 0.378 (0.216–0.575) | — | — | 0.754 (0.492–0.819) | <0.001 |
| Peak TSH (μIU/mL) | 93.070 (19.690–127.650) | — | — | 26.720 (11.490–72.505) | 0.100 |
| LT4 dose after 6 months (μg/day) | 75 (25–87.5) | — | — | 25 (0–68.75) | 0.007 |
| Positive TgAb (n) | 14 (tested in 15) (93.3%) | — | — | 4 (tested in 9) (44.4%) | 0.007 |
| Positive TPOAb (n) | 8 (tested in 15) (53.3%) | — | — | 2 (tested in 9) (22.2%) | 0.135 |
Data other than thyroid uptake on FDG-PET, TPOAb, and TgAb are expressed as medians (interquartile range). “Days” and “months” indicate days and months since the first ICI administration, respectively. fT3: free T3, fT4: free T4, TgAb: anti-thyroglobulin antibody, TPOAb: anti-thyroperoxidase antibody, LT4: levothyroxine. Statistical analyses compared the Toxic and Hypo groups with the Toxic-Hypo group. The Steel-Dwass test was used to assess fT3, fT4, and TSH before the first ICI administration. Pearson’s chi-square test was used to assess thyroid uptake on FDG-PET, positive TgAb, and positive TPOAb. The Mann-Whitney U test was used for other parameters. Bold type represents statistical significance.
Second, we analyzed the relationships between thyrotoxicosis severity, time of thyrotoxicosis onset, and TgAb positivity. A scatter plot of serum fT4 levels against time of thyrotoxicosis onset revealed that all patients in the Toxic-Hypo group developed thyrotoxicosis within 8 weeks after the first ICI administration (Fig. 3A). In addition, with a fT4 cut-off of 3 ng/dL, high serum fT4 levels were only seen in the Toxic-Hypo group (Fig. 3A). In particular, serum fT4 levels of patients who developed thyrotoxicosis within 4 weeks were significantly higher than those of patients who developed it after 5 weeks (p = 0.045 vs. 5–8 weeks, p = 0.003 vs. 9– weeks) (Fig. 3B). No significant associations were observed between TgAb titers and serum fT4 levels (Fig. 3C, 3D).

Correlations between factors involved in the outcome of thyroid irAEs. (A) Scatter plot of serum fT4 levels at thyrotoxicosis onset against the period since the first ICI administration. Red circles indicate the Toxic-Hypo group and pink circles indicate the Toxic group. (B) Box plot of serum fT4 levels for different periods between the first ICI administration and thyrotoxicosis onset: –4 indicates within 4 weeks, 5–8 indicates from 5 to 8 weeks, and 9– indicates after 9 weeks. (C) Scatter plot of serum fT4 levels against TgAb titers at thyrotoxicosis onset. (D) Box plot for different ranges of TgAb titers: –63 indicates 63 IU/mL or less, 64–511 indicates from 64 to 511 IU/mL, and 512– indicates 512 IU/mL or more. TgAb, anti-thyroglobulin antibody. Statistical analyses were performed for panels B and D using the Steel-Dwass test.
Third, we compared the Toxic-Hypo group with the Hypo group (Table 2). Regarding thyroid function before the first ICI administration, serum TSH levels were slightly higher in the Hypo group than in the Toxic-Hypo group (4.560 vs. 2.820 μIU/mL; p = 0.015). The incidence of thyroid uptake on FDG-PET was similar between the 2 groups. The duration from the first ICI administration to the onset of hypothyroidism was non-significantly shorter in the Toxic-Hypo group than in the Hypo group (70 vs. 94 days; p = 0.058). Furthermore, hypothyroidism was more severe in the Toxic-Hypo group than in the Hypo group, with lower nadir levels of both fT3 (1.15 vs. 2.17 pg/mL; p = 0.011) and fT4 (0.378 vs. 0.754 ng/dL; p < 0.001). Consequently, the Toxic-Hypo group required replacement therapy with larger doses of LT4 than the Hypo group (75 vs. 25 μg/day at 6 months after the first ICI administration; p = 0.007). The positive rate for TgAbs was higher in the Toxic-Hypo group (14 of 15 tested patients, 93.3%) than in the Hypo group (4 of 9 tested patients, 44.4%) (p = 0.007).
