2023 Volume 70 Issue 3 Pages 323-332
2023 Volume 70 Issue 3 Pages 323-332
Immune checkpoint inhibitors (ICIs) frequently cause immune-related adverse events (irAEs) that often involve endocrine organs. Pembrolizumab and atezolizumab are currently administered in combination with chemotherapy for several malignancies. Although transient thyrotoxicosis within 6 weeks after the first ICI dose is the typical course of thyroid irAEs with ICI monotherapy, we encountered a unique course of a thyroid irAE in a patient who received combination therapy consisting of pembrolizumab plus pemetrexed and carboplatin. Delayed onset of thyrotoxicosis occurred at 22 weeks after the first dose of pembrolizumab. To understand more about this curious event, we conducted a retrospective cohort study of the following groups: pembrolizumab monotherapy (Pem-mono), pembrolizumab plus chemotherapy (Pem-combi), atezolizumab monotherapy (Atezo-mono), and atezolizumab plus chemotherapy (Atezo-combi). There were no differences in the incidence of overt thyroid irAEs: Pem-mono, 12 of 151 patients (7.9%) versus Pem-combi, 4 of 56 patients (7.1%) (p = 0.85) and Atezo-mono, 5 of 27 patients (18.5%) versus Atezo-combi, 5 of 57 patients (8.8%) (p = 0.20). Through detailed analyses of patients with thyrotoxicosis, we found some patients with delayed-onset thyroid irAE, defined as development at 16 weeks or more after the first ICI dose. Delayed-onset thyroid irAEs were only observed in the combination therapy groups: Pem-combi or Atezo-combi, 3 of 8 patients versus Pem-mono or Atezo-mono, 0 of 10 patients. Our observation that thyroid irAE development can be delayed with ICIs when used in combination with chemotherapy suggests longer monitoring of thyroid function is needed to avoid missing irAEs.
IMMUNE CHECKPOINT INHIBITORS (ICIs) such as antibodies against cytotoxic T-lymphocyte–associated protein 4 (CTLA-4), programmed cell death-1 (PD-1), and programmed cell death ligand-1 (PD-L1), are used to treat various types of malignancies. Treatment with ICIs is often accompanied by immune-related adverse events (irAEs) that can involve several endocrine organs. Common endocrine irAEs include thyroid dysfunction and hypophysitis; insulin-dependent diabetes and primary adrenal insufficiency are rare [1]. PD-1 blockade with anti–PD-1 antibodies or anti–PD-L1 antibodies is frequently associated with irAEs involving the thyroid gland (thyroid irAEs). The incidence of overt thyroid irAEs with anti–PD-1 antibodies has been reported as 11.9–13.5% in clinical settings [2-5]. The typical clinical course of thyroid irAEs during PD-1 blockade is transient thyrotoxicosis within 2 to 6 weeks after the first dose of nivolumab with subsequent hypothyroidism requiring persistent levothyroxine replacement from 12 weeks [2, 6].
ICIs were previously used as monotherapy in second or later line treatment. Some combination ICI therapies have been developed as first-line treatment. For example, pembrolizumab, an anti–PD-1 antibody, and atezolizumab, an anti–PD-L1 antibody, were approved as first-line treatment for lung cancer in combination with chemotherapy [7]. Some studies report a higher frequency and earlier onset of endocrine irAEs during dual ICI therapy with anti–CTLA-4 and anti–PD-1 antibodies compared with PD-1 blockade monotherapy [8-12]. However, we could not find any evidence of differences in the characteristics of endocrine irAEs between ICI monotherapy and ICI combination therapy with cytotoxic agents.
The clinical features of thyroid irAEs by ICIs in combination with chemotherapy should be informative because such regimens are increasingly used as first-line treatment. We observed a unique clinical course distinguished by late onset of a thyroid irAE in a patient who received pembrolizumab plus pemetrexed and carboplatin combination therapy for non-small cell lung cancer. To characterize this event, we conducted a retrospective cohort study comparing pembrolizumab or atezolizumab monotherapy versus combination therapy with cytotoxic agents.
This retrospective cohort study was performed using the medical records of patients who started pembrolizumab or atezolizumab at Kyoto University Hospital between March 1, 2017 and December 31, 2020. Instead of obtaining informed consent, we provided each patient with the opportunity to opt out of the study using our website. We excluded patients who received drugs that could affect thyroid function according to following criteria: prior ICI therapy; subsequent therapy with other ICIs within 6 months; combination therapy with axitinib, a tyrosine kinase inhibitor (TKI); hepatocellular carcinoma that is often treated with a TKI; and ICI use as part of a clinical trial or patient-proposed healthcare services. In general, patients received pembrolizumab 200 mg every 3 weeks, atezolizumab 840 mg every 2 weeks for breast cancer, or atezolizumab 1,200 mg every 3 weeks for other cancers. The institutional review board and ethics committee of the Kyoto University Graduate School of Medicine approved this study (approval number, R1400). We conducted this study in accordance with the principles of the Declaration of Helsinki.
