2023 Volume 70 Issue 3 Pages 259-265
2023 Volume 70 Issue 3 Pages 259-265
Pheochromocytoma is a rare but life-threatening condition due to catecholamine release induced by drug treatments such as β-blockers or glucocorticoids. We present a case of hypertensive crisis due to pheochromocytoma, induced after the initiation of dexamethasone and landiolol during intensive care for severe coronavirus disease 2019 (COVID-19). Based on a detailed medical history review, the patient was previously diagnosed with primary aldosteronism by confirmatory tests, moreover, an abdominal computed tomography scan identified an adrenal tumor 2 years before current admission. We tentatively diagnosed the patient with pheochromocytoma and initiated α-blockers without conducting a catecholamine report, leading to stable hemodynamics. We present a successfully managed case of pheochromocytoma concomitant with COVID-19, which has become a global crisis.
DURING the coronavirus disease 2019 (COVID-19) pandemic, to prevent the risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, medical staff were required to minimize opportunities for patient contact, which may have caused delayed diagnosis and intervention for diseases [1, 2]. The definitive diagnosis of pheochromocytoma/paraganglioma (PPGL), which requires biochemical and radiological evaluations beyond routine practice, was more challenging than usual [3].
PPGL is a rare disease, with an incidence of 0.8–0.95 cases per person-year in the general population [4]. Although PPGL can be life-threatening due to hypertensive crisis in 7–18% of cases [5-7], 10–15% of the patients lack symptoms [8]. Hypertensive crises can be triggered by various factors, including physical manipulation of the tumor, systemic stress, and several medications [6]. Especially, COVID-19 can cause cytokine storms and require intensive care, including artificial ventilation, anesthetics [9], glucocorticoids [10], which are all potential risks for hypertensive crisis in PPGL.
A 71-year-old Japanese man was admitted to our hospital for COVID-19 treatment. Two years before admission, he visited a local hospital due to elevated blood pressure of 150/90 mmHg and was eventually diagnosed with normokalemic primary aldosteronism (PA) by two confirmatory tests (captopril challenge test and furosemide upright test) (Table 1). Since he refused surgical treatment, adrenal venous sampling (AVS) was not performed and treatment with esaxerenone 5 mg and amlodipine 5 mg was initiated 4 months before admission. However, even during medical treatment, the systolic blood pressure would occasionally rise paroxysmally to 200 mmHg with palpitations. Seven days before admission, he developed a fever, cough, and dyspnea associated with a positive real time-polymer chain reaction (PCR) test for COVID-19 at his local hospital. Despite 6 days of treatment with 6 mg dexamethasone tablets, he was transferred to the intensive care unit (ICU) at our hospital as a result of progressing respiratory failure. His past medical history included hypertension and gastroesophageal reflux disease. There was no family history of endocrinological disease or cancer. His usual medications were lansoprazole, amlodipine, and esaxerenone. He never smoked and rarely drank. Physical examination showed that body temperature was 36.0°C, blood pressure (BP) was 117/96 mmHg, pulse rate was 95/min, and respiratory rate was 24/min with a blood oxygen saturation (SpO2) of 93% on a nasal cannula of 3 L/min oxygen, with auscultated bilateral rales; however, no other findings were remarkable. Laboratory findings exhibited leukocytosis, lymphocytopenia, elevated ferritin, and C-reactive protein (CRP), suggesting severe inflammation, as shown in Table 2. Chest radiography showed bilateral ground-glass opacity and a 45% cardio-thoracic ratio. Because of his severe condition, he was admitted to the ICU in our hospital. His clinical course is shown in