Prompt LT4 replacement in Toxic-Hypo patients due to a high likelihood of subsequent hypothyroidismMore severe hypothyroidism was observed in the Toxic-Hypo group than in the Hypo group. Therefore, the diagnosis of thyrotoxicosis as a thyroid irAE may predict subsequent hypothyroidism, and initiating appropriate LT4 replacement may prevent symptoms due to a hypothyroid state. The LT4 dose should be promptly adjusted because hypothyroidism often progresses rapidly. We hypothesized that patients who were susceptible to subsequent hypothyroidism could benefit from prompt LT4 replacement, characterized by initiation after thyrotoxicosis improvement and quick dose titration.
In this context, we performed prompt LT4 replacement in 4 patients who met the following criteria: development of thyrotoxicosis within 4 weeks after the first ICI administration and serum fT4 levels >3 ng/dL at thyrotoxicosis onset. We compared these individuals with 10 patients of the cohort study who also met both criteria, designated as the conventional replacement group. The characteristics of all 14 patients are shown in Table 3. The clinical data on thyroid irAEs were very similar between the 4 patients with prompt LT4 replacement and the patients in the conventional replacement group (Table 4). The serum levels of fT3 and fT4 at thyrotoxicosis onset in patients 1–3 were higher than in the conventional replacement group, which resulted in the hospitalization of patients 1 and 3 for supportive care of thyrotoxicosis. Patient 3 presented with a low serum TSH level before the first ICI administration (Table 4); no action was taken, and the TSH level returned to normal by 1 week after the first ICI administration (fT3, 2.34 pg/mL; fT4 0.86 ng/dL; and TSH 0.712 μIU/mL).
Characteristics of patients who received prompt versus conventional LT4 replacement
| Prompt replacement | Conventional replacement | ||||
|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | n = 10 | |
| Age (years) | 80 | 62 | 47 | 63 | 66 (52–75) |
| Gender, n (%) Male/Female | Female | Female | Female | Female | 3 (30.0)/7 (70.0) |
| BMI | 24.5 | 20.9 | 23.4 | 25.4 | 25.6 (21.2–29.3) |
| Malignancy type, n (%) | Gastric cancer | Breast cancer | Breast cancer | Urothelial carcinoma |
NSCLC, 2 (20.0) MM, 4 (40.0) Others, 4 (40.0) |
| Regimen, n (%) | Nivolumab FOLFOX7 |
Pembrolizumab Gemcitabine Carboplatin |
Pembrolizumab Gemcitabine Carboplatin |
Pembrolizumab | Nivolumab, 7 (70.0) Pembrolizumab, 2 (20.0) Atezolizumab, 1 (10.0) |
| ICI administration data | |||||
| Number of administrations | 9 | 9 | 6 | 5 | 7 (3–10) |
| Duration of administration (days) | 168 | 176 | 127 | 168 | 113 (40–175) |
| Other irAEs, n (%) | |||||
| Pituitary irAEs | Not developed | 0 (0.0) | |||
| Insulin-dependent diabetes | Not developed | 0 (0.0) | |||
| Non-endocrine-related irAEs | Not developed | 3 (30.0) | |||
Age, BMI, and ICI administration data in the conventional replacement group are expressed as medians (interquartile range). The conventional replacement group included patients in the Toxic-Hypo group who developed thyrotoxicosis within 4 weeks after the first ICI administration and who had serum fT4 levels >3 ng/dL at thyrotoxicosis onset. NSCLC: Non-small cell lung cancer, MM: Malignant melanoma.
