2023 Volume 70 Issue 5 Pages 465-472
2023 Volume 70 Issue 5 Pages 465-472
Decidualization is a process of differentiation of human endometrial stromal cells (hESCs) accompanied by dramatic changes in cellular functions. This process is critical for embryo implantation and the establishment of pregnancy. Impairment of decidualization of hESCs leads to implantation failure, miscarriage, and unexplained infertility. The present review focuses on the metabolic changes in hESCs during decidualization. One of the changes taking place is in the glucose metabolism. Glucose uptake increases during decidualization because glucose is essential for the decidualization of hESCs. In hESCs, GLUT1 is highly expressed and involved in the increase of glucose uptake during decidualization. The up-regulation of GLUT1 is mediated by an epigenetic mechanism, which is regulated by CCAAT enhancer-binding protein β (C/EBPβ) and Wilms tumor 1 (WT1). Another metabolic change is in the lipid metabolism. Lipid accumulation in hESCs increases during decidualization. This increase is mediated by very low-density lipoprotein receptor (VLDLR). The up-regulation of VLDLR is regulated by WT1. In contrast to glucose, lipid is not essential for decidualization of hESCs. Endometrial cells have been implicated as important sources of nutrition for the embryo. hESCs may increase glucose and lipid storage so that they can supply them to the embryo during the implantation process. Taken together, decidualization is the process accompanied by metabolic changes, which may be associated with successful implantation.
Implantation of the human embryo in the maternal endometrium is a key factor in the establishment of pregnancy and requires a dialog between the embryo and the receptive endometrium [1]. In humans, this process is initiated by progesterone from the corpus luteum and involves morphological and functional changes in the endometrial cells [2]. Decidualization is a process by which the human endometrial stromal cells (hESCs), primed by estrogen in the proliferative phase, differentiate into decidualized hESCs by progesterone. This process is critical for embryo implantation and the establishment of pregnancy because a defective decidualization response is associated with reproductive disorders such as early pregnancy loss and recurrent miscarriage [3, 4]. Decidualization is regulated by many factors, including intracellular signaling pathways, their related molecules, and transcription factors [5-12]. These factors cause dramatic changes in gene expressions. In addition to transcription factors, these gene expressions are also regulated by a change in chromatin structure, which can be regulated by epigenetic mechanisms such as histone modifications and DNA methylation [13-15]. Recent genome-wide approaches have enabled us to observe the epigenetic status throughout the genome, which revealed that dramatic changes in genome-wide histone modifications occur with the alteration of a number of gene expressions during decidualization [16-19]. Therefore, epigenetic mechanisms also play key roles in the regulation of decidualization.
Along with these transcriptome changes, dramatic changes in various cellular functions occur in hESCs during decidualization [20-23]. Metabolically, decidualization stimulus activates the insulin signaling pathway and increases the glucose uptake in hESCs [11, 16, 24-30]. In addition to glucose, it has been shown that lipid storage in hESCs increases during decidualization [20, 31]. The metabolic changes taking place in hESCs are thus one of the most important aspects of decidualization contributing to successful implantation. In this review article, we describe recent findings on the metabolic changes in hESCs during decidualization, especially focusing on the glucose and lipid metabolisms.
Previous genome-wide analysis, using RNA-sequence and ChIP-sequence, showed that 881 genes are up-regulated in primary cultured hESCs by the induction of decidualization with estradiol (E) and medroxyprogesterone acetate (MPA) [16]. In these 881 genes, approximately 25% (223 genes) had an increase of active histone modifications, such as acetylation of histone H3 lysine-27 (H3K27ac) or trimethylation of histone H3 lysine-4 (H3K4me3), indicating that they were being up-regulated by the increases of H3K27ac and H3K4me3. In fact, the up-regulated genes with the increase of H3K27ac or H3K4me3 showed a higher increase in mRNA levels by decidualization than those without H3K27ac and H3K4me3 [16]. A pathway analysis revealed that the insulin signaling pathway is a significantly enriched pathway in these up-regulated genes with histone modifications. This indicated that H3K27ac and H3K4me3 preferentially deposit in a specific gene group such as the insulin signaling pathway-related genes [16]. These genes include the insulin receptor substrate 1 (IRS1), insulin receptor substrate 2 (IRS2), insulin receptor (INSR), forkhead box O1 (FOXO1), and mitogen-activated protein kinase 10 (MAPK10). The time course increases of mRNA and histone modifications during decidualization, shown in Fig. 1, were examined by RT-qPCR and ChIP-qPCR, respectively. These findings showed that epigenetic mechanisms are involved in the activation of the insulin signaling pathway during decidualization.
