Abstract
Cushing’s disease is associated with increased morbidity and mortality. Osilodrostat, a potent oral 11β-hydroxylase inhibitor, provided rapid, sustained mean urinary free cortisol (mUFC) normalization in Cushing’s disease patients in two Phase III studies (LINC 3, NCT02180217; LINC 4, NCT02697734). Here, we evaluate the efficacy and safety of osilodrostat in Cushing’s disease in patients of Asian origin compared with patients of non-Asian origin. Pooled data from LINC 3 and LINC 4 were analyzed. Outcomes were evaluated separately for Asian and non-Asian patients. For the analysis, 210 patients were included; 56 (27%) were of Asian origin. Median (minimum–maximum) osilodrostat dose was 3.8 (1–25) and 7.3 (1–47) mg/day in Asian and non-Asian patients, respectively. mUFC control was achieved at weeks 48 and 72 in 64.3% and 68.1% of Asian and 68.2% and 75.8% of non-Asian patients. Improvements in cardiovascular and metabolic-related parameters, physical manifestations of hypercortisolism, and quality of life were similar in both groups. Most common adverse events (AEs) were adrenal insufficiency (44.6%) in Asian and nausea (45.5%) in non-Asian patients. AEs related to hypocortisolism and pituitary tumor enlargement occurred in more Asian (58.9% and 21.4%) than non-Asian patients (40.3% and 9.1%). Of Asian and non-Asian patients, 23.2% and 13.6%, respectively, discontinued because of AEs. Asian patients with Cushing’s disease generally required numerically lower osilodrostat doses than non-Asian patients to achieve beneficial effects. Hypocortisolism-related AEs were reported in more Asian than non-Asian patients. Together, these findings suggest that Asian patients are more sensitive to osilodrostat than non-Asian patients.
Plain Language Summary
Why was this analysis carried out?
• People with Cushing’s disease have higher-than-normal levels of the hormone cortisol, caused by a benign (non-cancerous) tumor of the pituitary gland, known as an adenoma. Osilodrostat is a medicine that reduces and maintains normal cortisol levels in people with Cushing’s disease
• In people of Asian origin, the amount of osilodrostat in the blood from a tablet of the same dose is higher than in people of non-Asian origin. We wanted to examine whether osilodrostat would have similar beneficial effects in people of Asian and non-Asian origin, and if any side effects would be different between the two groups
How was this analysis carried out?
• The results of two previous studies were combined to allow a larger number of people of Asian and non-Asian origin to be included in this analysis
• The beneficial effects of osilodrostat and any side effects associated with the medicine were analyzed in people of Asian and non-Asian origin
What were the overall results?
• Cortisol rapidly dropped to normal levels in people of both Asian and non-Asian origin and remained at a normal level after osilodrostat had been taken for a long period of time in the majority of people
• People of Asian origin showed benefit from treatment with a lower dose of osilodrostat than people of non-Asian origin
• The symptoms of Cushing’s disease and a person’s quality of life were improved with osilodrostat in both groups
• Osilodrostat was well tolerated, with a few differences in side effects between the two groups
• More people of Asian origin experienced side effects related to levels of cortisol being too low, or to their existing pituitary tumor, than people of non-Asian origin
What do the results mean?
• Osilodrostat had similar benefits and was well tolerated in people of Asian and non-Asian origin, but people of Asian origin required lower doses than people of non-Asian origin
Where can I find more information?
• LINC 3 primary publication: Pivonello R et al. Lancet Diabetes Endocrinol 2020; 8: 748–761.
• LINC 4 primary publication: Gadelha M et al. J Clin Endocrinol Metab 2022; 107: e2882–e2895.
• LINC 3 long-term publication: Fleseriu M et al. Eur J Endocrinol 2022; 187: 531–541.
• LINC 4 long-term publication: Gadelha M et al. Front Endocrinol (Lausanne) 2023; 14: 1236465.
Introduction
Cushing’s disease is a rare disorder caused by an adrenocorticotropic hormone (ACTH)-producing pituitary adenoma, which in turn stimulates the adrenal glands to produce cortisol in excess. Chronic exposure to hypercortisolism is associated with numerous comorbidities that lead to an increased risk of morbidity and mortality [1, 2] and poor health-related quality of life (HRQoL) [3]. Differences in the phenotype of Cushing’s syndrome have previously been reported between East Asian and Caucasian patients [4].