Data on thyroid irAEs in patients who received prompt versus conventional LT4 replacement
| Prompt replacement | Conventional replacement | ||||
|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | n = 10 | |
| Before first ICI administration | |||||
| fT3 (pg/mL) | 2.59 | 2.54 | 3.02 | 2.21 | 2.75 (2.54–3.03) |
| fT4 (ng/dL) | 1.17 | 1.17 | 1.26 | 0.89 | 1.170 (1.012–1.235) |
| TSH (μIU/mL) | 1.798 | 1.200 | 0.060 | 3.239 | 2.820 (1.565–4.425) |
| At onset of thyrotoxicosis | |||||
| Period of onset (days) | 28 | 20 | 22 | 22 | 22 (19–25) |
| fT3 (pg/mL) | 17.51 | >25.00 | 17.41 | 11.95 | 10.81 (7.67–15.79) |
| fT4 (ng/dL) | 7.470 | >8.000 | 6.410 | 5.24 | 4.265 (3.370–5.475) |
| TSH (μIU/mL) | 0.009 | 0.031 | 0.007 | 0.040 | 0.028 (0.020–0.038) |
| Positive TgAb (n) | Positive | Positive | Positive | Positive | 14 (tested in 15) (93.3%) |
| TgAb titer (IU/mL) | 40.0 | 395.0 | 201.0 | 382.0 | 631.0 (391.0–705.0) |
| Positive TPOAb (n) | Positive | Positive | Negative | Positive | 8 (tested in 15) (53.3%) |
| TPOAb titer (IU/mL) | 335.0 | 319.0 | 10.0 | >600.0 | 371.0 (10.2–502.2) |
| At improvement of thyrotoxicosis | |||||
| Period of improvement (days) | 44 | 48 | 43 | 42 | 52 (44–66) |
| fT3 (pg/mL) | 2.33 | 1.94 | 1.71 | 2.35 | 2.05 (0.94–2.91) |
| fT4 (ng/dL) | 1.51 | 1.03 | 0.52 | 0.96 | 0.687 (0.499–1.193) |
| TSH (μIU/mL) | 0.013 | 0.817 | 0.360 | 0.054 | 4.440 (0.030–74.213) |
| At initiation of LT4 replacement | |||||
| Period of initiation (days) | 44 | 48 | 43 | 42 | 70 (67–94) |
| fT3 (pg/mL) | 2.33 | 1.94 | 1.71 | 2.35 | 0.98 (0.26–1.83) |
| fT4 (ng/dL) | 1.51 | 1.03 | 0.52 | 0.96 | 0.349 (0.111–0.542) |
| TSH (μIU/mL) | 0.013 | 0.817 | 0.360 | 0.054 | 97.335 (19.880–179.200) |
| LT4 dose after 6 months (μg/day, μg/kg/day) | 75, 1.36 | 75, 1.76 | 100, 1.85 | 100, 1.64 | 75 (50–100), 1.28 (0.95–1.53) |
Data other than positive TgAb and positive TPOAb in the conventional replacement group are expressed as medians (interquartile range). “Days” and “months” indicate days and months since the first ICI administration, respectively.
We considered it highly likely that the 4 patients in the prompt LT4 replacement group would develop subsequent hypothyroidism because they had severe, rapid-onset thyrotoxicosis and positive TgAbs. In addition, thyroid function tests performed at short intervals revealed very rapid decreases in the serum levels of fT3 and fT4. The diagnosis of thyroid irAEs in these patients was confirmed by TRAb measurements and imaging studies (Fig. 4): in patients 1–3, TRAbs were negative and a hypoechoic pattern was seen on thyroid ultrasonographic images (Fig. 4A, 4C, 4F), while patients 1 and 2 exhibited a reduction in thyroid size after thyrotoxicosis improvement (Fig. 4B, 4D). Even though patient 4 had positive TRAb titers (>40 IU/L), we excluded Graves’ disease based on the lack of thyroid uptake of 99mTcO4 (Fig. 4G) and negative TSAb.

Representative imaging results from patients who received prompt LT4 replacement. (A–F) Thyroid ultrasonography. (G) Thyroid uptake on 99mTcO4 scintigraphy. LT4, levothyroxine
Following this detailed assessment, we initiated LT4 replacement for the 4 patients as soon as we confirmed thyrotoxicosis improvement (Table 4). Longitudinal changes in thyroid function and LT4 dose are shown in Fig. 5. Prompt LT4 replacement kept serum levels of fT3 and fT4 within or near normal limits and prevented elevations of serum TSH levels (Fig. 5A–5C), whereas reduced fT3 and fT4 levels and marked elevations of TSH were often observed in the conventional replacement group (Fig. 5E–5G). During prompt LT4 replacement, we quickly increased the LT4 dosage to 75 or 100 μg/day, which differed from the approach taken in conventional replacement (Fig. 5D, 5H). Compared to the conventional replacement group, the 4 patients with prompt LT4 replacement had higher nadir levels of both fT3 (1.93 vs. 0.92 pg/mL; p = 0.047) and fT4 (0.96 vs. 0.33 ng/dL; p = 0.011), lower peak TSH levels (12.790 vs. 108.950 μIU/mL; p = 0.016), and higher LT4 doses at 8 weeks after the first ICI administration (62.5 vs. 0 μg/day; p = 0.001). We verified that hypothyroidism persisted in the 4 patients who received LT4 replacement as promptly as possible. Near subclinical hypothyroidism (fT4 0.99 ng/dL and TSH 4.362 μIU/mL) at 10 months was observed after the first ICI administration even though patient 1 received 75 μg/day of LT4; thyroid atrophy was seen in patient 2 during hypothyroidism (Fig. 4E); and finally, TSH was elevated to 21.242 μIU/mL with 50 μg/day of LT4 in patient 3, and to 23.782 μIU/mL with 75 μg/day of LT4 in patient 4 (Fig. 5C, 5D).