Laboratory dataSerum levels of free T3 (fT3), free T4 (fT4), TSH, and thyroglobulin (Tg) were measured with electrochemiluminescence immunoassays (Elecsys FT3 II kit, Elecsys FT4 kit, Elecsys TSH kit, and Elecsys Tg II kit, respectively; Roche Diagnostics, Mannheim, Germany). The reference ranges were 2.33–4.00 pg/mL, 0.880–1.620 ng/dL, 0.500–5.000 μIU/mL, and <33.7 ng/mL respectively. Anti-thyroid peroxidase antibody (TPOAb), anti-thyroglobulin antibody (TgAb), and anti-TSH receptor antibody (TRAb) were measured using Elecsys kits (Roche Diagnostics). The reference ranges were <16 IU/L, <28 IU/L, and <2.0 IU/L, respectively. Measurement of thyroid stimulating antibody (TSAb) titers was performed using a TSAb enzyme immunoassay kit (Yamasa Corp., Chiba, Japan); the cut-off level was 120%.
Assessments of irAEsAccording to the criteria that we previously reported, thyroid irAE was determined based on serum fT4 and TSH levels within 6 months after the administration of ICIs [2]. In the present study, we exclusively focused on overt thyroid irAEs, namely, situations where neither serum fT4 nor TSH levels were normal. Pituitary irAE was diagnosed by physicians according to clinical symptoms and low levels of ACTH and cortisol, as in our previous study [5]. Insulin-dependent diabetes as an irAE was defined as hyperglycemia with low serum C-peptide levels, exclusion of other causes, and requirement of continuous insulin treatment.
Statistical analysisContinuous variables were expressed as medians (interquartile range). Comparisons of parameters between two groups were performed with the Mann-Whitney U test or Pearson’s chi-square test, as appropriate. A p value of <0.05 was considered statistically significant. JMP Pro®, version 16.1.0 (SAS Institute Inc., Cary, NC, USA) was used to perform the statistical analyses.
A 72-year-old male was referred to our hospital for evaluation of possible lung cancer. He had smoked 50 cigarettes per day for 50 years. Chest computed tomography detected a 21 mm nodule in the left lower lobe with pleural infiltration and enlarged mediastinal lymph nodes. Biopsy of a mediastinal lymph node revealed adenocarcinoma. In addition, metastases to the left occipital lobe of the brain, right adrenal gland, and pubic bone were identified with 18F-fluorodeoxyglucose-positron emission tomography (18F-FDG-PET). Thus, the clinical stage was T3N3M1c (stage IVB). Driver mutations were not found in EGFR or BRAF. There were no ALK or ROS1 rearrangements. The PD-L1 tumor proportion score was 75%.
Combination therapy consisting of pembrolizumab, pemetrexed, and carboplatin every 3 weeks was initiated. His clinical course is shown in Fig. 1. Table 1 provides detailed data of the entire clinical course. After 22 weeks, which was 5 weeks after the sixth cycle, his periodic blood tests revealed thyrotoxicosis. Serum levels of fT3, fT4, and TSH were 8.54 pg/mL, 3.150 ng/dL, and <0.005 μIU/mL, respectively (Table 1). Although TRAb and TSAb were positive at 5.0 IU/L and 827%, respectively, thyroid ultrasonography did not show enhancement of blood flow (Fig. 2A). Thyroid scintigraphy with 99mTc-pertechnetate revealed low thyroid uptake (0.21%). Taken together, we diagnosed thyrotoxicosis as a thyroid irAE instead of thyrotoxicosis due to Graves’ disease. Combination therapy was discontinued. We administered a beta- blocker for his slight palpitations and observed the patient carefully. At the same time as the development of thyrotoxicosis, the size of the primary lesion and metastatic lesions decreased. Serum carcinoma embryonic antigen levels sharply decreased from 47.0 ng/mL to 5.1 ng/mL. Considering that he developed pneumonitis and then systemic erythema during the same period, anti-cancer therapy was not resumed.