The primary treatment goal for patients with Cushing’s disease is normalization of cortisol secretion [1, 3]. Transsphenoidal surgery is the recommended first-line treatment; however, around one-third of patients experience persistent or recurrent disease following surgery [5], and some patients are ineligible for or refuse surgery [5-7]. For these patients, medical therapies are an important treatment option, with steroidogenesis inhibitors usually considered as a first-choice medical therapy for most patients [7].
Osilodrostat is a potent oral inhibitor of 11β-hydroxylase, the enzyme that catalyzes the final step of cortisol synthesis. Findings from two Phase III studies (LINC 3 and LINC 4) showed that osilodrostat provided rapid and sustained reductions in mean urinary free cortisol (mUFC) in most patients with Cushing’s disease. These reductions were maintained long term [8-11].
There are few prospective studies reporting long-term efficacy and safety data for steroidogenesis inhibitors [8, 10, 12-15] and limited data describing the effects of medical treatments for Cushing’s disease in patients from different racial backgrounds. Here, we report efficacy and safety outcomes in patients of Asian and non-Asian origin during long-term osilodrostat treatment.
Materials and Methods
Study design and participants
Details of the LINC 3 and LINC 4 study designs have been published previously [9, 11]. In summary, LINC 3 was a Phase III, multicenter, open-label, double-blind, 48-week study that included an 8-week randomized-withdrawal period. LINC 4 was a Phase III, multicenter, 48-week study that included an initial 12-week, randomized, double-blind, placebo-controlled period followed by a 36-week open-label treatment period. Both studies were followed by an optional extension for patients benefiting from osilodrostat treatment at week 48, as assessed by the study investigator. In LINC 3, all patients received open-label osilodrostat treatment (starting at 2 mg twice daily [bid]), except those randomized to placebo during the randomized-withdrawal period (weeks 26–34). Osilodrostat dose titration was permitted every 2 weeks until week 12, then every 4 weeks thereafter. In LINC 4, patients were randomized to osilodrostat 2 mg bid or matching placebo for the first 12 weeks. Osilodrostat dose titration was permitted every 3 weeks until week 12; patients then restarted osilodrostat 2 mg bid unless they were on a lower dose at week 12, and dose titration was permitted every 3 weeks thereafter. In both studies, dose-titration decisions were based on efficacy and tolerability. Maximum osilodrostat dose was 30 mg bid in both studies (maximum dose during the first 12 weeks of LINC 4 was 20 mg bid). Patients aged 18–75 years with a confirmed diagnosis of persistent/recurrent Cushing’s disease after pituitary surgery and/or irradiation, or those with de novo disease (non-surgical candidates), as well as with mUFC >1.5 times the upper limit of normal (ULN) in LINC 3 or >1.3 × ULN in LINC 4, morning plasma ACTH above the lower limit of normal, and a confirmed source of excess ACTH from a pituitary origin, were enrolled in LINC 3 and LINC 4 [9, 11].
The studies were conducted in accordance with the Declaration of Helsinki, with an independent ethics committee/institutional review board at each site approving the study protocols; patients provided written informed consent to participate at the beginning of the studies and for the extension periods.
Assessments
Efficacy assessments reported in this analysis are: change from baseline in mUFC over time; proportion of mUFC responders over time; change from baseline in serum cortisol, morning salivary cortisol and late-night salivary cortisol (LNSC); mUFC and LNSC control status (defined as: both mUFC + LNSC controlled [mUFC ≤ ULN + LNSC ≤ ULN], only mUFC controlled [mUFC ≤ ULN + LNSC > ULN], only LNSC controlled [mUFC > ULN + LNSC ≤ ULN], and both mUFC + LNSC uncontrolled [mUFC > ULN + LNSC > ULN]); and cardiovascular and metabolic parameters, including fasting plasma glucose (FPG), glycated hemoglobin (HbA1c), total cholesterol, low-density lipoprotein and high-density lipoprotein cholesterol, triglycerides, weight, systolic blood pressure (SBP), diastolic blood pressure (DBP), body mass index (BMI) and waist circumference. Physical manifestations of hypercortisolism, including facial rubor, striae, dorsocervical and supraclavicular fat pads, proximal muscle atrophy, central obesity, ecchymoses and hirsutism, were assessed locally by site investigators from photographs from the shoulders up and of the trunk and rated subjectively on a semi-quantitative scale: 0 = absent; 1 = mild; 2 = moderate; 3 = severe. An improvement in physical manifestations of hypercortisolism was defined as a decrease in score from baseline to the given time point. HRQoL was assessed using the Cushing’s Quality of Life Questionnaire (CushingQoL) and the Beck Depression Inventory II (BDI-II).