Clinical courses of patients who received prompt LT4 replacement and comparisons with reference patients who received conventional LT4 replacement. (A–D) Thyroid function test results of patients who received prompt LT4 replacement (A–C) and their LT4 doses (D) (n = 4). (E–H) Thyroid function test results of patients who received conventional LT4 replacement (E–G) and their LT4 doses (H) (n = 10). See Tables 3 and 4 for detailed patient characteristics. (I–L) Comparisons of treatment outcomes between the groups. Nadir indicates the lowest value within 24 weeks after the first ICI administration, and peak indicates the highest value during the same time frame. Statistical analyses were performed using the Mann-Whitney U test.
This retrospective study focused on thyroid function following thyroid irAEs. The Toxic-Hypo group exhibited earlier onset of severe thyrotoxicosis than the Toxic group, and more severe hypothyroidism than the Hypo group. TgAb positivity at thyroid irAE onset was also distinctive of the Toxic-Hypo group. Based on these findings, we could predict which patients who received PD-1 blockade therapy were highly likely to develop hypothyroidism after thyrotoxicosis. We therefore started prompt LT4 replacement in 4 such patients, beginning as soon as thyrotoxicosis improved, and this successfully prevented a severely hypothyroid state.
A number of reports have discussed the epidemiology of thyroid irAEs and their predictive factors, and a few have focused on the clinical course after thyroid irAE onset. Following reports on small numbers of patients [17, 18], we previously confirmed that patients with lung cancer who developed overt thyroid irAEs had a better prognosis than those who did not [1]. So far, the effects of thyroid irAEs on prognosis have been reproducible, except in malignant melanoma [19-22]. The present study sheds light on thyroid function outcomes. Inaba et al. previously demonstrated that patients with persistent thyroid irAEs had higher serum fT4 levels than those in whom these irAEs were temporary [23]. The former group partially corresponds to our Toxic-Hypo and Hypo groups. It is critical to determine whether thyroid irAEs accompany hypothyroidism, because thyrotoxicosis improves without treatment but hypothyroidism often requires persistent LT4 replacement [12].
From a clinical perspective, the administration of ICIs is often suspended within 3 months due to progressive disease. In such cases, thyroid function monitoring is sometimes suspended before the onset of hypothyroidism, which can result in hypothyroidism being overlooked. Here we identified several risk factors for severe hypothyroidism, specifically prior thyrotoxicosis with rapid onset, severe thyrotoxicosis (defined by high serum fT4 levels), and positive TgAb titers. We believe that thyroid function should be monitored in patients with these risk factors even after ICIs are suspended.
These findings provide useful suggestions for the management of thyroid irAEs. Physicians can predict subsequent hypothyroidism in some patients who develop thyrotoxicosis while receiving PD-1 blockade therapy. Our case series involving prompt LT4 replacement suggests a potentially useful management strategy for thyroid irAEs, as summarized in Fig. 6. First, patients with rapid-onset thyrotoxicosis are susceptible to subsequent hypothyroidism. We consider that “rapid onset” can be defined as within 4 weeks after the first ICI administration, because 14 of our 15 patients (93.3%) who developed thyrotoxicosis within 4 weeks developed hypothyroidism (Fig. 3A). Considering that most ICI regimens are administered at intervals of 4-weeks or fewer, it is feasible in clinical settings to define “rapid onset” thyrotoxicosis as that occurring within 4 weeks after initial ICI administration if thyroid function tests are performed at the second ICI administration.