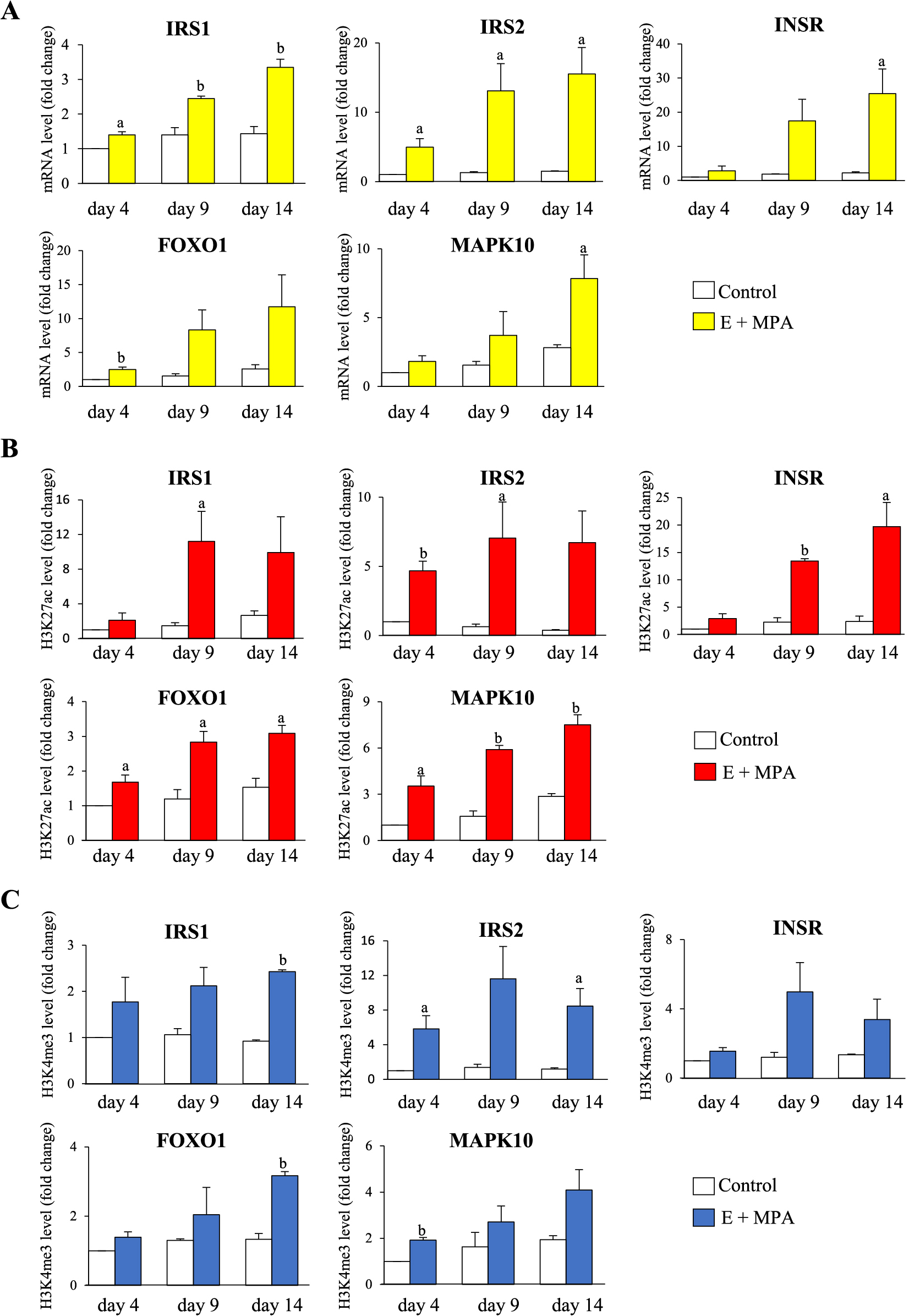
Time-course changes in mRNA, H3K27ac, and H3K4me3 of insulin signaling-related genes during decidualization. hESCs were treated with estradiol (E) (10–8 M) + medroxyprogesterone acetate (P) (10–6 M) for 14 days to induce decidualization. Cells treated without E + MPA were used as controls. (A) mRNA expression levels of the insulin signaling-related genes were examined at days 4, 9, and 14 after decidualization stimulation by real-time RT-qPCR. Values were normalized to those of GAPDH and expressed as a ratio of the control sample at day 4. (B) H3K27ac levels and (C) H3K4me3 levels in the promoter regions of the insulin signaling-related genes were examined by ChIP-qPCR. Values were normalized to those of input samples and expressed as a ratio of the control sample at day 4. Mean ± SE of three independent experiments. a, p < 0.05 vs. corresponding control sample; b, p < 0.01 vs. corresponding control sample (t-test).
The insulin signaling-related genes in Fig. 1 are involved in glucose uptake in various types of cells [29, 32]. Previous reports have demonstrated that the glucose uptake significantly increases in hESCs by decidualization [11, 16, 26-30]. Interestingly, when hESCs were incubated with decidualization stimulus, the increases in mRNA expression of IGF-binding protein-1 (IGFBP-1) and prolactin (PRL), which are well-recognized markers for decidualization [9, 30, 33, 34], were significantly lower at low glucose concentrations than at the normal ones [11, 16, 28]. This suggests that glucose is necessary for