Safety was assessed from core study baseline to end of extension by monitoring adverse events (AEs) according to Common Terminology Criteria for Adverse Events (version 4.03). AEs are presented as reported by the investigators. AEs of special interest, anticipated based on the mechanism of action of osilodrostat, included those related to hypocortisolism, accumulation of adrenal hormone precursors, pituitary tumor enlargement, and arrhythmogenic potential and QT prolongation. AEs that could potentially be linked to hypocortisolism were grouped in accordance with the study protocol. Adrenal insufficiency, as reported by the investigator, was included in this grouping; however, there was no protocol-mandated requirement for adrenal insufficiency to be confirmed by measuring serum cortisol levels. Change from baseline in ACTH, other hormonal markers (11-deoxycortisol, 11-deoxycorticosterone, aldosterone, estradiol, estrone, dehydroepiandrosterone sulfate [DHEAS], testosterone) and serum potassium were assessed. Tumor volume was evaluated in patients with magnetic resonance imaging assessments available. Pharmacokinetic (PK) parameters for plasma osilodrostat by incident dose are reported for the LINC 3 study only as they were not evaluated in LINC 4. To provide additional information on osilodrostat bioavailability in patients of Asian and non-Asian origin, data are reported from a Phase I, randomized, placebo-controlled study (A2102), which compared the PK of osilodrostat in healthy male Japanese and Caucasian subjects. In summary, healthy volunteers received daily osilodrostat doses of 0.5, 1.0 and 2.0 mg for 2 weeks. PK parameters included time to maximal plasma concentration (Cmax), area under the concentration–time curve between 0 and 12 hours (AUC0–12h), and area under the concentration–time curve between 12 and 24 hours (AUC12–24h) on day 14. To determine whether differences in osilodrostat bioavailability between Japanese and Caucasian subjects could be explained by body weight, effect of race on body-weight-adjusted AUC0–12h, AUC12–24h and Cmax was evaluated.
Statistical methods
Data from LINC 3 and LINC 4 were pooled, and efficacy and safety outcomes were evaluated separately in patients of Asian and non-Asian origin. Race was recorded on the case report form based on the assumption of the site investigator as Caucasian, black, Asian, or other in LINC 3 and as white, Asian, black or African American, unknown, American Indian or Alaska Native, or other in LINC 4. Periods during which patients received placebo were excluded from the analysis.
Categorical data are presented as frequencies and percentages, and continuous data are summarized as mean (standard deviation [SD]) or median (minimum–maximum) values. Results were analyzed descriptively for all patients with an assessment at both baseline and the given visit. No formal statistical testing was performed.
Results
Patient disposition, baseline characteristics and disease history
From a globally diverse population of 210 patients with Cushing’s disease, 56 (26.7%) were from a heterogeneous population of Asian origin (LINC 3, n = 39; LINC 4, n = 17) and 154 (73.3%) were of non-Asian origin (LINC 3, n = 98; LINC 4, n = 56). Patient enrollment by country is outlined in Fig. 1.
During the core phases, 25/56 (44.6%) and 48/154 (31.2%) Asian and non-Asian patients, respectively, discontinued treatment. During the extension phases, 17/45 (37.8%) and 24/121 (19.8%) Asian and non-Asian patients, respectively, discontinued treatment. The primary reason for treatment discontinuation at any time was because of an AE (Fig. 2).