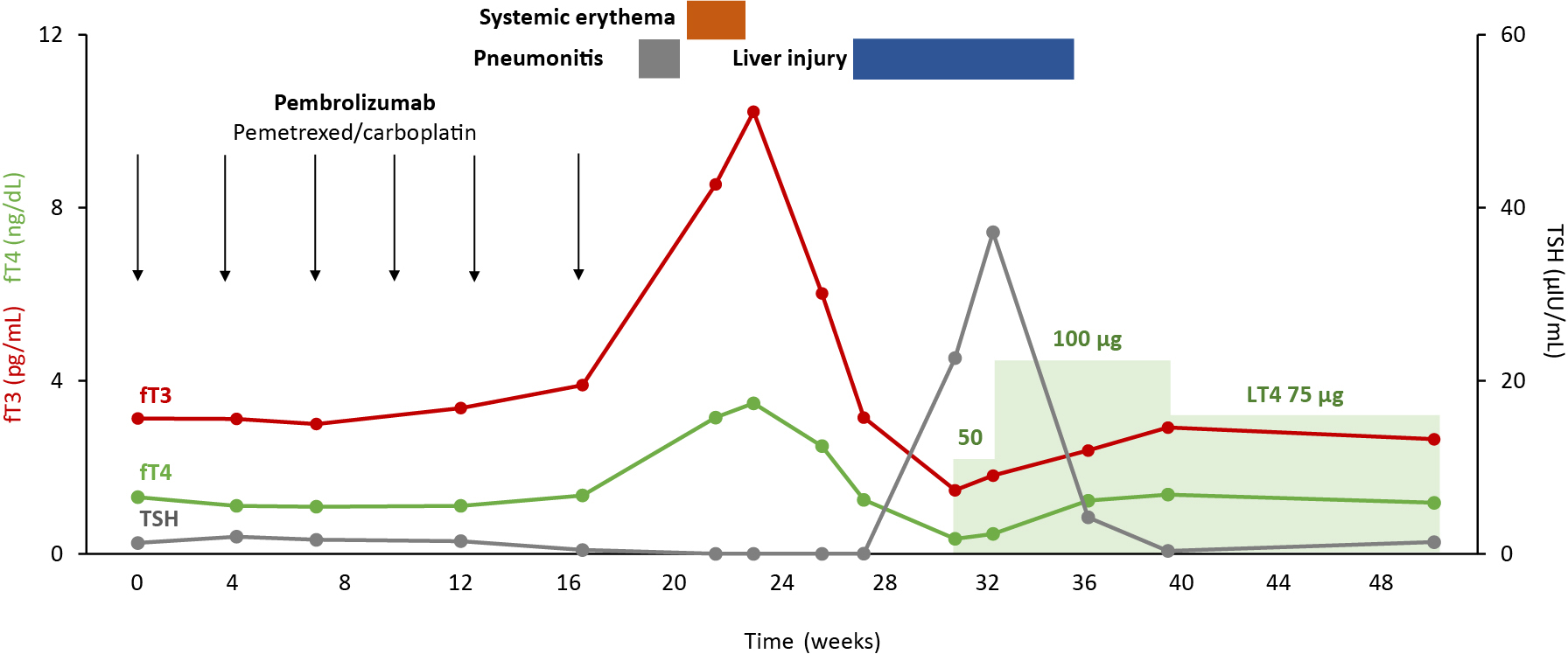
Clinical course of a patient who developed a delayed onset immune-related adverse event involving the thyroid gland (thyroid irAE). Week 0 was defined at the date when the patient received the first dose of pembrolizumab. fT3, free T3; fT4, free T4; TSH, thyroid stimulating hormone; LT4, levothyroxine.
| Week | 0 | 4 | 7 | 12 | 17 | 22 | 23 | 26 | 27 | 31 | 32 | 36 | 39 | 49 | 57 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| fT3 (pg/mL) | 3.13 | 3.12 | 3.00 | 3.37 | 3.90 | 8.54 | 10.22 | 6.02 | 3.15 | 1.47 | 1.81 | 2.39 | 2.92 | 2.65 | 3.02 |
| fT4 (ng/dL) | 1.310 | 1.110 | 1.090 | 1.110 | 1.350 | 3.150 | 3.480 | 2.49 | 1.250 | 0.348 | 0.461 | 1.23 | 1.370 | 1.180 | 1.020 |
| TSH (μIU/mL) | 1.260 | 1.980 | 1.630 | 1.470 | 0.448 | <0.005 | <0.005 | <0.005 | 0.009 | 22.640 | 37.170 | 4.220 | 0.333 | 1.350 | 0.484 |
| LT4 (μg/day) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 50 | 100 | 100 | 75 | 75 |
| Tg (ng/mL) | — | — | — | — | — | 411.8 | — | — | — | 10.3 | — | — | — | — | — |
| TPOAb (IU/mL) | — | — | — | — | — | <9 | — | — | — | <9 | — | — | — | — | — |
| TgAb (IU/mL) | — | — | — | — | — | 322 | — | — | — | 276 | — | — | — | — | — |
| TRAb (IU/L) | — | — | — | — | — | 5.0 | — | — | — | 4.4 | — | — | — | 1.4 | — |
| TSAb (%) | — | — | — | — | — | — | 827 | — | — | 613 | — | — | — | — | 119 |
| CEA (ng/mL) | 47.0 | 10.5 | 6.0 | 4.0 | 5.1 | 6.0 | — | 4.8 | — | 10.0 | — | — | 10.0 | 9.7 | 7.1 |
| AST (U/L) | 24 | 31 | 50 | 60 | 44 | 34 | 34 | 65 | 136 | 144 | 73 | 39 | 32 | 32 | 35 |
| ALT (U/L) | 23 | 31 | 73 | 89 | 59 | 40 | 34 | 108 | 192 | 283 | 131 | 51 | 36 | 37 | 42 |
Week was defined based on the date when the patient received the first dose of pembrolizumab. “—” indicates no measurement.
irAE, immune-related adverse event; fT3, free T3 (reference range: 2.33–4.00 pg/mL); fT4, free T4 (0.880–1.620 ng/dL); TSH, thyroid stimulating hormone (0.500–5.000 μIU/mL); LT4, levothyroxine; Tg, thyroglobulin (<33.7 ng/mL); TPOAb, anti-thyroperoxidase antibody (<16 IU/L); TgAb, anti-thyroglobulin antibody (<28 IU/L); TRAb, anti-TSH receptor antibody (<2.0 IU/L); TSAb, thyroid stimulating antibody (<120%); CEA, carcinoembryonic antigen (<5.0 ng/mL); AST, aspartate aminotransferase (13–30 IU/L); ALT, alanine aminotransferase (10–42 IU/L).

