2023 Volume 70 Issue 6 Pages 601-610
2023 Volume 70 Issue 6 Pages 601-610
The skeletal muscle is an endocrine organ that produces proteins and peptides, collectively termed as myokines. The temperature of skeletal muscles varies during exercise and/or with changes in ambient temperature. However, whether myokine secretion is regulated by heat stimulation is unclear. Thus, we aimed to explore the effects of environmental heat stimulation on myokine secretion. We initially investigated the secretome of C2C12 myotubes and identified several novel heat-responsive myokines. The concentration of C-C motif chemokine ligand 5 (CCL5) dramatically decreased by 0.3-fold in response to heat stress. After 3 h heat stimulation of C2C12 cells, the expression of heat shock protein 70 was induced, and the gene expression and secretion of CCL5 was significantly attenuated in C2C12 cells. We then examined the effects of acute heat stress on serum CCL5 levels in mice and Ccl5 gene expression in skeletal muscles. Mice were maintained at 23°C, exposed to 45°C for 30 min, and then returned to the 23°C chamber for recovery. The expression of Ccl5 in the skeletal muscle significantly decreased after 3 h of recovery. Serum CCL5 levels increased by approximately 1.9-fold after 30 min of heat exposure and then significantly decreased by approximately 0.7-fold after 23 h of recovery. This study suggests that heat stimulation decreases CCL5 secretion from the skeletal muscle in vitro and in vivo. Given its fundamental role in inflammation by recruiting several immune cells, CCL5 has a potential role in controlling inflammatory responses in the body after heat stimulation.
THE SKELETAL MUSCLE secretes many proteins and peptides, collectively termed as myokines [1, 2]. These myokines play important roles in the communication between muscles and other organs; for example, fluctuations in myokine secretion due to skeletal muscle cell contraction can influence the transduction of “exercise signals” from skeletal muscles to the whole body [3]. In addition to exercise, stresses such as changes in skeletal muscle temperatures, nutritional and oxygen supplementation, and chemical exposure can affect skeletal muscles. However, the regulation of myokines by stimuli other than exercise is not well documented.
Except when the environmental temperature is extremely low, exercise generally increases the heat content and temperature of the body, including skeletal muscles [4]. Passive heat stimulation also contributes to increasing the skeletal muscle temperature [5]. Collectively, these reports suggest that the skeletal muscle temperature drastically increases or decreases depending on stimuli, including exercise. Temperature changes in the skeletal muscle directly affect its physiological functions and relations, including force/velocity and power/velocity relations. De Ruiter et al. [6] clearly demonstrated that the maximal isometric force and maximal shortening velocity significantly decrease with decreasing muscle temperature. In addition, Naito et al. [7] reported that whole body heat stress in rats protects against skeletal muscle atrophy induced by hindlimb unweighting.
In vitro studies provided evidence that heat stress modifies the functions of skeletal muscle cells. C2C12 myocytes are a well-established model for studying muscle differentiation. A previous study used this model to show that mild heat stress induced by incubating cells at 40°C for 1 h promotes mitochondrial biogenesis, which activates AMP kinase and SIRT1 expression [8]. However, severe heat stress induced by culturing C2C12 cells at 41.5°C for 4–8 days attenuates cell differentiation into myotubes by suppressing MyoD and myogenin [9]. Hence, the responses of skeletal muscle cells may vary depending on the degree of heat stimulation.
Heat-dependent responses of skeletal muscle cells have been extensively studied. The most apparent of these responses is the induction of heat shock proteins (HSPs), which facilitate protein folding by acting as chaperones. HSP70 is induced by exercise and passive heat stimulation. The induction of HSP70 predominantly facilitates stress-adaptive responses. For instance, loss of stress-dependent HSP70 induction delays inflammatory responses after skeletal muscle injury and impairs muscle regeneration [10]. By contrast, HSP70 overexpression sufficiently reduces muscle damage and accelerates the recovery of muscle functions [11-13]. HSP70 induction is a hallmark of increasing temperature in skeletal muscles and regulates skeletal muscle plasticity under these conditions.