Fig. 2
Patient disposition
Patient demographics were generally similar across both patient groups (Table 1). Expected differences in baseline characteristics included numerically lower weight, height and BMI in Asian patients than in non-Asian patients. Baseline mean (SD) mUFC was 10.2 (16.0) and 4.2 (5.0) × ULN in Asian and non-Asian patients, respectively. 33.9% (n = 19) of Asian and 18.8% (n = 29) of non-Asian patients had a macroadenoma at baseline. 10.7% (n = 6) of Asian patients and 9.1% (n = 9) of non-Asian patients were taking spironolactone at baseline. The most common medications used to treat cardiovascular and metabolic comorbidities taken at baseline are summarized in Table 1.
Table 1 Patient demographics and disease history in patients of Asian and non-Asian origin
|
Asian patients
N = 56 |
Non-Asian patients
N = 154 |
| Median age, years (range) |
38.5 (19–70) |
41.0 (19–70) |
| Sex, n (%) |
|
|
| Female |
44 (78.6) |
123 (79.9) |
| Male |
12 (21.4) |
31 (20.1) |
| Mean weight, kg (SD) |
69.5 (16.0) |
83.7 (21.0) |
| Mean height, cm (SD) |
157.7 (7.1) |
164.3 (8.9) |
| Mean BMI, kg/m2 (SD) |
27.9 (5.8) |
31.0 (7.6) |
| Median time to first osilodrostat dose since diagnosis, months (range) |
62.2 (3–216) |
52.5 (2–287) |
| Disease status, n (%) |
|
|
| De novo |
7 (12.5) |
13 (8.4) |
| Persistent/recurrent |
49 (87.5) |
141 (91.6) |
| Previous surgery, n (%) |
37 (66.1) |
92 (59.7) |
| Previous pituitary irradiation, n (%) |
16 (28.6) |
51 (33.1) |
| Proportion of patients on concomitant medications, n (%) |
45 (80.4) |
146 (94.8) |
| Most common (>15% of patients in either group) concomitant medications, n (%) |
|
|
| Levothyroxine sodium |
10 (17.9) |
26 (16.9) |
| Lekovit CA
|
9 (16.1) |
7 (4.5) |
| Amlodipine
|
9 (16.1) |
11 (7.1) |
| Metformin
|
10 (14.3) |
46 (15.6) |
| Colecalciferol
|
2 (3.6) |
47 (30.5) |
| mUFC, nmol/24 h [×ULN] |
|
|
| Mean (SD) |
1,412.0 (2,210.9)
[10.2 (16.0)] |
586.1 (693.1)
[4.2 (5.0)] |
| Median (min–max) |
520.8 (21.0–9,612.0)
[3.8 (0.2–69.7)] |
362.9 (36.0–5,490.0)
[2.6 (0.3–39.8)] |
| Size of adenoma, n (%) |
|
|
| Macroadenoma (≥10 mm) |
19 (33.9) |
29 (18.8) |
| Microadenoma (<10 mm) |
36 (64.3) |
122 (79.2) |
| Unknown |
1 (1.8) |
3 (1.9) |
ULN for mUFC is 138 nmol/24 h (50 μg/24 h)
Median (minimum–maximum) osilodrostat exposure from core study baseline to end of extension was 105 (1–194) weeks in patients of Asian origin and 96 (2–245) weeks in patients of non-Asian origin. Median average osilodrostat dose throughout the study was 3.8 (1–25) mg/day in Asian patients and 7.3 (1–47) mg/day in non-Asian patients. Mean daily osilodrostat dose and mean dose by body weight was numerically lower over time in patients of Asian origin than in patients of non-Asian origin (Supplementary Fig. 1). Following initial increases in mean (SD) osilodrostat daily dose in accordance with protocol from baseline to weeks 2 and 8, osilodrostat dose remained stable (Supplementary Fig. 1A).
Efficacy of osilodrostat treatment
The proportion of patients with mUFC ≤ ULN appeared to be similar for both groups during the core phases; high response rates were maintained during the extension. The proportion (95% confidence interval) with mUFC ≤ ULN at week 12 was 70.8% (55.9–83.0) in Asian patients and 73.7% (65.5–80.9) in non-Asian patients. At week 72, 68.1% (52.9–80.9) of Asian patients and 75.8% (67.3–83.0) of non-Asian patients had mUFC ≤ ULN (Fig. 3A).