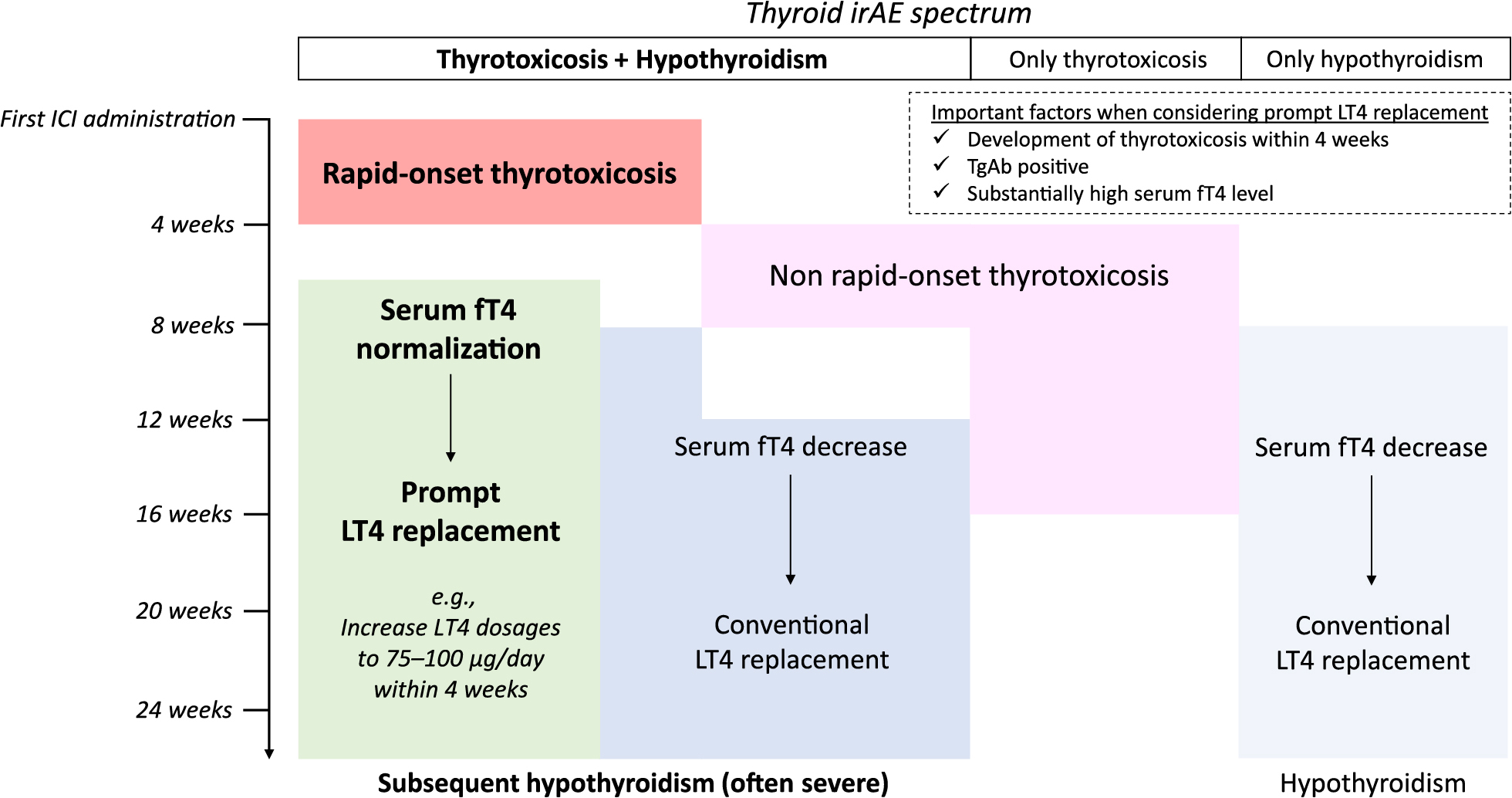
Schematic summary of a potential thyroid irAE management strategy based on the present study
Second, TgAb measurements at the onset of thyrotoxicosis are informative. Previous reports showed that patients with positive TgAb and/or TPOAb titers before ICI therapy were at risk of thyroid irAEs [6, 24, 25]. However, it would be more costly to measure these autoantibodies in all patients than only in those with thyrotoxicosis. Furthermore, even if patients have risk factors for thyroid irAEs, ICIs are used for advanced malignancies as long as informed consent has been obtained. Importantly, hypothyroidism should not be overlooked because hypothyroid patients often exhibit specific symptoms and organ disorders. In terms of chemotherapy, serum creatinine levels are elevated in the hypothyroid state unless the patient receives treatment [26], and this interferes with the completion of full-dose chemotherapy regimens. Predicting subsequent hypothyroidism is likely to contribute to the successful treatment of malignancies.