hESCs to undergo decidualization, which requires an increase of glucose uptake. It was reported that glucose contributes to an increase of the H3K27ac levels in the promoter region of FOXO1 and its mRNA expression [11]. FOXO1 is an important transcription factor for decidualization and regulates a number of genes related to decidualization [35], including IGFBP-1 and PRL. Therefore, a low-glucose environment inhibits FOXO1 expression by decreasing the H3K27ac levels of the promoter, which in turn suppresses the gene expressions of IGFBP-1 and PRL. Generally, acetylation of proteins starts with the uptake of glucose by the cells, which is then metabolized to acetyl CoA via glycolysis and the TCA cycle. Acetyl CoA thus serves as a source of acetyl modification of proteins, including histone proteins [36]. Therefore, a low glucose environment decreases the cellular levels of acetyl CoA [37, 38], and consequently the H3K27ac levels are also decreased as observed in hESCs. These findings showed that glucose contributes to decidualization through an epigenetic mechanism. In addition to the glucose metabolism, insulin signaling is involved in the regulation of amino acid metabolism [39]. It was reported that the decidualization stimulus increase the intracellular levels of several amino acids such as alanine, lysine, histidine, tyrosine, phenylalanine and proline, which are used as an energy to accomplish decidualization [40]. Therefore, it is possible that the activated insulin signaling affects decidualization through the regulation of amino acid metabolism.
