Abstract
The symptoms of diabetes insipidus may be masked by the concurrence of adrenal insufficiency and emerge after the administration of hydrocortisone, occasionally at high doses. To elucidate the mechanism underlying polyuria induced by the administration of high-dose corticosteroids in the deficiency of arginine vasopressin (AVP), we first examined the secretion of AVP in three patients in whom polyuria was observed only after the administration of high-dose corticosteroids. Next, we examined the effects of dexamethasone or aldosterone on water balance in wild-type and familial neurohypophyseal diabetes insipidus (FNDI) model mice. A hypertonic saline test showed that AVP secretion was partially impaired in all patients. In one patient, there were no apparent changes in AVP secretion before and after the administration of high-dose corticosteroids. In FNDI mice, unlike dexamethasone, the administration of aldosterone increased urine volumes and decreased urine osmolality. Immunohistochemical analyses showed that, after the administration of aldosterone in FNDI mice, aquaporin-2 expression was decreased in the apical membrane and increased in the basolateral membrane in the collecting duct. These changes were not observed in wild-type mice. The present data suggest that treatment with mineralocorticoids induces polyuria by reducing aquaporin-2 expression in the apical membrane of the kidney in partial AVP deficiency.
ARGININE VASOPRESSIN (AVP), an antidiuretic hormone, is secreted from the posterior pituitary and acts on V2 receptors in the kidney to promote reabsorption of free water [1-3]. Diabetes insipidus (DI), characterized by polyuria and polydipsia, is caused by a deficiency in either AVP release (central DI) or renal action (nephrogenic DI) [4]. Occasionally, the symptoms of DI are masked by the concurrence of adrenal insufficiency. This condition is termed masked DI, and polyuria emerges after glucocorticoid replacement. Thus far, the precise mechanisms by which polyuria is induced after the administration of glucocorticoids in patients with masked DI have not been fully clarified. However, previous studies suggested the involvement of both AVP-dependent and AVP-independent mechanisms: glucocorticoid deficiency resulted in increases in the plasma levels of AVP in Sprague–Dawley rats [5], and water permeability of the renal collecting ducts was increased after adrenalectomy compared with a sham operation in Brattleboro rats, which are genetically AVP-deficient [6].
Several case reports described how polyuria was induced after administration of high-dose hydrocortisone, which has both glucocorticoid and mineralocorticoid actions, in patients with partial DI [7-10]. In these studies, the investigators speculated that the patients had masked DI, and that the polyuria was induced by the administration of glucocorticoids. However, the replacement of aldosterone reportedly improved a defect in the urinary diluting ability in adrenalectomized Brattleboro rats [11]. Moreover, it has been shown that the administration of aldosterone increases urine volumes in a rat model of DI [12]. Thus, it is unclear which action, i.e., glucocorticoid or mineralocorticoid action, is involved in the polyuria after administration of a high dose of hydrocortisone in partial DI.
In this study, we first reported on three clinical cases in which polyuria developed during the administration of high-dose corticosteroids. Second, we examined the effects of dexamethasone (a glucocorticoid) or aldosterone (a mineralocorticoid) on water balance in familial neurohypophyseal diabetes insipidus (FNDI) model mice, in which AVP secretion was partially impaired [13].
Materials and Methods
Clinical data collection
All clinical data were collected at the Nagoya University Hospital. Plasma AVP concentrations were measured using a radioimmunoassay (Yamasa Shoyu Corporation, Choshi, Japan). Hypertonic saline infusion tests were performed and assessed as previously described [14]. Plasma levels of adrenocorticotropic hormone (ACTH) and serum levels of cortisol were determined using an electrochemiluminescence immunoassay (Roche Diagnostics, Indianapolis, IN, USA). Plasma renin activities were measured using an enzyme immunoassay (Yamasa Shoyu Corporation) and plasma aldosterone concentrations were measured using a radioimmunoassay (Fujirebio Co., Ltd., Tokyo, Japan).
Animals
The generation of FNDI mice heterozygous for the mutant Avp gene (Cys98stop) has been previously described [13]. Wild-type (WT) mice (C57BL/6J) were purchased from Japan SLC, Inc. (Shizuoka, Japan). In all animal experiments, 2-month-old male FNDI and WT mice were used. Mice were maintained under controlled conditions (23.0 ± 0.5°C; lights were on from 09:00 to 21:00).
Measurement of urine volumes, urine AVP concentrations, urine cyclic adenosine monophosphate concentrations, urine osmolality, and serum potassium levels
WT and FNDI mice were implanted with osmotic minipumps (Alzet model 2002; Muromachi Kikai Co., Tokyo, Japan) containing: 1) dimethyl sulfoxide (DMSO) (WT or FNDI + vehicle group); 2) aldosterone (A9477; Sigma–Aldrich, St. Louis, MO, USA; 0.025 μg/g body weight [BW]/day) in DMSO (WT or FNDI + aldosterone group); or 3) dexamethasone (D2915; Sigma–Aldrich; 0.2 μg/g BW/day) in DMSO (WT or FNDI + dexamethasone group). All groups had free access to water and food. Mice were housed in metabolic cages, and 24-h pooled urine was collected for 5 days. Urine AVP concentrations were measured using an Arg8-Vasopressin ELISA Kit (Enzo Life Sciences, Farmingdale, NY, USA) following the instructions provided by the manufacturer. Cyclic adenosine monophosphate (cAMP) levels in urine were measured using an ELISA kit (Cayman Chemical Company, Ann Arbor, MI, USA) according to the manufacturer’s instructions. Urine osmolality was determined using a cryoscopic method (Oriental Yeast Co., Ltd., Tokyo, Japan). The levels of potassium in the serum were determined using an ion-selective electrode method (Oriental Yeast Co., Ltd.).