Of patients with evaluable assessments at baseline (Asian, n = 44; non-Asian, n = 116), 4.5% and 1.7% of Asian and non-Asian patients, respectively, had both mUFC + LNSC controlled, and 79.5% and 87.1% had both mUFC + LNSC uncontrolled. At week 12 (Asian, n = 28; non-Asian, n = 85), 39.3% and 47.1% of Asian and non-Asian patients, respectively, had both mUFC + LNSC controlled, and 17.9% and 20.0% had both mUFC + LNSC uncontrolled (Fig. 3B and 3C). At week 72 (Asian, n = 31; non-Asian, n = 80), 58.1% and 45.0% of Asian and non-Asian patients, respectively, had both mUFC + LNSC controlled, and 12.9% and 8.8% had both mUFC + LNSC uncontrolled (Fig. 3B and 3C).
Mean (SD) mUFC levels decreased from 11.3 (16.9) × ULN at baseline (n = 48) to 0.6 (0.6) × ULN at week 12 (n = 43) in Asian patients. In non-Asian patients, mean mUFC levels also decreased, from 4.4 (5.2) × ULN at baseline (n = 137) to 0.8 (1.0) × ULN at week 12 (n = 126) (Fig. 4A). In terms of severity of disease at baseline, 14.3% of Asian and 31.8% of non-Asian patients had mild disease (mUFC <2 × ULN), 46.4% of Asian and 48.1% of non-Asian patients had moderate disease (mUFC 2–5 × ULN), and 39.3% of Asian and 20.1% of non-Asian patients had severe disease (mUFC >5 × ULN; Fig. 4B).

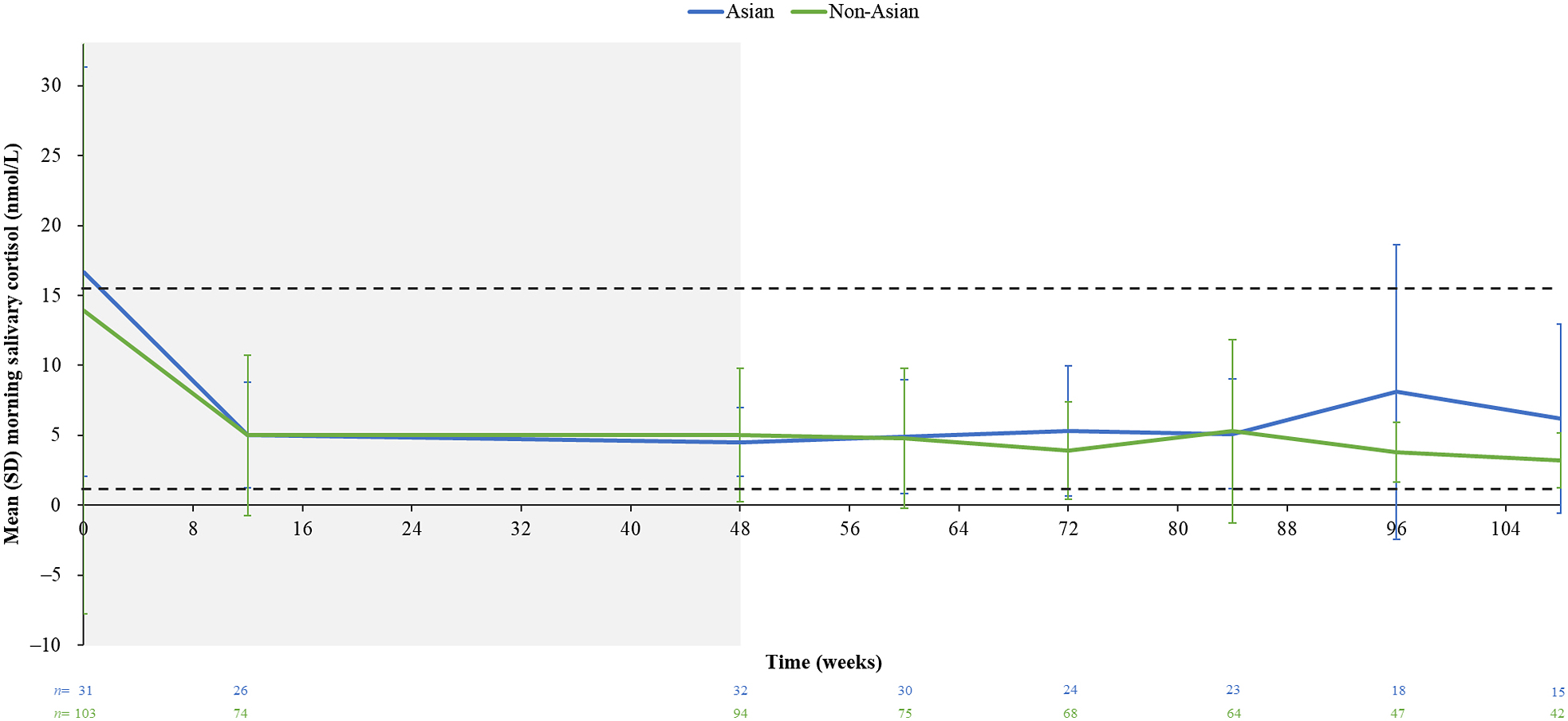
Mean (SD) serum cortisol levels decreased from baseline to week 12 in Asian (1.2 [0.5] [n = 47] and 0.5 [0.2] × ULN [n = 44], respectively) and non-Asian patients (1.0 [0.4] [n = 134] and 0.5 [0.3] × ULN [n = 126], respectively). Reductions in mean (SD) LNSC and morning salivary cortisol levels from baseline to week 12 were also observed in both groups: LNSC: Asian, baseline (n = 36), 5.4 (6.9) × ULN; week 12 (n = 28), 1.4 (1.0) × ULN; non-Asian, baseline (n = 99), 4.7 (8.5) × ULN; week 12 (n = 87), 1.4 (1.0) × ULN; morning salivary cortisol: Asian, baseline (n = 23), 1.2 (0.9) × ULN; week 12 (n = 26), 0.3 (0.2) × ULN; non-Asian, baseline (n = 86), 0.9 (1.5) nmol/L; week 12 (n = 74), 0.3 (0.4) × ULN. Mean serum cortisol levels remained within the normal range during long-term osilodrostat treatment, and mean LNSC levels remained ≥ULN in both groups (Fig. 4C and 4D). Mean morning salivary cortisol levels remained within the normal range during long-term treatment (Supplementary Fig. 2).
Changes in cardiovascular-related and metabolic parameters, physical manifestations of hypercortisolism, and patient-reported outcomes
Early improvements from baseline in cardiovascular-related and metabolic parameters were observed within the first 12 weeks of osilodrostat treatment in both groups, including reductions in FPG, HbA1c, SBP, DBP, waist circumference, weight, BMI and total cholesterol (Fig. 5). These improvements were maintained during long-term treatment. Triglycerides remained unchanged in both Asian and non-Asian patients (data not shown).
Most physical manifestations of hypercortisolism improved from baseline to week 12 in both Asian and non-Asian patients, and improvements were maintained up to week 72 (Supplementary Fig. 3). Improvements in muscle atrophy and hirsutism were observed during later stages of osilodrostat treatment in Asian patients than in non-Asian patients.
Improvements in mean CushingQoL and BDI-II scores were observed from baseline to week 48 and were maintained during long-term treatment in both groups (Supplementary Fig. 4).
Adverse events
The most common AEs observed in Asian patients were adrenal insufficiency (44.6%), nausea (33.9%), decreased appetite (26.8%) and headache (26.8%), and in non-Asian patients, they were nausea (45.5%), fatigue (40.9%) and headache (39.0%; Table 2). The most common grade 3/4 AE was hypertension, occurring in 12.5% of Asian patients and 11.7% of non-Asian patients (Table 2).