Previous studies have suggested that several physical conditions induce heat accumulation in skeletal muscles, modify intracellular signaling molecules, including HSP70, and induce cellular responses. However, whether heat stimulation regulates myokine expression/secretion in skeletal muscles remains unclear. Therefore, the present study aimed to determine whether myokine secretion is altered by environmental heat stimulation.
The mouse skeletal muscle cell line C2C12 was maintained as previously described [14, 15]. C2C12 myoblasts were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 30 μg/mL penicillin, and 100 μg/mL streptomycin at 37°C in a 5% CO2 atmosphere. For all experiments, cells were grown in 6-well plates (Corning Inc.) at a density of 1.5 × 105 cells/well in 2 mL growth medium. When the cells reached 100% confluence 2 days after plating, cell differentiation was induced by incubating them in differentiation medium (DMEM supplemented with 2% calf serum (CS), 30 μg/mL penicillin, and 100 μg/mL streptomycin) for 7 days. The medium was changed every 24 h after inducing differentiation. After heat stimulation, cell culture supernatants were passed through a 0.2-μm pore size filter unit (Minisart Syringe Filter; Sartorius Lab Instruments GmbH, Göttingen, Germany) and directly used in the following experiments. Cell viability in C2C12 myotubes was evaluated using the MTT Cell Count Kit (Nacalai Tesque) according to the manufacturer’s protocol.
Animal experimentsAll animal experiments were approved by the Animal Care Committee at Toyo University. Male C57B6/J mice (Charles-River Japan, Inc., Tokyo, Japan; 8 weeks of age at the beginning of the experimental protocol) were individually housed in a temperature- and humidity-controlled room. The animals were fed chow (CLEA Japan, Inc., Tokyo, Japan) and water ad libitum and then acclimatized to a 12-h light cycle (lights on between 0700 and 1900 h) for 1 week before experimental manipulation. During this week, the mice were assigned to individual cages. On the experimental day (Day 7), control mice, heat-treated mice (exposed to 45°C for 30 min), or recovered mice (exposed to 45°C for 30 min and then returned to the normal chamber for 3 or 23 h) were anesthetized, and heart blood samples were collected. The mice were immediately sacrificed, and the tibialis anterior (TA), extensor digitorum longus (EDL), quadriceps (Quad), and soleus (SOL) muscles were removed, washed in PBS(–), frozen in liquid nitrogen, and then stored at –80°C.
Cytokine arrayAfter heat stress, culture supernatants were collected. The proteins in the culture supernatant were comprehensively analyzed using Proteome ProfilerTM Arrays Mouse Cytokine Array Panel A (R&D systems, MN, USA) in accordance with the manufacturer’s protocol. The signals were measured using ChemiDocTM XRS+ (Bio-Rad, Hercules, CA, USA). The intensity of each spot was quantified using ImageJ (National Institutes of Health, Bethesda, MD, USA).
Enzyme-linked immunosorbent assay (ELISA)The concentration of each myokine secreted was measured using Mouse CCL5/RANTES DuoSet ELISA (R&D Systems) and Mouse CXCL10/IP-10/CRG-2 DuoSet ELISA (R&D Systems) in accordance with the manufacturer’s protocol. In brief, each standard reference material (CCL5/RANTES; 2000, 1000, 500, 250, 125, 62.5, and 31.25 pg/mL, CXCL10/IP-10/CRG-2; 4000, 2000, 1000, 500, 250, 125, and 62.5 pg/mL) and samples were incubated at 4°C overnight. Absorbance at 450 nm was measured using an xMarkTM Microplate Spectrophotometer (Bio-Rad). The absorbance of each standard reference material was used to create a standard curve, and the concentration of CCL5 in the samples was calculated.