Thyroid ultrasonography and 18F-fluorodeoxyglucose-positron emission tomography (18F-FDG-PET) of the case patient. (A, B) Ultrasonographic images of the thyroid gland at the onset of thyrotoxicosis and at the onset of hypothyroidism. (C, D) 18F-FDG-PET images focused on the thyroid gland before and after treatment with pembrolizumab in combination with chemotherapy. (E) 18F-FDG-PET revealed primary lung cancer in the left lower lobe and metastases in the mediastinal lymph nodes, right adrenal gland, and pubic bone. (F) 18F-FDG-PET after discontinuation of treatment revealed complete remission.
During follow-up, thyrotoxicosis decreased but hypothyroidism developed (Fig. 1). Simultaneously, his erythema improved and liver injury occurred. To treat hypothyroidism, levothyroxine therapy was started at 31 weeks after the initiation of anti-cancer therapy when fT3, fT4, and TSH levels were 1.47 pg/mL, 0.348 ng/dL, and 22.640 μIU/mL, respectively (Table 1). In accordance with the changes in thyroid function, his thyroid gland became atrophic and had a severely hypoechoic pattern (Fig. 2B). 18F-FDG-PET showed mild uptake of 18F-FDG in the thyroid gland after the onset of thyrotoxicosis (Fig. 2C, 2D). His thyroid function had remained stable with 75 μg of levothyroxine since week 39. He achieved complete remission and did not require additional therapy at 20 months, which was verified with 18F-FDG-PET (Fig. 2E, 2F).
Patient characteristics and the incidence of thyroid irAEs in the cohort studyWe performed a retrospective cohort study to learn more about the curious thyroid irAE seen in this patient, which was characterized by delayed onset at 22 weeks after the first dose of pembrolizumab. According to the criteria described in the Materials and Methods section, we included 207 patients who received pembrolizumab and 84 patients who received atezolizumab (Fig. 3). The baseline characteristics are presented in Table 2. Pembrolizumab was used as monotherapy in 151 patients (72.9%) and in combination with chemotherapy in 56 patients (27.1%). Atezolizumab was used as monotherapy in 27 patients (32.1%) and in combination with chemotherapy in 57 patients (67.9%).
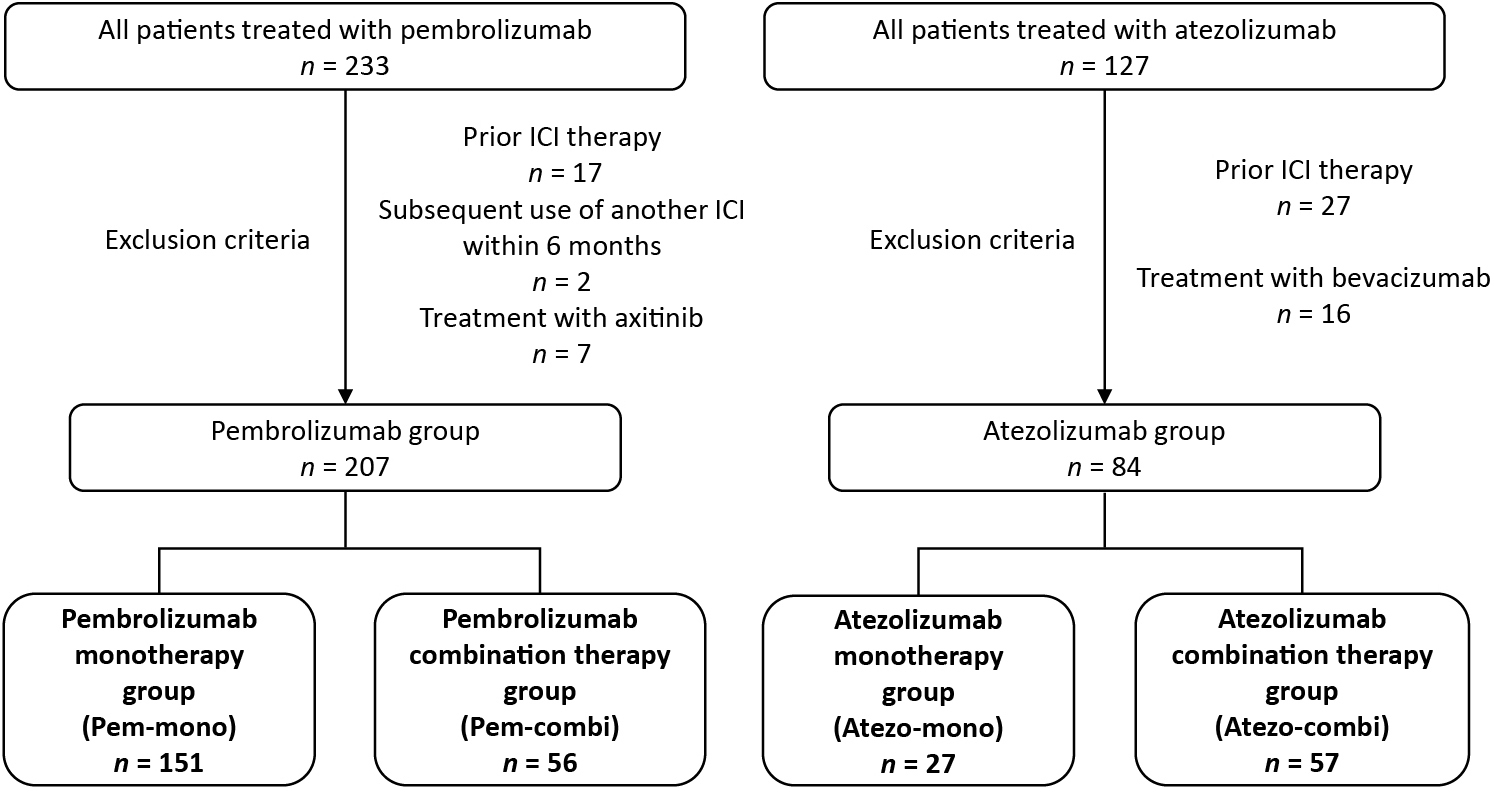
Patient flow diagram for the retrospective cohort study. ICI, immune checkpoint inhibitor.