Table 2 Summary of adverse events in patients of Asian and non-Asian origin
| n (%) |
Asian patients* (N
= 56) |
Non-Asian patients* (N = 154) |
| All grades |
Grade 3/4 |
All grades |
Grade 3/4 |
| Any AE |
56 (100.0) |
31 (55.4) |
153 (99.4) |
81 (52.6) |
| AE leading to discontinuation |
13 (23.2) |
8 (14.3) |
21 (13.6) |
11 (7.1) |
| AEs requiring dose adjustment/interruption |
44 (78.6) |
16 (28.6) |
108 (70.1) |
39 (25.3) |
| AEs requiring additional therapy |
56 (100.0) |
25 (44.6) |
143 (92.9) |
58 (37.7) |
| Most common AEs (occurring in >15% of
patients) |
|
|
|
|
| Adrenal insufficiency |
25 (44.6) |
3 (5.4) |
34 (22.1) |
6 (3.9) |
| Nausea |
19 (33.9) |
— |
70 (45.5) |
4 (2.6) |
| Decreased appetite |
15 (26.8) |
— |
41 (26.6) |
1 (0.6) |
| Headache |
15 (26.8) |
1 (1.8) |
60 (39.0) |
6 (3.9) |
| Fatigue |
11 (19.6) |
1 (1.8) |
63 (40.9) |
5 (3.2) |
| Glucocorticoid deficiency |
11 (19.6) |
— |
17 (11.0) |
5 (3.2) |
| Arthralgia |
10 (17.9) |
— |
52 (33.8) |
5 (3.2) |
| Nasopharyngitis |
10 (17.9) |
— |
27 (17.5) |
1 (0.6) |
| Vomiting |
9 (16.1) |
1 (1.8) |
34 (22.1) |
4 (2.6) |
| Dizziness |
8 (14.3) |
— |
40 (26.0) |
— |
| Hypertension |
8 (14.3) |
7 (12.5) |
32 (20.8) |
18 (11.7) |
| Back pain |
7 (12.5) |
— |
32 (20.8) |
1 (0.6) |
| Increased blood testosterone |
7 (12.5) |
— |
27 (17.5) |
— |
| Myalgia |
7 (12.5) |
1 (1.8) |
31 (20.1) |
4 (2.6) |
| Urinary tract infection |
7 (12.5) |
— |
30 (19.5) |
3 (1.9) |
| Asthenia |
5 (8.9) |
— |
39 (25.3) |
3 (1.9) |
| Edema peripheral |
5 (8.9) |
— |
29 (18.8) |
— |
| Diarrhea |
4 (7.1) |
— |
40 (26.0) |
1 (0.6) |
| Abdominal pain |
3 (5.4) |
— |
27 (17.5) |
5 (3.2) |
| Influenza |
5 (8.9) |
1 (1.8) |
25 (16.2) |
— |
A patient with multiple severity grades for an AE is only counted under the maximum grade. *Excludes data collected for placebo recipients during the 12-week randomized period
Overall, 23.2% (n = 13/56) of Asian patients and 13.6% (n = 21/154) of non-Asian patients discontinued the study because of an AE (AEs leading to discontinuation in ≥2 patients: Asian: pituitary tumor benign, n = 4; malignant pituitary tumor, n = 2; pituitary tumor, n = 2; adrenal insufficiency, n = 2; VIth nerve paralysis, n = 2; non-Asian: adrenal insufficiency, n = 6; pituitary tumor, n = 4; pituitary tumor benign, n = 2; hypokalemia, n = 2; headache, n = 2).
The most common serious AEs (incidence >5%) in Asian patients were adrenal insufficiency (5.4%, n = 3), pituitary tumor (5.4%, n = 3) and pituitary tumor benign (5.4%, n = 3). In non-Asian patients, the most common serious AE was adrenal insufficiency (5.2%, n = 8).
Hypocortisolism-related AEs occurred in 58.9% of Asian patients and 40.3% of non-Asian patients, most commonly adrenal insufficiency (Asian, 44.6%; non-Asian, 22.1%). AEs related to hypocortisolism were less frequent during long-term treatment (weeks 48–72 and week 72 onwards) than during dose titration (first 12 weeks) in Asian and non-Asian patients (Supplementary Fig. 5). These AEs were mostly manageable with drug interruption (Asian, 50.0%; non-Asian, 35.7%) and/or glucocorticoid therapy (Asian, 25.0%; non-Asian, 20.1%).