Reverse transcription-qPCRTotal RNA was isolated using TRIzolTM reagent (Thermo Fisher Scientific Inc., Waltham, MA, USA) in accordance with the manufacturer’s protocol. The concentration of total RNA was quantified using NanoDropTM 2000/2000c (Thermo Fisher Scientific Inc.). Reverse transcription was performed using PrimeScriptTM RT Master Mix (Perfect Real Time) (Takara Bio, Shiga, Japan), and the obtained cDNA was diluted 10-fold with EASY Dilution (for real-time PCR; Takara Bio). Quantitative PCR (qPCR) was performed using the StepOneTM or StepOnePlusTM real-time PCR system (Applied Biosystems, Waltham, MA, USA). The sequences of the primers used were mouse Ccl5, 5'-CATATGGCTCGGACACCA-3' and 5'-ACACACTTGGCGGTTCCT-3'; mouse Hspa1a, 5'-TGGTGCAGTCCGACATGAAG-3' and 5'-GCTGAGAGTCGTTGAAGTAGGC-3'; and mouse Gapdh, 5'-TGTGTCCGTCGTGGATCTGA-3' and 5'-CGTGCTTCACCACCTTCTTGA-3'.
Western blottingCells were harvested using a cell extract solution [50 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1 mM EDTA, 1% TritonX-100, 0.1% Protease Inhibitor Cocktail (Nacalai Tesque, Kyoto, Japan)] and then centrifuged at 4°C, 15,000 × g for 10 min. The supernatant was added with 2× sample buffer [0.1 M Tris-HCl (pH 6.8), 4% sodium dodecyl sulfate (SDS), 20% glycerol, 0.01% bromophenol blue] and 1% 2-mercaptoethanol. The cells were resolved by 10% SDS-polyacrylamide gel electrophoresis. Proteins were transferred onto polyvinylidene difluoride membranes (MERCK Millipore, Billerica, MA, USA). The membranes were blocked with 3% bovine serum albumin in TBS-T at room temperature for 30 min and then incubated at 4°C overnight with primary antibodies, including anti-CCL5 antibody (R&D Systems, 1:1000), anti-HSP70 antibody (Cell Signaling Technology, Danvers, MA, USA, 1:1000), and anti-β-actin antibody (Cell Signaling Technology, 1:1000). The membranes were washed three times with TBS-T for 10 min and then incubated with a secondary antibody [horseradish peroxidase-linked anti-mouse or anti-rabbit IgG (Cell Signaling Technology, 1:5000)] at room temperature for 1 h. The signal for each protein was visualized using enhanced chemiluminescence detection (Nacalai Tesque) and ChemiDocTM XRS+. Each band was quantified using ImageJ software (https://imagej.nih.gov/ij/).
Statistical analysisAll experiments were repeated at least three times. Statistical analyses were performed using GraphPad Prism software (GraphPad Software, CA, USA). Student’s t-test was used for two-group comparisons. Differences were considered significant at p values below 0.05. Comparisons among treatment groups were performed using one-way ANOVA with Tukey’s post-test or Student’s t-test, and p values below 0.05 were considered statistically significant.
Differentiated C2C12 myotubes were cultured at 37, 39, 40, 41, or 42°C for 24 h to optimize the thermal condition for inducing heat-dependent stress in C2C12 myotubes. The expression of HSP70 was analyzed using western blotting. When myotubes were cultured at 42°C, the expression of HSP70 significantly increased (Fig. 1A; *p < 0.05, n = 3). In addition, the induction of HSP70 was clearly observed 3 h after heat loading at 42°C and gradually increased up to 24 h (Fig. 1B). These results clearly demonstrated that heat treatment at 42°C for 3 h induced heat stress in C2C12 myotubes. Heat stimulation at 42°C for 24 h reduced cell viability by approximately 0.7-fold (Supplementary Fig. 1A; *p < 0.05, n = 3).

HSP70 was induced by heat stimulation in C2C12 myotubes
(A) Fully differentiated C2C12 cells were cultured at 37, 39, 40, 41, and 42°C for 24 h. Cell lysates were prepared, and HSP70 expression was analyzed using western blotting. The graph represents the mean of HSP70 expression (normalized by β-actin expression) ± SEM (*p < 0.05, n = 3). (B) Differentiated C2C12 cells were cultured at 37 or 42°C for indicated hours, and HSP70 expression was analyzed using western blotting. The experiment was repeated three times, and a representative result is shown.