The efficiency of glucose uptake is determined by a family of facilitative glucose transporters (GLUTs) [41]. Among several GLUTs identified in the human endometrium, GLUT1 is highly expressed in hESCs, and its expression was increased during decidualization whereas the expressions of other GLUTs were very low or not detected [26, 28, 42]. Therefore, it has been suggested that GLUT1 is responsible for glucose uptake during decidualization. A recent report showed that GLUT1 knockdown suppressed the increase of glucose uptake by decidualization in hESCs with the suppression of decidualization marker expressions [26]. These findings showed that the glucose uptake is regulated by GLUT1 and that the up-regulation of GLUT1 is an essential event for decidualization and the subsequent establishment of pregnancy. In fact, reduced stromal expression of GLUT1 has been observed in infertile women suffering from implantation failure [42]. Regarding the regulation of GLUT1 expression, CCAAT enhancer-binding protein β (C/EBPβ) and Wilms tumor 1 (WT1) are involved in the up-regulation of GLUT1 [26]. These transcription factors are known to be key transcription factors for decidualization because they regulate a number of gene expressions [5, 19, 20, 33, 43]. The promoter region of GLUT1 has binding sequences for both C/EBPβ and WT1. Decidualization stimulus induces their recruitment to the promoter region [26]. It is interesting to note that GLUT1 expression is also regulated by histone modifications (Fig. 2). The up-regulation of GLUT1 is accompanied by an increase of H3K27ac at the promoter region. This increase of H3K27ac is regulated by the recruitment of C/EBPβ and WT1 because the knockdown of these transcription factors decreased the H3K27ac level [26]. In general, histone modifications are induced by the recruitment of pioneer transcription factors, which are responsible for the initiation of the epigenetic changes [44-47]. These factors recruit the cofactors that have histone acetyltransferase (HAT) activities, such as p300, to induce H3K27ac [48, 49]. Decidualization stimulus induced the recruitment of p300 to the GLUT1 promoter region, and the knockdown of C/EBPβ or WT1 suppressed it. Therefore, C/EBPβ and WT1 work as pioneer factors by recruiting p300 and inducting H3K27ac to the GLUT1 promoter region during decidualization (Fig. 2). It was reported that multiple pioneer factors can simultaneously bind to cis-elements and recruit HAT factors cooperatively when their DNA binding sites are close together [50, 51]. DNA binding sites of C/EBPβ and WT1 in the GLUT1 promoter region are located close to each other (+224–+238 bp for WT1 binding site, +310–+321 bp for C/EBPβ binding site). Therefore, it is not surprising that they both work as pioneer factors by cooperatively recruiting p300 to the GLUT1 promoter region. In addition, another recent report showed a signaling pathway involved in glucose uptake. Decidualization stimulus activates MAPK and PI3K/AKT pathways through the increase of IRS2 expression. This pathway is also involved in the increase of GLUT1 expression and glucose uptake [30].
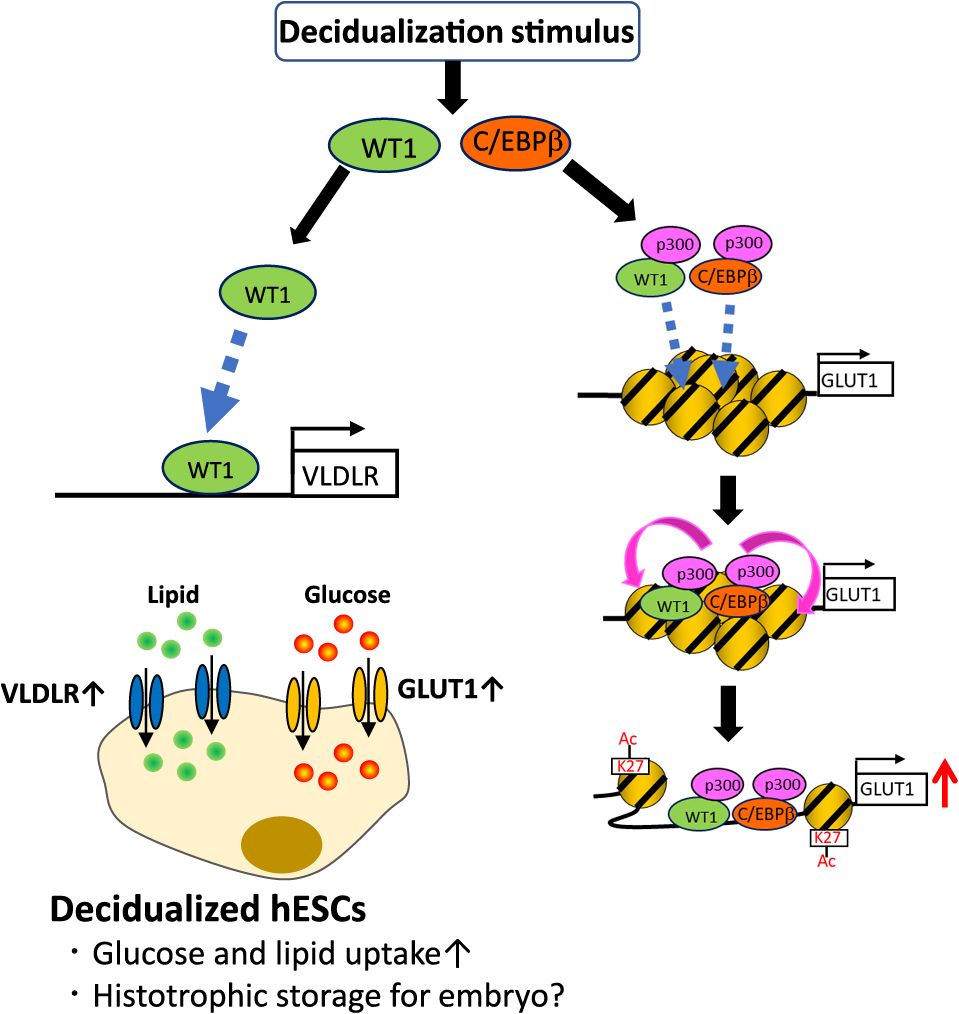