Fig. 1. Remdesivir 200 mg per day for four days, tocilizumab 560 mg infused once, and 6.6 mg of dexamethasone were intravenously administered for COVID-19 treatment. On the 6th day, continuous intravenous infusion of landiolol was initiated for tachycardia atrial fibrillation. Following that, diaphoresis and constipation developed, and BP fluctuated dramatically; systolic BP ranged from 80–220 mmHg and diastolic BP from 50–160 mmHg. His breathing situation was not improving. Although we first planned imaging studies to identify the cause of tachyarrhythmia and fluctuated BP, imaging studies were minimized as a means of infection control during the COVID-19 pandemic. We therefore obtained a detailed medical record and imaging from his local hospital, which indicated a 30-mm right adrenal tumor with a relatively high unenhanced computed tomography (CT) attenuation value (40 Hounsfield units [HU]) 2 years before admission (Fig. 2a). Although plasma and urinary free metanephrine reports had not yet arrived, a suspected diagnosis of hypertensive crisis due to pheochromocytoma was made, and a shot of phentolamine followed by continuous intravenous infusion was initiated, leading to a stable BP. On the tenth day, because of severe constipation and redeveloped hypertension, metyrosine was added, which resulted in dramatic improvement of those symptoms. Then, intravenous administration of phentolamine was switched to a doxazosin oral tablet on the fourteenth day. He was released from isolation and transferred to the endocrine unit for further examination of pheochromocytoma. On the 14th day, we finally obtained the catecholamine profile; both plasma and urinary free metanephrine and adrenaline levels were remarkably elevated as expected (Table 3). 123I-metaiodobenzylguanidine (MIBG) scintigraphy showed significant uptake in the right adrenal tumor, confirming the diagnosis of pheochromocytoma (Fig. 2b). However, the anesthesiologist decided to postpone adrenalectomy because the patient’s respiratory function had not fully recovered due to severe COVID-19-related lung damage at the time (Fig. 2c). Laparoscopic adrenalectomy was performed 154 days after the initial admission. Pathological findings revealed typical features of pheochromocytoma with well-preserved chromogranin A and succinate dehydrogenase B (SDHB) expression, suggesting that the tumor was not associated with hereditary pheochromocytoma/paraganglioma syndrome (Fig. 3a–d). Pheochromocytoma of the Adrenal Gland Scaled Score was one point and Grading of Adrenal Pheochromocytoma and Paraganglioma was three points, suggesting that the tumor was low to moderate malignancy. Focal CYP11B2 positive cell clusters were found within the associated adrenal cortex, supporting the diagnosis of PA (Fig. 3e). HE staining showed no findings of necrosis or thrombus, but those of slight hemorrhage in the tumor (Fig. 3a) and CD-31 immunostaining showed no ruptured blood vessel (Fig. 3f). We also confirmed the normalized catecholamine profile after adrenalectomy, indicating that pheochromocytoma was cured. Three months after surgery and discontinuation of esaxerenone, plasma aldosterone concentration (PAC) was 122 pg/mL and plasma renin activity (PRA) was 0.5 ng/mL/hr, suggesting residual autonomous aldosterone secretion from the opposite side of the adrenalectomized lesion. He therefore restarted eplerenone; subsequently, his blood pressure was normalized.
| Parameter | Value |
|---|---|
| At baseline | |
| PAC (pg/mL) | 218 |
| PRA (ng/mL/hr) | 0.3 |
| Captopril-challenge test | |
| PAC (60 min after taking captopril) | 172 |
| PRA (60 min after taking captopril) | 0.4 |
| Furosemide upright test | |
| PAC (2 hours after loading) | 476 |
| PRA (2 hours after loading) | 1.1 |
Criteria of each confirmatory test for positive result: Captopril-challenge test was PAC/PRA >200 60 minutes after load, Furosemide upright test was PRA <2.0 ng/mL/hr 2 hour after load.