We investigated whether heat stimulation alters the secretome of C2C12 myotubes. C2C12 myotubes were cultured at 37°C or 42°C for 24 h, and the cell culture medium was collected. The collected medium was assayed using the Proteome Profiler Mouse Cytokine Array Kit (Panel A), which can potentially evaluate the secretion of 40 mouse cytokines and chemokines. At least 10 chemokine/cytokine spots were reproducibly observed in three independent experiments (Fig. 2), and the secretion of five cytokines/chemokines, namely, C-X-C motif chemokine ligand 10 (CXCL10), macrophage colony stimulating factor (M-CSF), C-C motif chemokine ligand 2 (CCL2), C-C motif chemokine ligand 5 (CCL5), and interleukin-1 receptor antagonist (IL-1ra), was altered by heat stimulation. As shown in Fig. 2, the secretion of CXCL10 and CCL5 decreased by approximately 0.3-fold after heat stimulation (Fig. 2; **p < 0.01, n = 3). In addition, the secretion of M-CSF and CCL2 decreased by approximately 0.8-fold (*p < 0.05, n = 3). By contrast, the secretion of IL-1ra increased by approximately 1.4-fold after heat stimulation (*p < 0.05, n = 3). Of these heat-responsive myokines, CXCL10 and CCL5 showed prominent changes in response to heat stimulation and confirmed the changes in the secretion of these myokines by ELISA. Thus, they were used in subsequent experiments. The secretion of CCL5 significantly decreased by approximately 200 pg/mL to 100 pg/mL after heat stimulation (Fig. 3A, *p < 0.05, n = 3). Although the secretion of CXCL10 also decreased after heat stimulation, no statistical significance was detected (data not shown). These results suggested that the secretion of CCL5 was controlled by heat stimulation.

Heat stimulation altered the secretome in C2C12 myotubes
Fully differentiated C2C12 myotubes were cultured in the differentiation medium at 37 or 42°C for 24 h. After 24 h, culture supernatants were subjected to cytokine array analysis. Three independent experiments were performed, and the intensity of each spot was measured using ImageJ software. The graph represents the mean ± SEM obtained from these experiments (*p < 0.05, ** p < 0.01, n = 3).

Heat stimulation decreased CCL5 expression in C2C12 myotubes
Fully differentiated C2C12 myotubes were cultured at 37 or 42°C for 24 h. (A) Culture supernatants were analyzed by ELISA to measure CCL5 concentration. The graph represents the mean ± SEM (*p < 0.05, n = 3). (B) After 24 h, total protein was subjected to western blotting to monitor intracellular CCL5 expression. The graph represents the mean ± SEM (*p < 0.05, n = 5). (C) After 24 h, total RNA was isolated and Ccl5 expression was measured using quantitative PCR (*p < 0.05, n = 3).
We also analyzed CCL5 expression in the cells using western blotting. As shown in Fig. 3B, CCL5 expression decreased by approximately 0.6-fold after heat stimulation (*p < 0.05, n = 5). Similarly, Ccl5 expression decreased (approximately 0.4-fold) after heat stimulation (Fig. 3C, *p < 0.05, n = 3). These results indicated that heat stimulation decreased the expression of CCL5, at least in part, by regulating its gene expression in C2C12 myotubes.
Acute heat stimulation sustained the reduction of CCL5 expression from C2C12 myotubesIn addition to chronic heat stimulation, we investigated the effect of acute heat stimulation on CCL5 expression. Differentiated C2C12 myotubes were treated at 42°C for 3 h and then continuously cultured at 37°C. Samples were collected at 0 (prior to heat stimulation), 3, 6, 12, and 24 h. We initially confirmed the expression of Hspa1a and HSP70 in C2C12 myotubes. The expression of Hspa1a was elevated by more than 400-fold after 3 h heat stimulation and remained high for up to 6 h, even when the cells were returned to 37°C (Fig. 4A, **p < 0.01, n = 3). The expression of HSP70 was similarly induced by 3 h of heat stimulation and then gradually increased until 24 h, even when the cells were returned to 37°C (Fig. 4B, *p < 0.05, n = 3). The viability of C2C12 cells did not significantly change under this condition, that is, stimulating at 42°C for 3 h followed by continuous culturing at 37°C for 21 h (Supplementary Fig. 1B; n = 3).