Western blotting analysis
Homogenates of the inner medullae separated from the kidneys of mice were prepared. Semiquantitative immunoblotting was carried out to assess the relative expression levels of proteins of interest, as previously described [15]. The blots were probed with the following primary antibodies: rabbit anti–aquaporin-2 (anti-AQP2, SPC-503D; StressMarq Biosciences, Victoria, Canada; 1:1,000) and rabbit anti-glyceraldehyde-3-phosphate dehydrogenase (anti-GAPDH; ab181602; Abcam, Cambridge, UK; 1:10,000). Horse radish peroxidase-conjugated donkey anti-rabbit immunoglobulin G (IgG) (NA934; GE Healthcare, Little Chalfont, UK; Research Resource Identifier: AB_772206) was used as a secondary antibody. Can Get Signal Immunoreaction Enhancer Solution (Toyobo, Osaka, Japan) was used for the dilution of primary and secondary antibodies. ECL Prime Western Blotting Detection Reagent (GE Healthcare, Chicago, IL, USA) was used to detect signals. The intensities of bands in western blots were quantified with ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Immunohistochemistry and immunofluorescence analysis
Kidneys were fixed by perfusion (through the left ventricle) with periodate lysine (0.2 M) and paraformaldehyde (2%) in phosphate-buffered saline. Tissue samples were soaked for several hours in 20% sucrose in phosphate-buffered saline, embedded in Tissue-Tek O.C.T. compound (Sakura Finetechnical, Tokyo, Japan), and stored at –80°C until sectioning. Kidneys were cut into sections (thickness: 10 μm) using a cryostat at –20°C, thaw mounted on Superfrost Plus microscope slides (Matsunami, Tokyo, Japan), and stored at –80°C until immunohistochemical analysis, which was performed as previously described [16]. Briefly, sections were incubated with the primary antibody, anti-AQP2 (ab199975; Abcam; 1:3,000) overnight at 4°C. The sections were rinsed and incubated with secondary antibody, Alexa Fluor 488-conjugated goat anti-mouse IgG (H + L) highly cross-adsorbed (1:1,000; A11029; Invitrogen, San Diego, CA, USA), for 2 h at room temperature. Immunofluorescence images were captured by a laser-scanning confocal microscope (LSM 5 Pascal; Carl Zeiss, Oberkochen, Germany). The distribution of AQP2 was semi-quantitatively analyzed. The fluorescence intensities of AQP2 in the kidneys were quantified with ImageJ software, according to previous studies [17-19]. The AQP2 intensities were measured from the apical to the basolateral membrane. These data were obtained using principal cells constructing tubule segments (n = 9 collecting ducts from three animals per group).
Statistical analysis
The statistical significance of differences between groups was analyzed using an unpaired t-test, one-way analysis of variance (ANOVA), or two-way ANOVA, with repeated measures followed by a Bonferroni test as appropriate. Results are expressed as the mean ± standard error, and p-values <0.05 denoted statistically significant differences.
Study approval
The Ethics Committee of Nagoya University Graduate School of Medicine does not require approval for case reports. Written informed consent for the collection and use of retrospective clinical data was provided by the patients. All animal procedures were approved by the Animal Experimentation Committee of the Nagoya University Graduate School of Medicine and performed in accordance with institutional guidelines for animal care and use.