Third, a high serum fT4 level at thyrotoxicosis onset is another risk factor for subsequent hypothyroidism. Although we observed a correlation between rapid-onset thyrotoxicosis and high serum fT4 level (Fig. 3B), 1 patient in whom thyrotoxicosis occurred rapidly did not develop hypothyroidism (Fig. 3A). Moreover, among patients who developed thyrotoxicosis after 4 weeks, serum fT4 levels in the Toxic group were not very high (Fig. 3A). If we used a serum fT4 cut-off level >3 ng/dL at thyrotoxicosis onset, all such patients in our cohort would be considered to have developed subsequent hypothyroidism.
This study also considered how best to optimize LT4 replacement therapy for Toxic-Hypo patients who were highly likely to develop severe hypothyroidism. Unfortunately, the conventional LT4 replacement strategy used at our hospital kept some patients in the severely hypothyroid state (Fig. 5A–5D). Similar clinical courses have been reported in other cohort studies [2, 17, 27]. Since LT4 has a long half-life of about 7 days, doses are usually adjusted at 4- to 6-week intervals [28]. However, hypothyroidism in thyroid irAEs can drastically progress even within a period of 4 weeks, and may require substantial LT4 doses as seen in Fig. 5. Previous studies demonstrated that ICI-associated hypothyroidism required LT4 replacement dosages of 1.2–1.45 μg/kg/day to maintain the euthyroid state [13, 29, 30].
Based on the above information, we performed prompt LT4 replacement when we could closely monitor thyroid function. LT4 dosages were quickly increased to 75–100 μg/day (1.36–1.85 μg/kg/day) within 4 weeks (Fig. 5D, Table 4), which was earlier than conventional replacement (Fig. 5H, 5L). Furthermore, we initiated LT4 replacement once we confirmed the improvement of thyrotoxicosis, defined as normalization of serum fT4 levels. This strategy was possible based on the prediction of subsequent hypothyroidism as discussed above. Prompt LT4 replacement successfully kept patients in the euthyroid state and prevented severe, prolonged hypothyroidism. It should be noted that an accurate diagnosis of thyroid irAEs is the basis of prompt LT4 replacement. We carefully excluded Graves’ disease based on TRAb titers, thyroid ultrasonography, and thyroid uptake of 99mTcO4 if needed.
There were several limitations to this study. First, several patients were not tested for TgAbs or TPOAbs at thyroid irAE onset. Second, multivariate analyses could not be performed due to an insufficient number of patients. Third, we included only patients who were treated with ICIs as monotherapy. Different clinical courses may be seen in patients who receive combination treatment with chemotherapy [15], CTLA-4 inhibitors [5], and probably tyrosine kinase inhibitors [31-33]. Fourth, unlike a previous report [23], we could not determine the serum fT4 cut-off level using statistics such as the receiver operating characteristic curve due to the insufficient number of patients. Lastly, while we demonstrated the successful management of 4 representative patients, further clinical experience is necessary to establish the indications for prompt LT4 replacement. In addition, careful discussions are necessary regarding the best protocol for prompt LT4 replacement. We quickly increased LT4 dosages to 75–100 μg/day, but lower LT4 dosages might be acceptable. For example, to avoid LT4 replacement for patients who do not develop severe subsequent hypothyroidism, patients can initially receive 50 μg/day of LT4, with the addition of more dosages only when their TSH levels abnormally increase.
In conclusion, this retrospective study focused on thyroid function outcomes related to thyroid irAEs, and clarified that patients with rapid-onset severe thyrotoxicosis and positive TgAbs titers were highly likely to develop subsequent hypothyroidism. Based on this prediction, we performed prompt LT4 replacement as soon as thyrotoxicosis improved, and thereby prevented a severely hypothyroid state.
This work was supported by Japan Society for the Promotion of Science KAKENHI Grant Number 19K23942.
IY received lecture fees from Ono Pharmaceutical Co., Ltd., and Bristol-Myers Squibb. NI received lecture fees from MSD KK, Ono Pharmaceutical Co., Ltd., and Sanofi KK. NI received scholarship grants from Sanofi KK, MSD KK, Ono Pharmaceutical Co., Ltd., and Novartis Pharma.