Abbreviation: PAC, plasma aldosterone concentration; PRA, plasma renin activity
| Parameter | Value | Reference range |
|---|---|---|
| White-cell count (/μL) | 9,600 | 4,500–8,000 |
| Neutrophils (%) | 88.7 | 40–70 |
| Eosinophils (%) | 0.0 | 0.0–7.0 |
| Basophils (%) | 0.1 | 0.0–1.0 |
| Monocytes (%) | 4.8 | 2.0–7.0 |
| Lymphocytes (%) | 6.4 | 27–47 |
| Hemoglobin(g/dL) | 17.1 | 12–16 |
| Platelet count (/μL) | 243,000 | 100,000–330,000 |
| Total protein (g/dL) | 6.7 | 6.3–8.2 |
| Albumin (g/dL) | 3.5 | 3.5–5.0 |
| Urea nitrogen (mg/dL) | 27.5 | 8.0–20.0 |
| Creatinine (mg/dL) | 1.09 | 0.5–1.2 |
| Sodium (mEq/L) | 132 | 135–147 |
| Potassium (mEq/L) | 5.0 | 3.3–4.8 |
| Chloride (mEq/L) | 97 | 98–108 |
| Aspartate aminotransferase (U/L) | 86 | 8–38 |
| Alanine aminotransferase (U/L) | 94 | 4–44 |
| Lactate dehydrogenase (U/L) | 483 | 106–211 |
| Alkaline phosphatase (U/L) | 113 | 104–338 |
| γ-glutamyl transpeptidase (U/L) | 288 | 16–73 |
| Creatine kinase (U/L) | 255 | 25–170 |
| C-reactive protein (mg/dL) | 2.19 | 0.0–0.3 |
| Ferritin (ng/mL) | 1,306 | 13–277 |
| Glucose (mg/dL) | 326 | 73–109 |
| HbA1c (%) | 7.0 | 4.9–6.0 |
| PT-INR | 0.92 | 0.85–1.15 |
| APTT (sec) | 32.4 | 26–38 |
| D-dimer (μg/mL) | 0.5 | <1.0 |
Abbreviation: HbA1c, hemoglobin A1c; PT-INR, prothrombin time/international normalized ratio; APTT, activated partial thromboplastin time

Clinical course during ICU stay
Abbreviation: TCZ, tocilizumab; RDV, remdesivir; DEX, dexamethasone; LAN, landiolol; PHE, phentolamine; MET, metyrosine; DOX, doxazosin
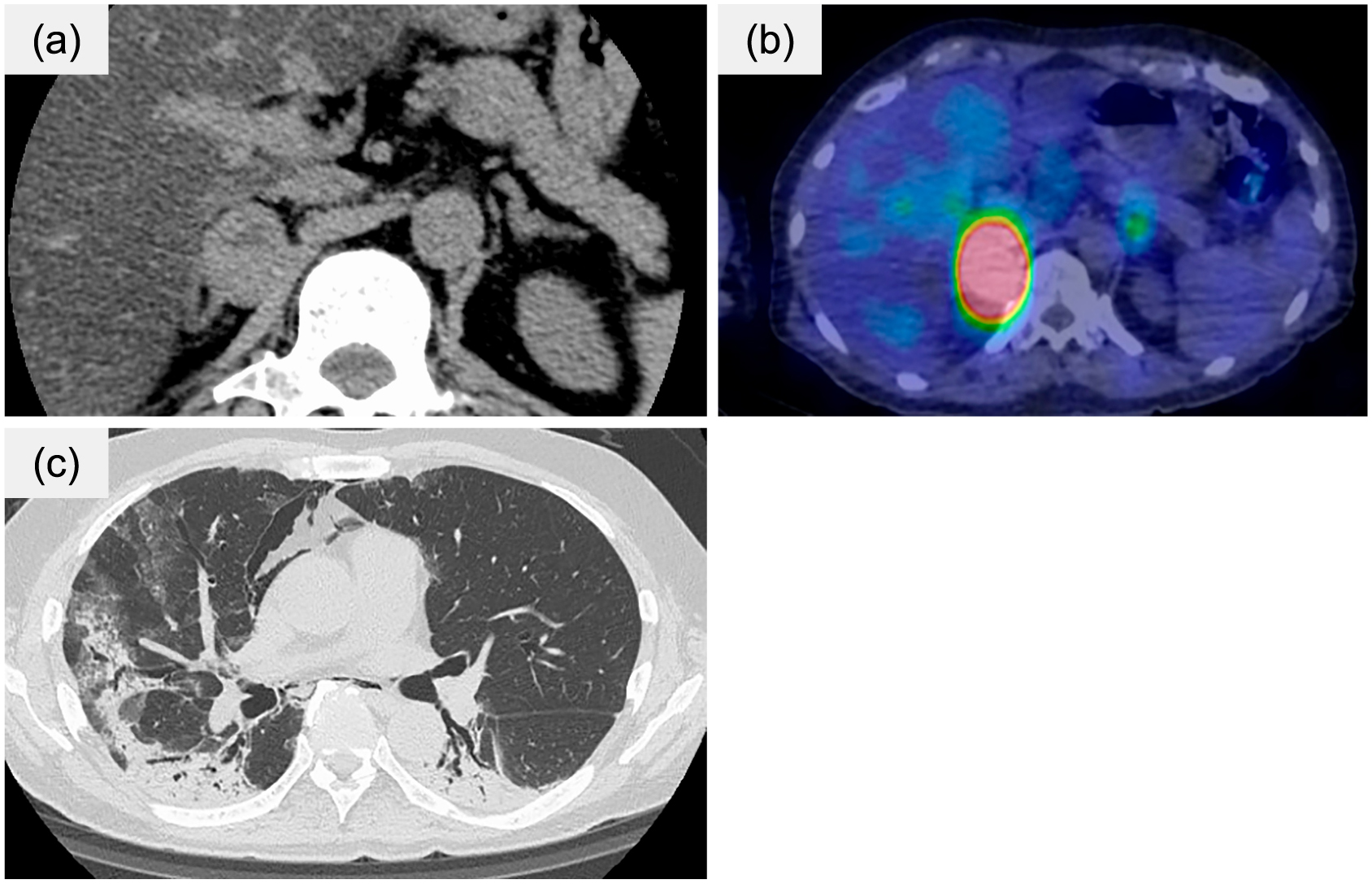
Radiological findings
(a) Abdominal CT findings 2 years before admission at his local hospital, (b) 123I-MIBG scintigraphy 15 days after admission, (c) Chest CT findings 15 days after admission
Abbreviation: CT, computed tomography; 123I-MIBG, 123I-metaiodobenzylguanidine
| Parameter | Value | Reference range |
|---|---|---|
| free metanephrine (pg/mL) | 781 | 0–130 |
| free normetanephrine (pg/mL) | 272 | 0–506 |
| adrenalin (ng/mL) | 4.5 | 0–0.17 |
| noradrenalin (ng/mL) | 1.0 | 0.15–0.57 |
| dopamine (ng/mL) | 0.06 | 0–0.03 |
| PAC (pg/mL) | 61.9 | 3.0–82.1 |
| ARC (pg/mL) | 0.88 | 2.21–39.4 |
| Urine | ||
| free metanephrine (mg/day) | 3.8 | 0.05–0.2 |
| free normetanephrine (mg/day) | 437 | 0.1–0.28 |
| adrenalin (μg/day) | 829 | 1.1–22.5 |
| noradrenalin (μg/day) | 437 | 29.2–118 |
| dopamine (μg/day) | 630 | 100–1,000 |
| VMA (mg/day) | 4.6 | 1.4–4.9 |
Abbreviation: PAC, plasma aldosterone concentration; ARC, active renin concentration; VMA, vanillylmandelic acid

Pathological findings in the right adrenal tumor