Acute heat stimulation was sufficient to reduce CCL5 expression in C2C12 myotubes
Fully differentiated C2C12 myotubes were cultured at 37 or 42°C for 3 h, followed by continuous culture at 37°C for 21 h. (A, B) The expression of HSP70 protein and Hspa1a gene was measured using western blotting and quantitative PCR, respectively. The graph represents the mean ± SEM (*p < 0.05, n = 3). (C) At each time point, the culture supernatant was collected and analyzed by ELISA to measure CCL5 concentration. The graph represents the mean ± SEM (*p < 0.05, ** p < 0.01, ****p < 0.001, n = 3). (D) Expression of Ccl5 was analyzed using quantitative PCR. The graph represents the mean ± SEM (*p < 0.05, n = 3).
We investigated CCL5 regulation after acute heat stimulation and recovery. CCL5 secretion from C2C12 myotubes decreased after 3 h of heat stimulation and remained significantly low until 24 h of recovery (Fig. 4C, *p < 0.05, **p < 0.01, ****p < 0.001, n = 3). By contrast, Ccl5 expression did not change after 3 h of heat stimulation; however, it gradually decreased up to 24 h of recovery (Fig. 4D, *p < 0.05, n = 3). The heat stimulation-induced reduction in CCL5 secretion occurred before Ccl5 expression changes, suggesting that heat stimulation suppressed the secretion and gene expression of CCL5. Overall, the results suggested that acute (3 h) heat stimulation, which was the minimum stimulation to induce HSP70, was sufficient to evoke prolonged chronic reduction of CCL5 expression in C2C12 myotubes.
Effects of rising temperature on serum CCL5 levels and skeletal muscle Ccl5 expression in miceWe examined whether acute heat stress in mice affects serum CCL5 levels and Ccl5 expression in skeletal muscles. As described in Materials and Methods, male C57B6/J mice maintained at 23°C were exposed to 45°C for 30 min and then returned to the 23°C chamber (recovery time). At each time point (0 min (Ctrl), 30 min heat stimulation (Heat), 3 and 23 h after heat stimulation (Rec 3 h, Rec 23 h, respectively)), the mice were anesthetized, and heart blood samples were collected. The mice were immediately sacrificed, and skeletal muscle samples (TA, EDL, Quad, and SOL) were prepared.
In all skeletal muscles we tested, Hspa1a expression was not induced after 30 min of heat exposure but significantly increased after 3 h of recovery and then returned to basal levels after 23 h of recovery (Fig. 5A–D, ***p < 0.05, ****p < 0.001 vs. Ctrl, n = 5). By contrast, Ccl5 expression significantly decreased in EDL and SOL after 30 min of heat stimulation but significantly decreased in all four muscles by approximately 0.1- to 0.5-fold after 3 h of recovery (Fig. 5E–H). These changes were only temporal, and Ccl5 expression gradually returned to basal levels after 23 h of recovery (Fig. 5E–H, *p < 0.05, **p < 0.01, ***p < 0.005 vs. Ctrl, n = 5). Serum CCL5 levels increased in response to 30 min of heat exposure by approximately 1.9-fold but significantly decreased by approximately 0.7-fold after 23 h of recovery (Fig. 6, *p < 0.05, ***p < 0.005 vs. Ctrl, n = 5).