Results
Case reports
Case 1
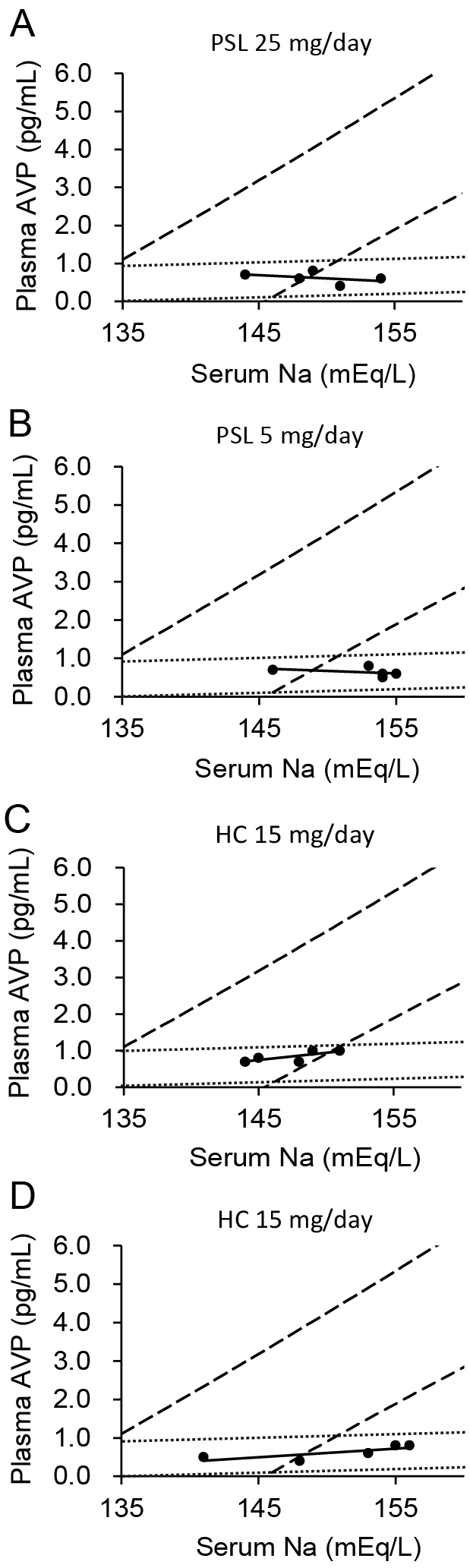
A 75-year-old man diagnosed with IgG4-related autoimmune pancreatitis 4 years earlier was admitted to hospital due to hyponatremia. Blood testing showed elevated IgG4 levels, and magnetic resonance imaging revealed a strongly contrasted enlarged pituitary gland, collectively leading to a diagnosis of IgG4-related hypophysitis. The plasma level of ACTH was 2.8 pg/mL and the serum level of cortisol was 0.5 μg/dL at 2:00 PM. The plasma aldosterone level was 53.0 pg/mL and plasma renin activity was 0.4 ng/mL/h. In addition, the ACTH level showed no response in a corticotropin-releasing hormone stimulation test (data not shown), indicating secondary adrenal insufficiency. Following the initiation of treatment with 15 mg/day hydrocortisone, the patient complained of decreased visual acuity and bitemporal hemianopsia; hence, treatment was switched from 15 mg/day hydrocortisone to 40 mg/day prednisolone, of which glucocorticoid and mineralocorticoid activity were equivalent to 160 and 32 mg hydrocortisone, respectively [20]. Subsequently, the patient complained of thirst, polydipsia, and polyuria. On day 14 of hospitalization (12 days after the initiation of treatment with prednisolone), pituitary gland enlargement was reduced on magnetic resonance imaging and ocular symptoms improved, although the symptom of DI continued (Fig. 1A). A hyperintense signal in the posterior pituitary gland was absent on T1-weighted magnetic resonance imaging (data not shown). On day 23 of hospitalization, when the patient presented with polyuria and was receiving 25 mg/day prednisolone, a hypertonic saline infusion test revealed insufficient vasopressin secretion (Fig. 2A). Based on the diagnosis of central DI, the administration of oral desmopressin (DDAVP) (60 μg/day) was initiated. As the dose of prednisolone was decreased, the symptoms of DI were resolved and DDAVP was discontinued. During follow-up examination 2 years later, when polyuria was not evident, a hypertonic saline infusion test showed persistent insufficient vasopressin secretion (Fig. 2B).
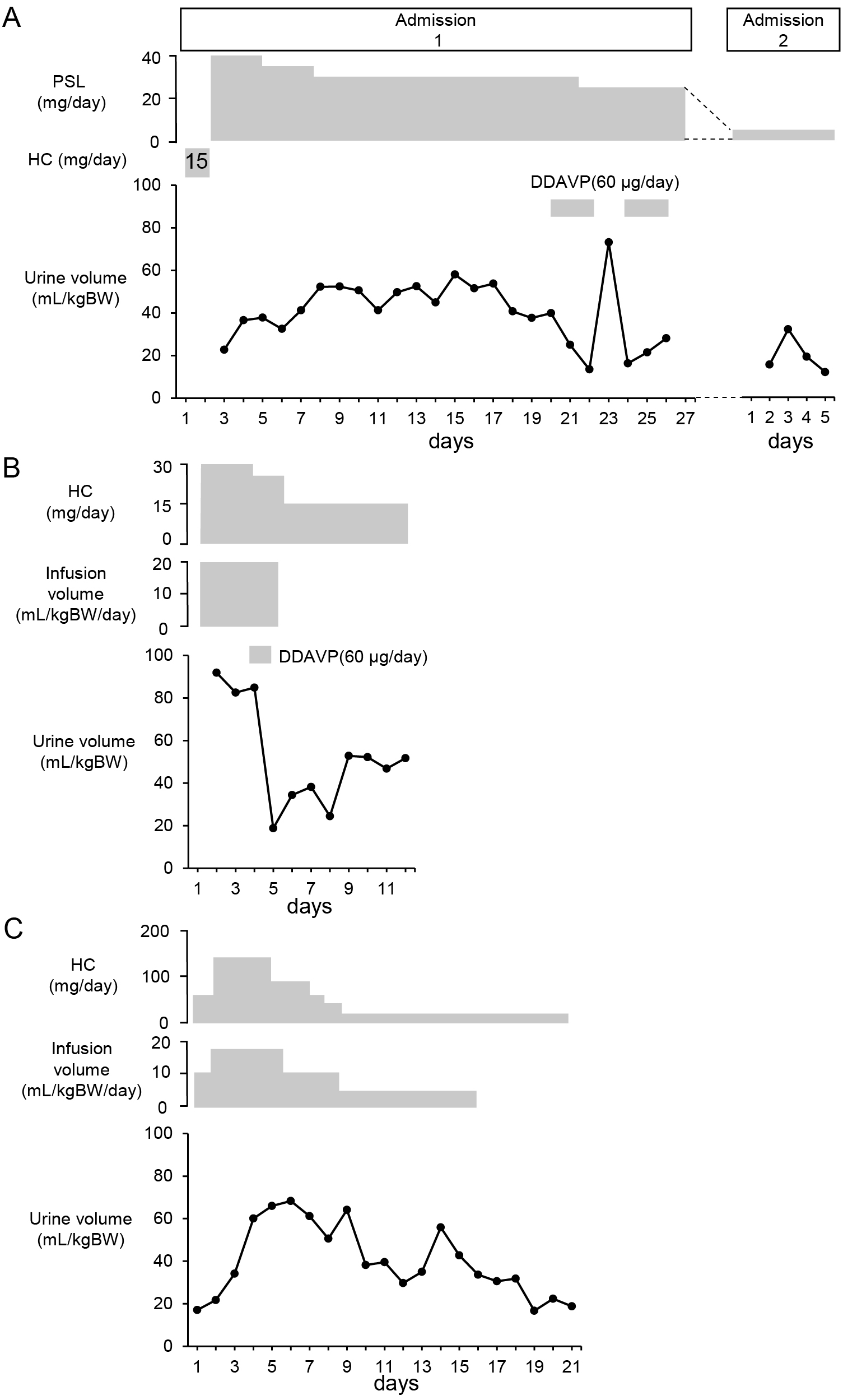
A 53-year-old man visited our hospital with a complaint of fatigue. He had been treated with levothyroxine following the diagnosis of hypothyroidism based on the results of blood tests (thyrotropin: 5.47 μIU/mL; free thyroxine: 0.625 ng/dL, anti-thyroperoxidase antibody: 17.5 IU/mL); nevertheless, his symptoms had not been ameliorated. Additional blood testing revealed that the plasma level of ACTH was 1.8 pg/mL and the serum level of cortisol was 0.1 μg/dL at 11:30 AM, indicating secondary adrenal