Regulation of glucose and lipid uptake during decidualization. Decidualization stimulus activates WT1 and C/EBPβ, and induces their recruitment to the GLUT1 promoter region. These transcription factors work as pioneer factors by recruiting histone acetyltransferase (p300) and inducing histone acetylation (H3K27ac) in the GLUT1 promoter region. The up-regulation of GLUT1 contributes to the increase of glucose uptake during decidualization. WT1 is also involved in lipid uptake. WT1 binds to the promoter region of VLDLR. The up-regulation of VLDLR contributes to the increase of lipid uptake during decidualization. Decidualized hESCs may work as histotrophic storage for embryos during the implantation process.
Another significant metabolic change occurring during decidualization is the increase of lipid storage in hESCs. Lipids are stored in cells in monolayer membrane-encased organelles called lipid droplets [52]. Decidualization stimulus increases the lipid droplet accumulation in hESCs, which was demonstrated by staining with a lipophilic fluorescent dye [20, 31]. Furthermore, the levels of intracellular triglyceride in hESCs were also increased by decidualization [20]. Because the abilities of lipogenesis and lipolysis are not altered in hESCs by decidualization, lipid accumulation during decidualization results from the increase of lipid uptake into the hESCs. Previous microarray analysis showed that WT1 up-regulates the genes associated with lipid transport during decidualization, suggesting the key role of WT1 in lipid metabolism [20]. In fact, WT1 knockdown suppressed lipid accumulation during decidualization. To identify the downstream factor of WT1 contributing lipid accumulation, we focused on very low-density lipoprotein receptor (VLDLR) [20]. VLDLR is a member of the low-density lipoprotein (LDL) receptor family. It mediates the uptake of lipoproteins by endocytosis and contributes to lipid accumulation in cells [53, 54]. VLDLR is expressed in the human endometrium and its expression is increased by decidualization. VLDLR knockdown suppressed lipid accumulation during decidualization. The up-regulation of VLDLR during decidualization was induced by the recruitment of WT1 to the promoter region of VLDLR (Fig. 2). Furthermore, VLDLR overexpression in hESCs reverted the suppression of lipid accumulation caused by the WT1 knockdown. Therefore, the WT1-VLDLR system contributes to lipid accumulation during decidualization (Fig. 2). Other than VLDLR, fatty acid-binding proteins (FABPs), fatty acid transport protein (FATPs), and CD36 are known to participate in lipid uptake and lipid accumulation [53, 55, 56]. Among these proteins, FABP3, FATP1, FATP4, and FATP5 were detected at the mRNA level in hESCs by microarray analysis. Their expression levels were neither increased by decidualization stimulus nor decreased by WT1 knockdown [20], suggesting that WT1-VLDLR pathway is the main regulator of the lipid uptake in ESCs during decidualization. WT1 is an essential transcription factor regulating mammalian urogenital development [57]. Mutations in WT1 cause Wilms tumor, a form of pediatric kidney cancer [58, 59], as well as a variety of human syndromes. In contrast to these roles of WT1, it is interesting to note that WT1 is associated with the metabolic regulation of glucose and lipid in hESCs.