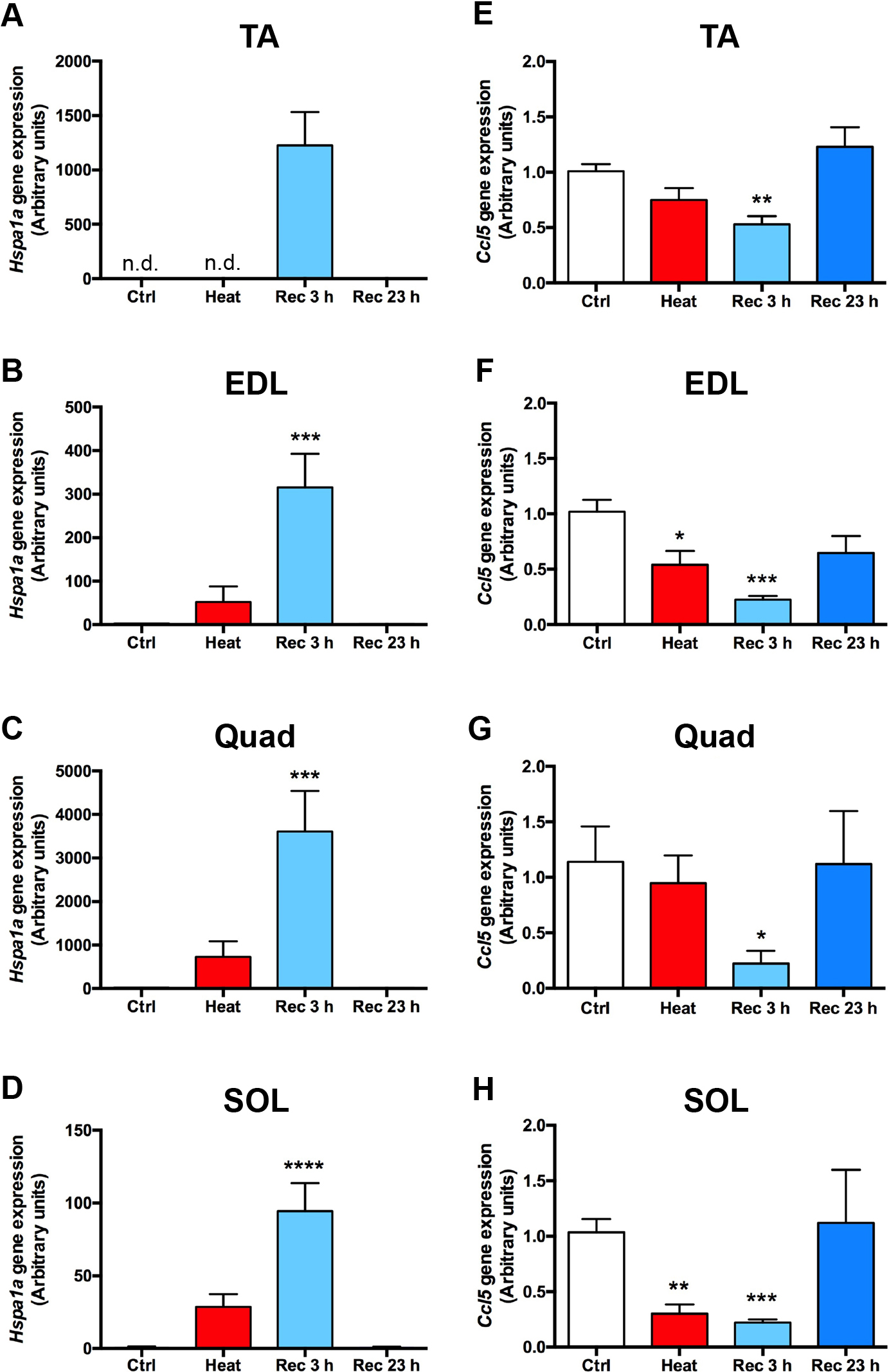
Heat stimulation on mice modulates Ccl5 gene expression in skeletal muscles.