insufficiency. The plasma aldosterone level was 137.0 pg/mL and plasma renin activity was 4.5 ng/mL/h. Polyuria developed after initiating treatment with 30 mg/day hydrocortisone (Fig. 1B). T1-weighted magnetic resonance imaging demonstrated the loss of a hyperintense signal in the posterior pituitary gland (data not shown). DDAVP was administered on day 4, and urine volumes were decreased on day 5. As the dose of hydrocortisone was tapered, the urine volume decreased and the administration of DDAVP was discontinued. A hypertonic saline infusion test performed during treatment with 15 mg/day hydrocortisone showed insufficient vasopressin secretion (Fig. 2C).
Case 3
A 59-year-old woman who had received chemotherapy, including pembrolizumab (an immune checkpoint inhibitor), for lung adenocarcinoma presented to the emergency department with fever. She was diagnosed with septic shock due to leg cellulitis. Despite the clinical improvement of infection by antibacterial treatment for 10 days, persistent unexplained hypotension was noted. Endocrinological examinations revealed that the plasma level of ACTH was 6.9 pg/mL and the serum level of cortisol at 06:30 AM was 0.3 μg/dL. These levels did not show increases in the corticotropin-releasing hormone stimulation test (data not shown). Thus, she was diagnosed with ACTH deficiency induced by pembrolizumab. Neither the plasma aldosterone level nor plasma renin activity were measured. Treatment with high-dose hydrocortisone was initiated, which led to stabilization of circulation dynamics. During treatment with 50–150 mg/day hydrocortisone, polyuria was observed and fluid infusion volumes were increased (Fig. 1C). A hyperintense signal in the posterior pituitary gland was absent on T1-weighted magnetic resonance imaging (data not shown). However, as the dose of hydrocortisone was tapered, the urine volume was decreased to <40 mL/kgBW/day (Fig. 1C). On day 17 after the initiation of steroid replacement, in the absence of polyuria, a hypertonic saline infusion test was performed, which showed insufficient AVP secretion (Fig. 2D).
Unlike dexamethasone, aldosterone induced polyuria in FNDI mice
There were no significant differences in urine volumes or water intake for 5 days during the administration of vehicle, dexamethasone, and aldosterone in WT mice (Fig. 3A, B, Supplementary Table 1). In FNDI mice, there were no significant differences in urine volumes or water intake between vehicle and dexamethasone groups. However, 2–5 days after the initiation of treatment, these parameters were significantly increased in the aldosterone group compared with the vehicle group (Fig. 3C, D, Supplementary Table 2; p < 0.01).