| All patients (n = 291) | Pembrolizumab (n = 207) | Atezolizumab (n = 84) | |
|---|---|---|---|
| Median age (range), (years) | 70 (63–75) | 71 (64–77) | 67 (59–73) |
| Gender, n (%) | |||
| Male | 197 (67.7) | 149 (72.0) | 48 (57.1) |
| Female | 94 (32.3) | 58 (28.0) | 36 (42.9) |
| Primary site, n (%) | |||
| Non-small cell lung cancer | 169 (58.1) | 115 (55.6) | 54 (64.3) |
| Urothelial carcinoma | 49 (16.8) | 49 (23.7) | |
| MSI-high cancer | 23 (7.9) | 23 (11.1) | |
| Malignant melanoma | 13 (4.5) | 13 (6.3) | |
| Head and neck cancer | 7 (2.4) | 7 (3.4) | |
| Small cell lung cancer | 21 (7.2) | 21 (25.0) | |
| Breast cancer | 9 (3.1) | 9 (10.7) | |
| Endocrine-related irAE, n (%) | |||
| Overt thyroid irAE | 26 (8.9) | 16 (7.7) | 10 (11.9) |
| Pituitary irAE | 4 (1.4) | 3 (1.4) | 1 (1.2) |
| Insulin-dependent diabetes | 3 (1.0) | 3 (1.4) | 0 (0.0) |
| Treatment, n (%) | |||
| ICI monotherapy | 178 (61.2) | 151 (72.9) | 27 (32.1) |
| ICI combination therapy | 113 (38.8) | 56 (27.1) | 57 (67.9) |
| Agents in combination | CBDCA + PEM 40 (19.3) CBDCA + ABR 15 (7.2) CDDP + 5-FU 1 (0.5) |
CBDCA + ETP 21 (25.0) CBDCA + PTX + Bev 20 (23.8) ABR 9 (10.7) CBDCA + PEM 5 (6.0) CBDCA + ABR 2 (2.4) |
MSI, microsatellite instability; ICI, immune checkpoint inhibitor; CBDCA, carboplatin; PEM, pemetrexed; ABR, nanoparticle formulation of paclitaxel; CDDP, cisplatin; 5-FU, 5-fluorouracil; ETP, etoposide; PTX, paclitaxel; Bev, bevacizumab.
We divided patients into groups receiving monotherapy (Pem-mono and Atezo-mono) or combination therapy (Pem-combi and Atezo-combi) by ICI (Fig. 3). The incidence of overt thyroid irAEs was 7.9% (12 of 151 patients) in the Pem-mono group and 7.1% (4 of 56 patients) in the Pem-combi group (p = 0.85) (Table 3). The incidence of thyrotoxicosis (4.0% in the Pem-mono group vs. 7.1% in the Pem-combi group) and hypothyroidism (6.6% vs. 5.4%) was comparable across groups. In the Pem-mono group, a pituitary irAE was observed in 1 patient (0.7%) and insulin-dependent diabetes was observed in 2 patients (1.3%) There were 2 patients (3.6%) with pituitary irAEs and 1 patient (1.8%) with insulin-dependent diabetes in the Pem-combi group.