As described above, sufficient glucose uptake is essential for decidualization of hESCs because the hESCs themselves need glucose for proper decidualization. On the other hand, the decreased lipid accumulation by VLDLR knockdown does not suppress decidualization [20]. Therefore, although lipids are not essential for decidualization reaction, hESCs may store them for some other reason. Implantation is the process by which an embryo attaches itself to the receptive endometrial luminal epithelium and then invades the decidualized stroma under the epithelium [1]. Embryo implantation and subsequent placentation are tightly regulated by the trophoblast-endometrial micro-environment [60]. Before a full invasion of the trophoblast and the establishment of a functional placenta, the endometrial cells have been implicated as important sources of nutrition for the embryo [21, 61-64]. Previous reports have indicated that the endometrial glands are connected to the intervillous space in order to supply nutrients to the embryo [63, 64]. Since the embryo is surrounded by decidualized hESCs after being embedded within the superficial layer of the endometrium, it is likely that the decidualized hESCs also work as a histotrophic storage site for the embryo. Therefore, hESCs may increase lipid storage during decidualization so that they can later supply the embryo with them. In fact, it has been reported that lipids are an essential source of nutrition for the development of the embryos [65]. Not only lipids, but also glucose is needed for embryo development [66]. Since the decidual layer before the establishment of placental circulation is under hypoxic conditions [67], the embryo is dependent on anaerobic glycolysis for its energy supply. Given that anaerobic glycolysis critically depends on the availability of glucose, the decidualized hESCs may work as glucose storage for embryos. The increase of glucose uptake in hESCs during decidualization may have two purposes: to complete the decidualization process, and to serve as glucose storage for the embryo.
This review has focused on the changes in cellular metabolisms including glucose and lipids. hESCs increase the uptake of glucose and lipids during decidualization. These metabolic changes are regulated by the up-regulation of the transcription factors and their downstream transporters. In addition to being utilized for decidualization, hESCs may also store glucose as a source of nutrition for the embryo. Because it has been impossible to directly observe the implantation process of humans for ethical reasons, solid evidence of endometrial cells as a histotrophic storage site has yet to be established. Recently, a human endometrial organoid has been developed, which is a 3D endometrium-like structure with epithelial cells [68, 69]. Furthermore, as a model of the human embryo, blastocyst-like cells, called blastoids, have been developed from human pluripotent stem cells [70, 71]. By co-culturing these newly developed cells, real-time monitoring of the nutrient transfer from hESCs to an embryo may one day become possible. This may help to provide insight into the role of hESCs as histotrophic storage for the embryo. It is expected that further studies will demonstrate the roles of decidualized hESCs for implantation.
We wish to thank Dr. Hiroshi Kimura (Tokyo Institute of Technology, Tokyo, Japan) for the gift of anti-H3K27ac and H3K4me3 antibodies. This research was supported by JSPS KAKENHI grants (JP21K09542, JP20K09645, JP20K18191, JP20H03825 and JP21K09517), the Yamaguchi Endocrine Research Foundation, and the Setsuro Fujii Memorial, Osaka Foundation for Promotion of Fundamental Medical Research.
The authors declare they have nothing to disclose with regard to the contents of this review article.