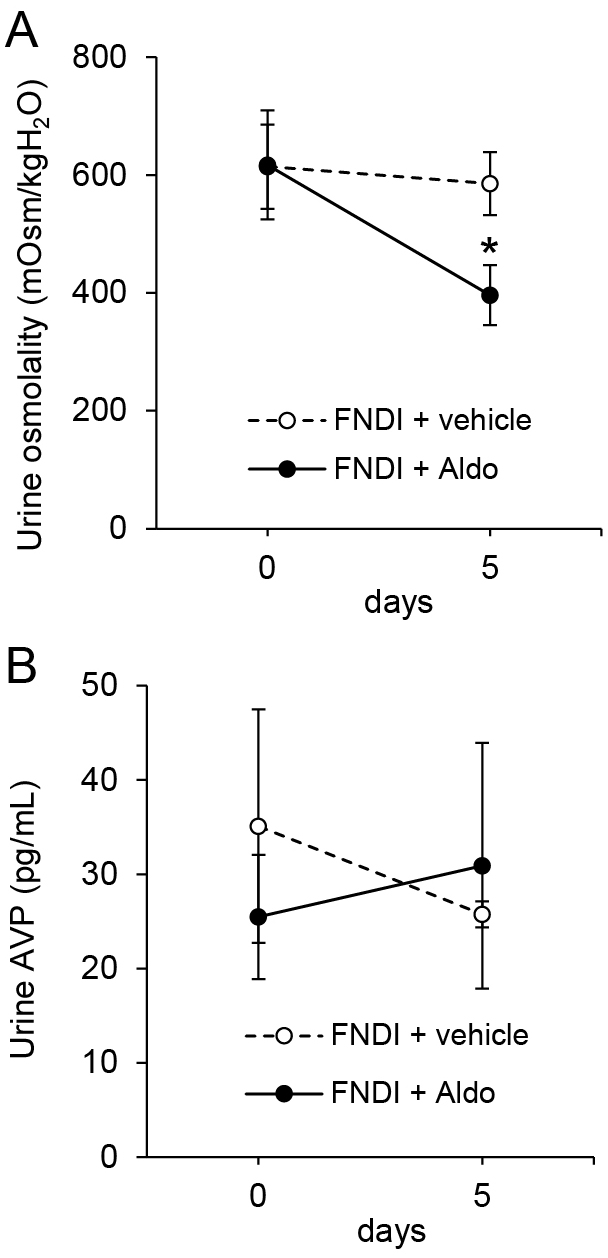
Aldosterone-induced hypotonic polyuria in FNDI mice without a decrease in urine AVP secretion or hypokalemia
In FNDI mice, the urine osmolality was significantly lower in the aldosterone group versus the vehicle group on day 5 after the initiation of treatment (Fig. 4A; p < 0.05). However, there were no significant differences in the concentrations of urine AVP or cAMP between the vehicle and aldosterone groups in WT and FNDI mice (Fig. 4B, Supplementary Fig. 1, Supplementary Tables 1 and 2). Differences were also not observed in daily amounts of urinary AVP or cAMP between days 0 and 5 in FNDI mice (Supplementary Fig. 2). On day 5, the levels of potassium in the serum were not significantly different between the vehicle and aldosterone groups of FNDI mice (vehicle: 6.91 ± 0.21 mEq/L; aldosterone: 6.72 ± 0.42 mEq/L; p = 0.67). These results demonstrated that the increase in urine volumes was not associated with a decrease in AVP secretion or hypokalemia.
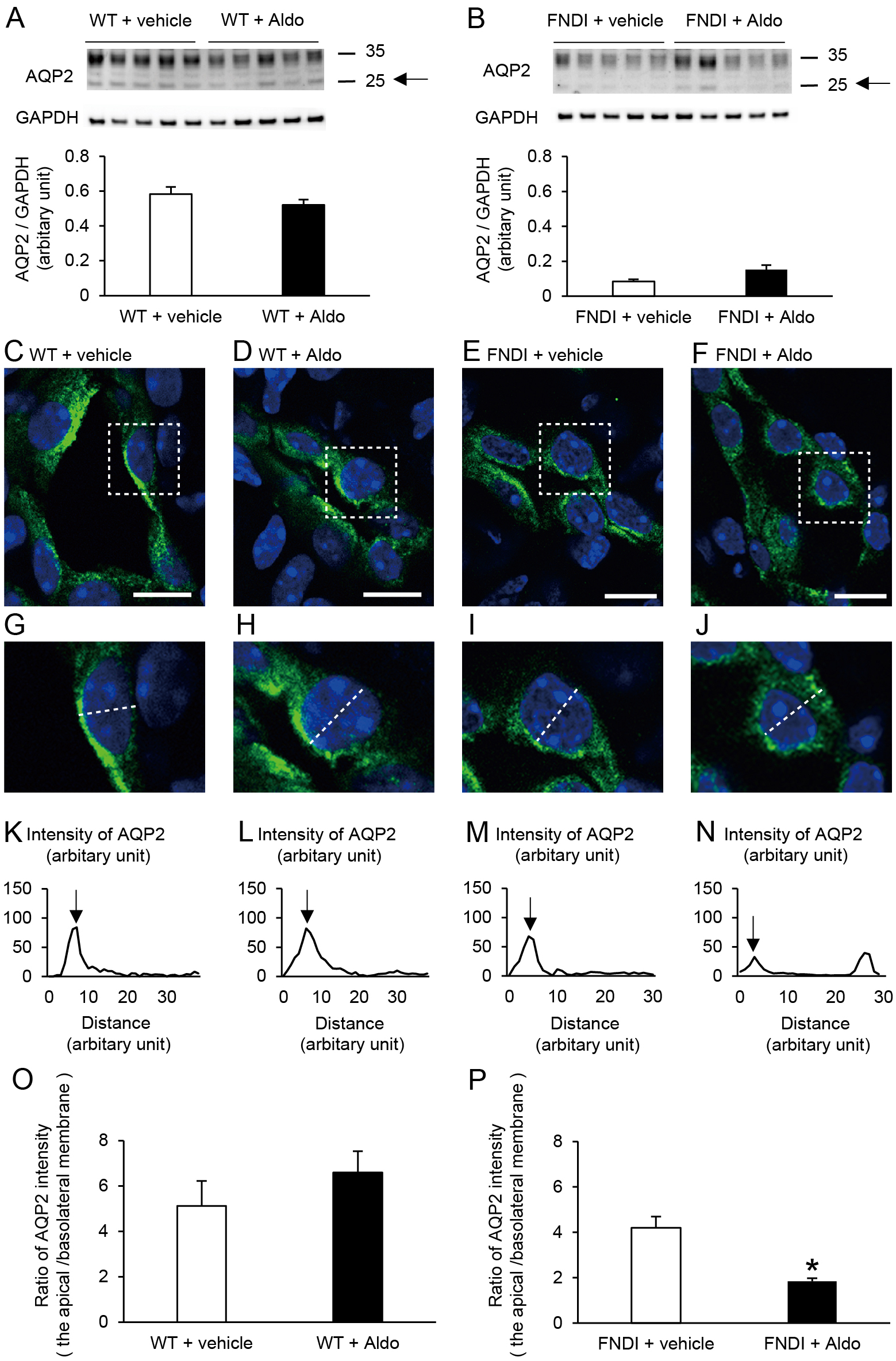
Aldosterone altered the distribution of AQP2 and reduced its expression in the apical membrane in the collecting ducts
The protein abundance of AQP2 in the inner medulla was significantly lower in FNDI mice than in WT mice under basal conditions (Supplementary Fig. 3; p < 0.05). However, there were no significant differences in AQP2 between the vehicle and aldosterone-treated groups in WT (Fig. 5A) or FNDI mice (Fig. 5B). Nevertheless, the fluorescence intensities of AQP2 were decreased in the apical membrane and increased in the basolateral membrane in the aldosterone-treated group compared with the vehicle-treated group in FNDI mice (Fig. 5E, F, I, J, M, N). Semiquantitative analyses revealed that the ratio of AQP2 intensity in the apical membrane to that in the basolateral membrane was significantly decreased in aldosterone-treated FNDI mice versus vehicle-treated FNDI mice (Fig. 5P; p < 0.05). In WT mice, treatment with aldosterone did not affect the distribution of AQP2 (Fig. 5C, D, G, H, K, L, O). All immunohistochemical images and AQP2 intensities used for analyses are shown in Supplementary Figs. 4 and 5.
Discussion
In the three patients of this study, polyuria was only observed following the administration of high-dose corticosteroids. Hypertonic saline testing showed that AVP secretion was impaired even in the absence of polyuria. In FNDI mice, unlike dexamethasone, the administration of aldosterone increased urine volumes and decreased urine osmolality. The immunohistochemical analyses showed that AQP2 expression was decreased in the apical membrane but increased in the basolateral membrane of the collecting duct following the administration of aldosterone in FNDI mice. These changes were not observed in WT mice treated with aldosterone.