| Pembrolizumab (n = 207) | Atezolizumab (n = 84) | |||||
|---|---|---|---|---|---|---|
| Pem-mono (n = 151) | Pem-combi (n = 56) | p | Atezo-mono (n = 27) | Atezo-combi (n = 57) | p | |
| Median age (range), (years) | 72 (65–77) | 70 (59–73) | 0.01 | 70 (64–74) | 67 (56–73) | 0.04 |
| Gender, n (%) | ||||||
| Male | 105 (69.5) | 44 (78.6) | 0.20 | 16 (59.3) | 32 (56.1) | 0.79 |
| Female | 46 (30.5) | 12 (21.4) | 11 (40.7) | 25 (43.9) | ||
| Primary site, n (%) | ||||||
| Non-small cell lung cancer | 60 (39.7) | 55 (98.2) | <0.01 | 0 (0.0) | 27 (47.4) | <0.01 |
| Urothelial carcinoma | 49 (32.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| MSI-high cancer | 23 (15.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Malignant melanoma | 13 (8.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Head and neck cancer | 6 (4.0) | 1 (1.8) | 0 (0.0) | 0 (0.0) | ||
| Small cell lung cancer | 0 (0.0) | 0 (0.0) | 27 (100.0) | 21 (36.8) | ||
| Breast cancer | 0 (0.0) | 0 (0.0) | 0 (0.0) | 9 (15.8) | ||
| Endocrine-related irAE, n (%) | ||||||
| Overt thyroid irAE | 12 (7.9) | 4 (7.1) | 0.85 | 5 (18.5) | 5 (8.8) | 0.20 |
| Thyrotoxicosis, n (%) | 6 (4.0) | 4 (7.1) | 4 (14.8) | 4 (7.0) | ||
| Hypothyroidism, n (%) | 10 (6.6) | 3 (5.4) | 2 (7.4) | 4 (7.0) | ||
| Pituitary irAE | 1 (0.7) | 2 (3.6) | — | 0 (0.0) | 1 (1.8) | — |
| Insulin-dependent diabetes | 2 (1.3) | 1 (1.8) | — | 0 (0.0) | 0 (0.0) | — |
Statistical analyses for pituitary irAE and insulin-dependent diabetes were not performed due to the small number of patients.
Pem-mono, Pembrolizumab monotherapy; Pem-combi, Pembrolizumab combination therapy; Atezo-mono, Atezolizumab monotherapy; Atezo-combi, Atezolizumab combination therapy. Bold type represents statistical significance.
In the atezolizumab groups, the incidence of overt thyroid irAEs was 18.5% (5 of 27 patients) in the Atezo-mono group and 8.8% (5 of 57 patients) in the Atezo-combi group (p = 0.20) (Table 3). The incidence of thyrotoxicosis (14.8% in the Atezo-mono group vs. 7.0% in the Atezo-combi group) and hypothyroidism (7.4% vs. 7.0%) was similar across groups. A pituitary irAE was observed in 1 patient (1.8%) in the Atezo-combi group and in 0 patients in the Atezo-mono group. Insulin-dependent diabetes was not observed in either atezolizumab group.
Clinical course of thyroid irAEs in the cohort studySince the incidence of thyroid irAEs was similar among patients receiving monotherapy versus combination therapy, we subsequently analyzed the period of onset of thyroid irAEs with reference to the case presented. We examined thyroid function test results of the 26 patients who developed overt thyroid irAEs. Thyrotoxicosis was generally observed within 9 weeks after the first ICI dose in the monotherapy groups: Pem-mono group, n = 12; and Atezo-mono group, n = 5 (Fig. 4A–4C). Meanwhile, there was no consistent pattern in the period of thyrotoxicosis onset in the combination therapy groups: Pem-combi group, n = 4; and Atezo-combi group, n = 5 (Fig. 4D–4F).

Clinical courses of patients who developed overt thyroid irAEs. (A–C) Thyroid function of 17 patients who received pembrolizumab monotherapy (n = 12) or atezolizumab monotherapy (n = 5). (D–F) Thyroid function of 9 patients who received combination therapy that included pembrolizumab (n = 4) or atezolizumab (n = 5).
fT3, free T3; fT4, free T4; irAE, immune-related adverse event; TSH, thyroid stimulating hormone.
We focused on 18 patients who developed thyrotoxicosis. A histogram of thyrotoxicosis onset revealed that early onset within 9 weeks after the first dose was observed with both monotherapy (7 of 178 patients, 3.9%) and combination therapy (4 of 113 patients, 3.5%) (Fig. 5A). Later onset (week 10 or later) was often seen with combination therapy (4 of 113 patients, 3.5%) but relatively rare with monotherapy (3 of 178 patients, 1.7%) (Fig. 5A). The severity of thyrotoxicosis within 9 weeks of the two groups was similar; 2 patients who received combination therapy exclusively presented with high peak serum fT4 levels (>2 ng/mL) during thyrotoxicosis after week 10 (Fig. 5B, 5C). Furthermore, of the 7 patients who had thyrotoxicosis of later onset, all 4 patients who received combination therapy developed subsequent hypothyroidism but all 3 patients in the monotherapy group did not (Fig. 5C).