(A–H) Eight-week-old male C57B6/J mice were housed individually in a temperature- and humidity-controlled room. On the experimental day, control mice, heat-treated mice (exposed to 45°C for 30 min), or recovered mice (exposed to 45°C for 30 min and then returned to the normal chamber for 3 or 23 h) were anesthetized, and tibialis anterior (TA), extensor digitorum longus (EDL), quadriceps (Quad), and soleus (SOL) muscles were removed as described in Materials and Methods. Relative expression of Hspa1a (A–D) or Ccl5 (E-H) in TA (A, E), EDL (B, F), Quad (C, G), and SOL (D, H) was analyzed using quantitative PCR. The graph represents the mean ± SEM, and the data were analyzed using a one-way ANOVA followed by Tukey’s multiple comparison test (*p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.001 vs. Ctrl, n = 5).

Heat stimulation on mice modulates serum CCL5 levels
Eight-week-old male C57B6/J mice were housed individually in a temperature- and humidity-controlled room. On the experimental day, control mice, heat-treated mice (exposed to 45°C for 30 min), or recovered mice (exposed to 45°C for 30 min and then returned to the normal chamber for 3 or 23 h) were anesthetized, and heart blood samples were collected. Serum CCL5 concentration was determined using ELISA. The graph represents the mean ± SEM, and the data were analyzed using a one-way ANOVA followed by Tukey’s multiple comparison test (*p < 0.05, ***p < 0.005 vs. Ctrl, n = 5).
These data suggest that heat stimulation in individual animals causes heat stress in skeletal muscles, as evidenced by the upregulation of Hspa1a expression and downregulation of Ccl5 expression. Although further studies are required to understand the effect of Ccl5 downregulation on skeletal muscle, serum CCL5 levels also decreased after 30 min of heat stimulation and 23 h of recovery.
Excessive heat production due to exercise or an increase in environmental temperature can affect the whole body and even cause heat stroke. The skeletal muscle is particularly sensitive to heat stimulation [16]. Thus, skeletal muscle cells are predicted to be influenced by such temperature changes. The results of the present study revealed that myokine secretion fluctuated when the temperature around C2C12 cells was changed. Similar to the contraction-dependent myokine secretion in C2C12 cells [14, 15], the secretion of several myokines, such as CXCL10 and CCL5, was significantly reduced. Specifically, Ccl5 expression in skeletal muscles and serum CCL5 levels in mice were significantly modified by changes in the temperature of the breeding environment.
CCL5, also known as regulated upon activation, normal T cell expressed, and secreted (RANTES), plays a fundamental role in inflammation by recruiting several immune cells, including T cells, macrophages, dendritic cells, and NK cells [17, 18]. In vitro experiments clearly showed that prolonged (up to 24 h) and acute (3 h) heat stimulation reduced CCL5 secretion from C2C12 myotubes, suggesting that the reduction in CCL5 secretion was mainly stimulated by heat but not by decreased temperature after heat stimulation. In addition, in vivo experiments revealed that acute heat stimulation in mice temporally increased serum CCL5 levels, whereas prolonged resting time reduced serum CCL5 levels and Ccl5 expression in skeletal muscles (Fig. 6). Overall, blood CCL5 levels showed biphasic changes in response to heat stimulation, with transient increases and sustained decreases. Several lines of evidence suggest that heat induces muscle damage, such as rhabdomyolysis; therefore, although we did not identify the cells that control the initial upregulation of serum CCL5 levels, this might be a signature of heat-dependent acute inflammation [19]. In fact, the serum levels of several inflammatory cytokines are upregulated by elevated temperatures [20, 21]. By contrast, Ccl5 expression in skeletal muscle significantly decreased with increasing ambient temperature. In addition, serum CCL5 levels significantly decreased 23 h after heat stimulation. Hence, although further studies are required to elucidate if the decrease in Ccl5 expression in skeletal muscle directly regulates serum CCL5 level, it is possible that decreased CCL5 expression in skeletal muscles after heat stimulation may contribute to, at least partially, the reduction in serum CCL5 level after recovery.