When DI is accompanied by either primary or secondary adrenal insufficiency, polyuria is masked, which has been attributed to a deficiency of glucocorticoids but not mineralocorticoids. Indeed, decreased urine volumes in masked DI are usually increased after substitution of physiological dose of glucocorticoids. However, some cases, including the three cases in this study, showed polyuria only after a high dose of corticosteroids. Although the possibility that high-dose glucocorticoids suppressed AVP secretion or inhibited its action cannot be completely excluded in Case 1 of this study, hypertonic saline tests were performed both when polyuria was evident and urine volumes were in the normal range, showing similar results. These findings demonstrated that the administration of high-dose corticosteroids induced polyuria in an AVP-independent manner, at least in Case 1 of this study. On the other hand, while the three patients of this study were diagnosed with DI based on hypertonic saline tests [14], basal plasma AVP levels were detectable in all cases. This may explain why the three patients did not require DDAVP treatment unless high-dose corticosteroids were administered.
In Case 2, the increased level of plasma aldosterone measured suggests that the patient had been dehydrated. In this situation, not only the plasma level of aldosterone but also that of AVP may be increased given that the patient had partial DI. Therefore, the effects of endogenous aldosterone on AQP2 and urine volumes may be less apparent. In Case 3, the patient had been treated with immune checkpoint inhibitors for lung adenocarcinoma. It is, however, unclear whether central DI was related to the treatment, as it is rare that central DI is caused by immune checkpoint inhibitors [21, 22].
As both hydrocortisone and prednisolone have mineralocorticoid as well as glucocorticoid activities [20], it was unclear which activity was responsible for increases in urine volumes in the three patients of this study. A previous study reported that treatment with high-dose aldosterone increased urine volumes in a rat model of DI [12]. However, AVP secretion was not examined, and high-dose mineralocorticoids were administered in the previous study, which could decrease levels of potassium in serum [23]. In this study, we assessed AVP secretion and the level of potassium in serum of FNDI mice treated with a relatively low dose of aldosterone. The results demonstrated that urine volumes increased following the administration of aldosterone without affecting AVP secretion or the level of potassium in serum. In comparison, dexamethasone did not affect urine volumes in FNDI mice in this study. Thus, this suggests that mineralocorticoids, but not glucocorticoids, were responsible for polyuria during treatment with high-dose corticosteroids in partial DI.