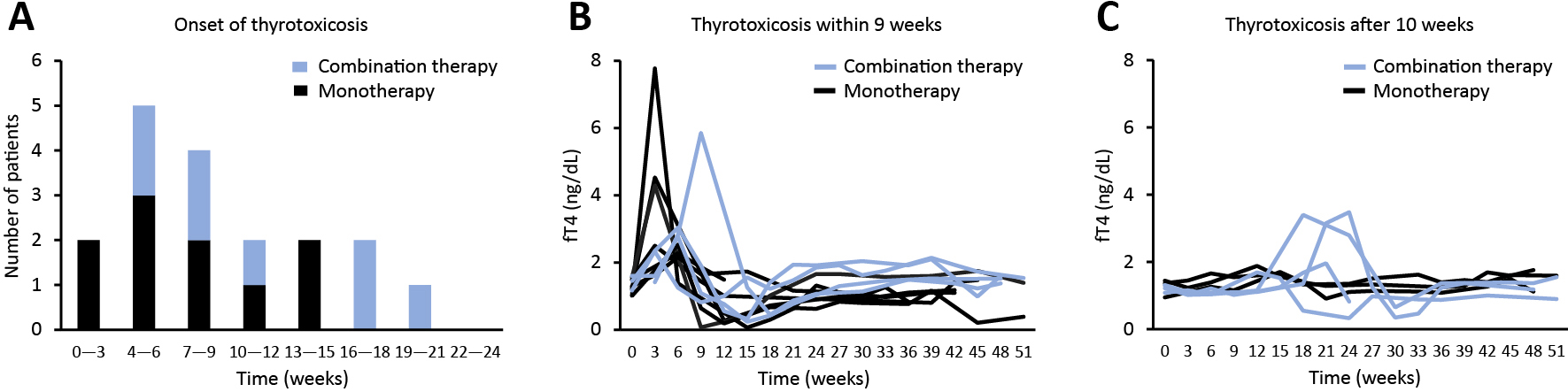
Clinical features of patients who developed thyrotoxicosis. (A) Histogram showing the number of patients by thyrotoxicosis onset. Blue bars represent combination therapy and black bars represent monotherapy. (B) Line graphs of thyroid function in 10 patients with thyrotoxicosis that developed within 9 weeks of the first ICI dose. (C) Line graphs of thyroid function in 8 patients with thyrotoxicosis that developed at 10 weeks or later.
fT4, free T4.
In the 18 patients who developed thyrotoxicosis, age, gender, ICI use, and thyrotoxicosis severity were similar between the monotherapy and combination therapy groups (Table 4). In the combination therapy groups, prophylactic glucocorticoids were used in all 8 patients, while grade 2 or higher decreases in neutrophil or lymphocyte count according to common terminology criteria for adverse events version 5.0 were not seen in any patients. Subsequent hypothyroidism seemed to be more frequent in the combination therapy groups (8 of 8 patients, 100%) than in the monotherapy groups (5 of 10 patients, 50.0%) (Table 4). We defined delayed-onset thyroid irAE as thyrotoxicosis that develops after 16 weeks based on the histogram in Fig. 5A. This delayed-onset thyroid irAE was only seen in the combination therapy groups (3 of 8 patients, 37.5%) (Table 4).
| Monotherapy (n = 10) | Combination therapy (n = 8) | |
|---|---|---|
| Age (years) | 74 (61–81) | 69 (60–79) |
| Gender (male/female), n (%) | 9 (90.0)/1 (10.0) | 5 (62.5)/3 (37.5) |
| ICI (pembrolizumab/atezolizumab), n (%) | 6 (60.0)/4 (40.0) | 4 (50.0)/4 (50.0) |
| Primary site, n (%) | ||
| Non-small cell lung cancer | 4 (40.0) | 5 (62.5) |
| Urothelial carcinoma | 3 (30.0) | 0 (0.0) |
| Malignant melanoma | 2 (20.0) | 0 (0.0) |
| Head and neck cancer | 1 (10.0) | 0 (0.0) |
| Small cell lung cancer | 0 (0.0) | 3 (37.5) |
| Peak fT3 (pg/mL) | 5.58 (3.44–9.88) | 6.19 (5.16–9.89) |
| Peak fT4 (ng/dL) | 2.39 (1.69–4.35) | 2.56 (1.85–3.46) |
| TSH nadir (μIU/mL) | 0.041 (0.010–0.204) | 0.058 (0.007–0.082) |
| Subsequent hypothyroidism, n (%) | 5 (50.0) | 8 (100.0) |
| Delayed-onset thyroid irAE, n (%) | 0 (0.0) | 3 (37.5) |
fT3, free T3 (reference range: 2.33–4.00 pg/mL); fT4, free T4 (0.880–1.620 ng/dL); TSH, thyroid stimulating hormone (0.500–5.000 μIU/mL). Bold type represents a distinct difference.
The patient presented in this report received pembrolizumab in combination with chemotherapy for treatment of advanced non-small cell lung cancer. His clinical course was unique from the perspective of his thyroid irAE. He developed thyrotoxicosis at 22 week after the first dose of pembrolizumab and subsequently developed hypothyroidism, even though ICI-containing therapy was not resumed. The timing of thyrotoxicosis onset was inconsistent with a previous report on ICI monotherapy, in which patients often developed thyrotoxicosis within 6 weeks [2]. We conducted a retrospective cohort study to determine whether the clinical features of thyroid irAEs are affected by the use of cytotoxic agents along with ICIs. We clarified that delayed-onset thyroid irAEs were only observed in patients who received combination therapy.