Cytokine array analysis of heat-dependent myokines revealed that CCL5 secretion decreased upon the heat stimulation of C2C12 cells (Figs. 3A, 4C). Intriguingly, we have previously observed that exercise reduced Ccl5 and Cxcl10 expression in skeletal muscles and CCL5 levels in blood [14, 15]. In other words, CCL5 expression decreases with exercise and heat stimulation. Considering that exercise increases skeletal muscle temperature [22, 23], we can hypothesize that the contraction-dependent reduction of CCL5 expression in skeletal muscles may be partly mediated by the heat increases associated with the contraction. Another possibility is that the exercise- or heat-dependent reduction of CCL5 expression shares the same mechanism(s). We previously reported that the contraction-dependent reduction of CCL5 expression is partly mediated by AMP kinase activation, which is often caused by ATP depletion, in C2C12 myotubes [14]. Together with the previous finding that mild heat shock at 40°C for 1 h upregulates AMPK activity in C2C12 myotubes [8], these reports suggest that exercise- or heat-evoked ATP depletion followed by AMP kinase activation participates in the reduction of CCL5 expression in skeletal muscles. Interleukin-6 (IL-6) expression in skeletal muscles is also induced by exercise and heat stimulation but via distinct mechanisms [24-26]. The TRPV1/PKC/CREB pathway is responsible for the heat-dependent induction of IL-6 expression [26], whereas the contraction-dependent induction of IL-6 expression is inhibited by cyclosporine A, a calcineurin inhibitor [27]. In any case, one of the next important questions is the identification of intracellular mechanism(s) responsible for heat-dependent reduction of CCL5 expression in skeletal muscles.
Prolonged high ambient temperature conditions affect the immune system of animals [21, 28, 29]. Activation of the hypothalamic–pituitary–adrenocortical axis is a major consequence of heat stress responses [30], and increased blood glucocorticoid concentration slows immunological defenses [31]. By contrast, the induction of HSPs by heat stimulation increases innate immunity [32]. One of the main functions of HSPs is protein folding, which acts as a molecular chaperone [33-36]. However, recent studies have clearly shown that HSPs are released from cells and have cytokine-like functions [37]. The released HSPs often induce the release of proinflammatory cytokines, such as tumor necrosis factor-α, IL-1, IL-6, and IL-12, from monocytes, macrophages, and dendritic cells [37-45]. Overall, heat substantially affects several cells, tissues, and organs that are responsible for controlling the immune system and may produce complex consequences in response to temperature and exposure time. The present study shows for the first time that increased ambient temperature directly affects skeletal muscles and modifies myokine profiles, especially regulating CCL5 secretion. Although further studies are required to elucidate the precise mechanisms underlying heat-dependent CCL5 reduction in skeletal muscles, previous studies reported that elevated blood CCL5 levels are associated with normal aging, atherosclerosis, and obesity [46-49], all of which increase inflammatory responses in the body [50-53]. Together with the fact that exercise also reduces serum CCL5 levels [14], heat stimulation may also control the body in the same anti-inflammatory direction via this mechanism.
Heat-dependent reduction of CCL5 from skeletal muscle may not only regulate immune responses but also control other physiological responses. Studies have shown that elevated ambient temperature and exercise increase insulin sensitivity and ameliorate type 2 diabetes [54, 55]; HSP70 expression increases with both elevated ambient temperature and exercise and appears to be at least partially involved in this process [56]. On the contrary, mice with a global deletion of Ccl5 have been shown to be protected from high-fat diet (HFD)-induced obesity and insulin resistance [57]. These findings suggest that in addition to the previously known HSP-dependent increase in insulin sensitivity, a heat-dependent decrease in myokine CCL5 may contribute to increased insulin sensitivity.
In conclusion, the present study suggests for the first time that heat stimulation decreases CCL5 expression in skeletal muscles in vivo and in vitro. Serum CCL5 levels decreased with increasing ambient temperature in mice, suggesting that this mechanism has potential role(s) in controlling inflammatory and metabolic responses in the body after heat stimulation.
None.
We are grateful to Ayano Sonoda, Chiaki Nose, Kei Sato, Natsuki Fujinuma, Chiho Miyashita, Ayaka Saito, Shunya Okawa, and Takumi Watanabe (Faculty of Life Sciences, Toyo University) for their technical assistance.
This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (C-19K06442 to T.N.).