Patients with primary aldosteronism are known to have mild hypernatremia, suggesting an adjustment of the osmostat [24]. In Case 1 of this study, such an adjustment of osmostat (i.e., a shift of threshold at which AVP is released) was not observed in the hypertonic saline infusion test during treatment with a high dose of corticosteroids. Neither were significant differences noted in urinary AVP levels in wild-type or FNDI mice treated with aldosterone. Thus, the effects of aldosterone on AVP release in patients or mice in this study were seemingly little, if any.
Previous studies showed that mineralocorticoid receptors are expressed in both principal and intercalated cells in the collecting duct [25, 26]. While the cellular colocalization of vasopressin V2 receptor (V2R) and MR in renal collecting duct cells has not been shown, a previous study showed that aldosterone decreases water reabsorption in isolated rat inner medullary collecting ducts by affecting AQP2 trafficking through a non-genomic mechanism, which was observed in the presence of vasopressin [27]. While it has been shown that AVP increases AQP2 expression in the apical membrane [28-30], previous studies also demonstrated that lithium-induced nephrogenic DI rats treated with aldosterone showed increased urine volumes and decreased apical AQP2 expression [12]. In the present study, while aldosterone treatment significantly increased urine volumes in FNDI mice, urinary cAMP levels were not significantly different between vehicle and aldosterone groups. Taken together, it is suggested that aldosterone may regulate AQP2 localization downstream of cAMP in the AVP–V2R–cAMP pathway.
To clarify the mechanism through which mineralocorticoids induced polyuria in FNDI mice, we examined the expression of AQP2 in the kidney. The present data showed that mineralocorticoids reduced AQP2 expression in the apical membrane in FNDI mice. On the other hand, aldosterone did not affect AQP2 expression or water balance in WT mice in this study. As shown in Supplementary Fig. 3, the AQP2 levels in the inner medulla of the kidney were much higher in WT than in FNDI mice. Under conditions where the AVP–V2R-cAMP pathway, the main regulator of AQP2 levels [30-32], is intact, the effects of aldosterone on AQP2 expression may not manifest. Collectively, it is suggested that aldosterone may interfere with water reabsorption only when AVP release is partially impaired.
In this study, we did not compare the effects of glucocorticoids and aldosterone on urine volumes in patients with DI. Thus, it is unclear whether the findings observed in FNDI mice are applicable to patients. This is the limitation of the present study.
In conclusion, our analyses in patients with DI and animal models suggest that treatment with mineralocorticoids induces polyuria in partial DI in an AVP-independent manner.
Acknowledgments
This work was supported by the Suzuken Memorial Foundation (to HA) and a research grant from Aichi Kidney Foundation (to HT and JK). We thank Michiko Yamada and the staff of the Division of Experimental Animals, Nagoya University Graduate School of Medicine, for their technical support.
Author Contributions
HT and HA designed the project. JK and HT performed the experiments and analyzed the data with technical help and advice from TM, YK, YH, TT, DH, T. Kobayashi, YY, MS, TO, SI, HS, RB, and T. Katsuki. FA and SU supervised the study and helped to prepare the manuscript. JK, HT, and HA wrote the manuscript.
Disclosure
The authors declare no competing interests.
References
- 1 Coleman RA, Wu DC, Liu J, Wade JB (2000) Expression of aquaporins in the renal connecting tubule. Am J Physiol Renal Physiol 279: F874–F883.
- 2 Fushimi K, Uchida S, Hara Y, Hirata Y, Marumo F, et al. (1993) Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature 361: 549–552.
- 3 Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, et al. (1995) Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci U S A 92: 1013–1017.
- 4 Arima H, Azuma Y, Morishita Y, Hagiwara D (2016) Central diabetes insipidus. Nagoya J Med Sci 78: 349–358.
- 5 Ishikawa S, Schrier RW (1982) Effect of arginine vasopressin antagonist on renal water excretion in glucocorticoid and mineralocorticoid deficient rats. Kidney Int 22: 587–593.
- 6 Hirsch DJ, Hirtle RW (1986) Effect of adrenalectomy on distal tubule water permeability of the Brattleboro rat. Can J Physiol Pharmacol 64: 1170–1176.
- 7 Chin HX, Quek TP, Leow MK (2017) Central diabetes insipidus unmasked by corticosteroid therapy for cerebral metastases: beware the case with pituitary involvement and hypopituitarism. J R Coll Physicians Edinb 47: 247–249.
- 8 Yang D, Newman SK, Katz K, Agrawal N (2019) Central diabetes insipidus emerging after steroid replacement in pituitary apoplexy. CMAJ 191: E501–E504.
- 9 Non L, Brito D, Anastasopoulou C (2015) Neurosarcoidosis-associated central diabetes insipidus masked by adrenal insufficiency. BMJ Case Rep 2015: bcr2014206390.
- 10 Yatabe MS, Watanabe K, Hayashi Y, Yatabe J, Morimoto S, et al. (2017) Overlap of post-obstructive diuresis and unmasked diabetes insipidus in a case of IgG4-related retroperitoneal fibrosis and tuberoinfundibular hypophysitis: a case report and review of the literature. Intern Med 56: 47–53.