(a) Diffuse growth of basophilic polygonal tumor cells observed by hematoxylin and eosin staining, (b) Tumor cells were diffusely positive for chromogranin A, (c) succinate dehydrogenase B expression in tumor cells was retained. (d) Ki-67 proliferation index was less than 1%, and (e) CYP11B2 positive cell clusters of less than 1 mm were found in the adrenal cortex. (f) CD-31 immunostaining showed no ruptured blood vessel.
Although there were several cases of hypertensive crisis due to pheochromocytoma with COVID-19 [11, 12], our case is noteworthy in that the treatment with an α-blocker and metyrosine was initiated before definitive diagnosis of pheochromocytoma, resulting in immediate recovery from hypertensive crisis and stabilized hemodynamics. Several situations, including drug-induced diseases, are known to precipitate pheochromocytoma crisis [6]. In the present case, possible risk factors for hypertensive crisis included critical illness due to severe COVID-19, dexamethasone, β-blocker administration without α-blockers, and refractory hypertension was partially affected by coexisting PA.
In previous cases, an intratumoral hemorrhage in pheochromocytoma or ischemic brain infarction presumably triggered the hypertensive crisis [11, 12]. COVID-19 is known to be associated with coagulopathy [13] and catecholamines cause a greater risk of thrombosis by promoting Th2 cytokine response through α1-adrenergic receptors [14]. Furthermore, excessive catecholamines can induce platelet aggregation and thrombosis mediated through α2-adrenergic receptors [15]. However, our case had no abnormality in the coagulation and fibrinolysis system and pathological findings showed slight intratumoral hemorrhage on HE staining and no collapsed blood vessel in the tumor on CD-31 immunostaining, suggesting that coagulation abnormalities and bleeding due to COVID-19 were not considered to be the primary cause of hypertensive crisis in this case. Glucocorticoid administration can also trigger hypertensive crisis under the coexistence of PPGL via phenylethanolamine N-methyltransferase (PNMT) induction, which synthesizes adrenaline from noradrenaline [16]. High doses of dexamethasone have been shown to pose a relatively higher risk of crisis [17]. In our case, we consider that the administration of 6 mg dexamethasone for eight days contributed to the development of hypertensive crisis. However, dexamethasone was unlikely to be the direct cause of hypertensive crisis because he did not show any signs of hypertensive crisis for 6 days of sole dexamethasone prior to initiation of β-blocker.