The thyroid irAE in the case patient was carefully diagnosed. Although TRAb and TSAb were positive when he developed thyrotoxicosis, poor blood flow during thyroid ultrasonography and low uptake during thyroid scintigraphy ruled out Graves’ disease. Systemic erythema and liver injury occurred simultaneously, which were judged as irAEs by experts. Throughout his entire clinical course, which included subsequent hypothyroidism with an atrophic and hypoechoic pattern on thyroid ultrasonography, we were convinced that his thyroid dysfunction was due to a thyroid irAE. In addition, thyroid uptake during 18F-FDG-PET was supportive of thyroid irAE [2, 13, 14].
We conducted a retrospective cohort study to clarify the clinical features of thyroid irAEs associated with combination therapy consisting of ICIs and cytotoxic agents. Our results suggested that incidence of thyroid irAEs was not changed with concomitant chemotherapy. As there are no previous reports with similar methodology, we reviewed clinical trials of ICIs. With pembrolizumab plus chemotherapy, the incidence of hypothyroidism was reported as 6.7% and 7.9% and the incidence of hyperthyroidism was reported as 4.0% and 4.9% in the KEYNOTE-189 and KEYNOTE-407 trials, respectively [15, 16]. As for atezolizumab plus chemotherapy, the incidence was reported as 12.7%, 17.3%, and 12.6% for hypothyroidism and 4.1%, 4.4%, and 5.6% for hyperthyroidism in the IMpower150, IMpassion130, and IMpower133 trials, respectively [17-19]. For pembrolizumab monotherapy, the incidence of hypothyroidism was 9.1% and the incidence of hyperthyroidism was 7.8% in the KEYNOTE-024 trial [20]. Although comparisons might not be appropriate due to variations in patient characteristics, study design, and irAE criteria, concomitant chemotherapy does not seem to alter the incidence of thyroid irAEs.
The typical clinical course of thyroid irAEs during monotherapy is transient thyrotoxicosis during 2–6 weeks after the first ICI dose followed by hypothyroidism [4, 11]. Reports on dual ICI therapy with anti–CTLA-4 and anti–PD-1 antibodies suggest that thyroid and pituitary irAEs develop earlier than with PD-1 blockade monotherapy [9-11]. In contrast, here we demonstrated delayed onset of thyroid irAEs during combination therapy of ICI with cytotoxic agents. Our cohort study revealed that some patients who received combination therapy developed thyroid irAEs at 16 weeks or more after the first ICI dose. Meanwhile, early onset of thyroid irAEs was also observed in patients who received either combination therapy or monotherapy. To summarize, concomitant chemotherapy delayed thyroid irAE onset in some patients.
The delayed onset of thyroid irAEs should be an important clinical matter because it might be overlooked. Over time, ICI administration and thyroid function monitoring will be discontinued. In fact, ICIs were no longer administered at the time of thyroid irAE onset in 2 of our 3 patients with delayed-onset thyroid irAE. With combination therapy, hypothyroidism was rather more commonly observed. We should detect thyroid irAEs because patients suffer from subsequent hypothyroidism unless they receive levothyroxine replacement. Longer monitoring of thyroid function seems to be beneficial for patients who receive ICIs in combination with chemotherapy.
We hypothesize that cytotoxic agents or prophylactic glucocorticoids might attenuate immunological effects of ICIs, leading to delayed-onset thyroid irAEs. However, no differences in leukopenia or glucocorticoid use were seen in our 8 patients who developed thyrotoxicosis during ICI combination therapy. The development of irAEs after ICI discontinuation might be explained by a previous experimental finding that anti–PD-1 antibodies continue to bind to lymphocytes for more than 20 weeks after the last infusion [21]. Investigation of the underlying mechanisms might be important for the development of future cancer immunotherapy strategies. Whether delayed-onset irAEs can occur in non-endocrine organs is another topic of interest suggested by the case patient because he developed systemic erythema and liver injury as irAEs around the same time as delayed-onset thyroid irAE.
This study has some limitations. First, as the number of patients who developed thyroid irAEs was relatively small, we could not adjust for heterogeneity in cancer type or stage and prior treatments. Although we clearly demonstrated that delayed onset of thyroid irAEs, further studies are required to clarify the incidence of delayed onset, which patients are susceptible, and factors that predict delayed onset in larger cohorts. Regarding prediction, patients with TPOAb and TgAb were reported to be more likely to develop thyroid irAEs [22], but we did not have data on these titers at baseline.
We demonstrated that thyroid irAE onset was delayed in a patient who received pembrolizumab in combination with chemotherapy. The retrospective study of our cohort confirmed that ICIs in combination with cytotoxic agents could cause delayed-onset thyroid irAEs. When using ICIs with chemotherapy, careful and long-term monitoring, even after discontinuation of ICI administration, is necessary in order to not overlook irAEs.
This work was supported by Japan Society for the Promotion of Science KAKENHI Grant Number 19K23942.
IY received lecture fees from Ono Pharmaceutical Co., Ltd. and Bristol-Myers Squibb. NI received lecture fees from MSD KK, Ono Pharmaceutical Co., Ltd., and Sanofi KK. NI received scholarship grants from Sanofi KK, MSD KK, Ono Pharmaceutical Co., Ltd., and Novartis Pharma.