- 11 Green HH, Harrington AR, Valtin H (1970) On the role of antidiuretic hormone in the inhibition of acute water diuresis in adrenal insufficiency and the effects of gluco- and mineralocorticoids in reversing the inhibition. J Clin Invest 49: 1724–1736.
- 12 Nielsen J, Kwon TH, Praetorius J, Frøkiær J, Knepper MA, et al. (2006) Aldosterone increases urine production and decreases apical AQP2 expression in rats with diabetes insipidus. Am J Physiol Renal Physiol 290: F438–F449.
- 13 Hayashi M, Arima H, Ozaki N, Morishita Y, Hiroi M, et al. (2009) Progressive polyuria without vasopressin neuron loss in a mouse model for familial neurohypophysial diabetes insipidus. Am J Physiol Regul Integr Comp Physiol 296: R1641–R1649.
- 14 Takagi H, Hagiwara D, Handa T, Sugiyama M, Onoue T, et al. (2020) Diagnosis of central diabetes insipidus using a vasopressin radioimmunoassay during hypertonic saline infusion. Endocr J 67: 267–274.
- 15 Ando F, Mori S, Yui N, Morimoto T, Nomura N, et al. (2018) AKAPs-PKA disruptors increase AQP2 activity independently of vasopressin in a model of nephrogenic diabetes insipidus. Nat Commun 9: 1411.
- 16 Hagiwara D, Arima H, Morishita Y, Wenjun L, Azuma Y, et al. (2014) Arginine vasopressin neuronal loss results from autophagy-associated cell death in a mouse model for familial neurohypophysial diabetes insipidus. Cell Death Dis 5: e1148.
- 17 Ando F, Sohara E, Morimoto T, Yui N, Nomura N, et al. (2016) Wnt5a induces renal AQP2 expression by activating calcineurin signalling pathway. Nat Commun 7: 13636.
- 18 Wang A, Hirose T, Ohsaki Y, Takahashi C, Sato E, et al. (2019) Hydrochlorothiazide ameliorates polyuria caused by tolvaptan treatment of polycystic kidney disease in PCK rats. Clin Exp Nephrol 23: 455–464.
- 19 Vukicevic T, Hinze C, Baltzer S, Himmerkus N, Quintanova C, et al. (2019) Fluconazole increases osmotic water transport in renal collecting duct through effects on aquaporin-2 trafficking. J Am Soc Nephrol 30: 795–810.
- 20 Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, et al. (2013) A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol 9: 30.
- 21 Deligiorgi MV, Siasos G, Vergadis C, Trafalis DT (2020) Central diabetes insipidus related to anti-programmed cell-death 1 protein active immunotherapy. Int Immunopharmacol 83: 106427.
- 22 Zhao C, Tella SH, Del Rivero J, Kommalapati A, Ebenuwa I, et al. (2018) Anti-PD-L1 treatment induced central diabetes insipidus. J Clin Endocrinol Metab 103: 365–369.
- 23 de Seigneux S, Nielsen J, Olesen ETB, Dimke H, Kwon TH, et al. (2007) Long-term aldosterone treatment induces decreased apical but increased basolateral expression of AQP2 in CCD of rat kidney. Am J Physiol Renal Physiol 293: F87–F99.
- 24 Gregoire JR (1994) Adjustment of the osmostat in primary aldosteronism. Mayo Clin Proc 69: 1108–1110.
- 25 Ackermann D, Gresko N, Carrel M, Loffing-Cueni D, Habermehl D, et al. (2010) In vivo nuclear translocation of mineralocorticoid and glucocorticoid receptors in rat kidney: differential effect of corticosteroids along the distal tubule. Am J Physiol Renal Physiol 299: F1473–F1485.
- 26 Stokes JB (2000) Physiologic resistance to the action of aldosterone. Kidney Int 57: 1319–1323.
- 27 Wang Y, Ma F, Rodriguez EL, Klein JD, Sands JM (2020) Aldosterone decreases vasopressin-stimulated water reabsorption in rat inner medullary collecting ducts. Cells 9: 967.
- 28 Christensen BM, Zelenina M, Aperia A, Nielsen S (2000) Localization and regulation of PKA-phosphorylated AQP2 in response to V2-receptor agonist/antagonist treatment. Am J Physiol Renal Physiol 278: F29–F42.
- 29 Nishimoto G, Zelenina M, Li D, Yasui M, Aperia A, et al. (1999) Arginine vasopressin stimulates phosphorylation of aquaporin-2 in rat renal tissue. Am J Physiol 276: F254–F259.
- 30 Jung HJ, Kwon TH (2016) Molecular mechanisms regulating aquaporin-2 in kidney collecting duct. Am J Physiol Renal Physiol 311: F1318–F1328.
- 31 Deshpande V, Kao A, Raghuram V, Datta A, Chou CL, et al. (2019) Phosphoproteomic identification of vasopressin V2 receptor-dependent signaling in the renal collecting duct. Am J Physiol Renal Physiol 317: F789–F804.
- 32 Rieg T, Tang T, Murray F, Schroth J, Insel PA, et al. (2010) Adenylate cyclase 6 determines cAMP formation and aquaporin-2 phosphorylation and trafficking in inner medulla. J Am Soc Nephrol 21: 2059–2068.