The administration of a β-blocker alone in the absence of α-blockers enhances the α-adrenergic receptor signal, which causes hypertensive crisis due to systemic arterial vasoconstriction [18]. Our patient exhibited diaphoresis, constipation, and fluctuated BP after the initiation of landiolol, suggesting that the β-blocker directly triggered the hypertensive crisis.
Concomitant pheochromocytoma and PA were rarely reported although the prevalence of primary hyperaldosteronism in patients with resistant hypertension was high [19]. In a retrospective case series and literature reviews in a tertiary care institution, 87% of cases of concomitant pheochromocytoma and PA showed hypertension or hypokalemia, while symptoms due to catecholamine excess were recorded in only 40% of cases [20]. It is generally known that plasma renin activity (PRA) increases in patients with PPGL, reflecting a decrease in circulating blood volume [21]. However, in a previous report, 13 of 15 patients with PA combined with PPGL exhibited a PRA of less than 1.0 ng/mL/hr. Since PRA was suppressed to less than 1.0 ng/mL/hr at the time of diagnosis of PA in this case as well, PPGL may not have been suspected [20]. In this case, AVS was not performed because the patient had no desire for surgery at the time of PA diagnosis, and he was followed up with mineralocorticoid receptor antagonist. Previously, of the nine patients with concomitant pheochromocytoma and PA who underwent AVS, five (56%) were diagnosed as bilateral PA and four (44%) as unilateral, two of whom were ipsilateral to the pheochromocytoma and two of whom were contralateral to it [20]. AVS should be performed with careful attention to the risk of crisis in patients with coexisting PPGL. Perioperative hypertension and hypertensive crisis have been reported to occur when surgery for PA is performed without confirmation of the presence of a pheochromocytoma [22]. Thus, PPGL should be screened when an adrenal tumor is found with hypertension, even in the absence of symptoms due to catecholamine excess.
In this case, early use of alpha-blocker and metyrosine under COVID-19 infection might have been effective in treating hypertensive crisis caused by pheochromocytoma. COVID-19 can influence a variety of systems such as cardiovascular, respiratory, metabolic, immune, and hematological systems, and they are regulated by catecholamines [23]. Patients with severe COVID-19 develop cytokine storms and it is reportedly associated with severity and mortality [24-26]. Catecholamines affect the hypothalamic–pituitary–adrenal axis and result in influencing glucocorticoid-mediated immune signals [27]. It is generally considered that catecholamines selectively inhibit Th1 cytokine response (interleukin(IL)-1, IL-2, IL-12, tumor necrosis factor-α, etc.) and promote Th2 cytokine response (IL-4, IL-6, IL-10, etc.) [14]. We were unable to measure those cytokine levels due to insufficient residual samples. In our case, CRP which is induced by IL-6 was elevated, therefore, which might have promoted Th2 cytokine response. On the other hand, alpha-blockers and metyrosine, which suppresses catecholamine synthesis by inhibiting tyrosine hydroxylase, have been reported to be able to suppress excessive immune responses [28, 29].
In this case, although hypertensive crisis episodes after the β-blocker administration led us to suspect PPGL, obtaining a catecholamine profile required several days, and further imaging studies were difficult due to severe COVID-19. We carefully reviewed CT images taken two years prior at a local hospital, and the CT value of 40 HU was much higher than in typical adrenocortical adenoma of PA. We gave the tentative diagnosis as pheochromocytoma and immediately started phentolamine and additional metyrosine, which resulted in improved symptoms and stabilized hemodynamics. Since PPGL is an extremely rare disease, it is not commonly suspected as a differential diagnosis. During the COVID-19 pandemic, routine examinations were limited, as in this case, and so a detailed review of the patient’s past medical history and images and careful observation of symptoms and clinical course were very important for diagnosis.
Conflict of interest: The authors declare no conflict of interest.
Ethical approval: Not applicable.
Consent to participate: Not applicable.
Consent for publication: Informed consent for publication of the clinical data was obtained from the patient.
Acknowledgments: We would like to thank Editage (www.editage.com) for English language editing.