AEs related to accumulation of adrenal hormone precursors occurred in 53.6% of Asian and 61.7% of non-Asian patients, most commonly acne (14.3%), hypertension (14.3%), increased blood testosterone (12.5%) and hypokalemia (12.5%) in Asian patients and hypertension (20.8%), peripheral edema (18.8%), increased blood testosterone (17.5%), hypokalemia (12.3%) and hirsutism (11.0%) in non-Asian patients. AEs related to accumulation of adrenal hormone precursors occurred in fewer patients during long-term treatment (weeks 48–72 and week 72 onwards) than during the dose-titration phases (first 12 weeks) in Asian and non‑Asian patients (Supplementary Fig. 5).
AEs related to pituitary tumor enlargement occurred in 21.4% of Asian and 9.1% of non-Asian patients, most commonly benign pituitary tumor (Asian patients, 12.5%; non-Asian patients, 3.9%). There were no trends observed in ACTH levels in patients who experienced an AE related to pituitary tumor enlargement (data not shown).
AEs related to arrhythmogenic potential and QT prolongation were infrequent throughout the study (Supplementary Fig. 5), occurring in 3.6% of Asian and 4.5% of non-Asian patients overall.
Throughout the study, 98.2% (n = 55/56) of Asian and 92.2% (n = 142/154) of non-Asian patients required additional therapy to manage AEs. Two other patients also required additional therapy to manage AEs but were not included in the analysis as the type of additional therapy needed was unknown. The most common concomitant medications used to manage AEs in >15% of patients requiring additional therapy were paracetamol (47.3%, n = 26/55) and spironolactone (16.4%, n = 9/55) in Asian patients and paracetamol (35.9%, n = 51/142), hydrocortisone (20.4%, n = 29/142) and ibuprofen (19.7%, n = 28/142) in non-Asian patients.
Changes in hormone levels
After an initial increase in mean (SD) testosterone levels from 1.8 (1.5) and 1.0 (0.7) nmol/L at baseline to 3.4 (3.4) and 2.3 (2.0) nmol/L at week 48 in Asian and non-Asian female patients, respectively, levels decreased over long-term treatment (week 72: 2.9 [2.5] and 2.1 [1.9] nmol/L; Supplementary Fig. 6A). In Asian and non-Asian male patients, mean testosterone levels increased from baseline to week 12 but remained within the normal range during treatment (Supplementary Fig. 6B). Mean (SD) ACTH levels increased from 32.3 (62.5) pmol/L at baseline to 153.8 (284.0) pmol/L at week 72 in Asian patients. In non-Asian patients, mean (SD) ACTH levels also increased, from 12.7 (10.0) pmol/L at baseline to 49.3 (71.6) pmol/L at week 72 (Supplementary Fig. 6C). In Asian and non-Asian patients, respectively, mean 11-deoxycortisol and 11-deoxycorticosterone levels increased from baseline to week 48 and stabilized up to week 72 (Supplementary Fig. 6D and 6E). Potassium levels remained unchanged and within the normal range during treatment (Supplementary Fig. 6F). Changes in aldosterone, estradiol, DHEAS and estrone are shown in Supplementary Fig. 6G–M.
Median (minimum–maximum) tumor volume in Asian and non-Asian patients, respectively, was 83.0 (4.6–1,265.5; n = 43) and 78.6 (10.0–6,112.9; n = 86) mm3 at baseline and 69.4 (21.0–1,513.0; n = 29) and 77.2 (8.2–3,638.0; n = 53) mm3 at week 72.
In patients with a microadenoma at baseline, median (minimum–maximum) tumor volume at baseline and week 72, respectively, was 84.5 (4.6–244.6; n = 29) and 68.2 (21.1–1,513.0; n = 18) mm3 in Asian patients and 60.0 (10.0–1,370.8; n = 62) and 68.0 (8.2–745.3; n = 41) mm3 in non-Asian patients.
In patients with a macroadenoma at baseline, median (minimum–maximum) tumor volume at baseline and week 72, respectively, was 78.2 (26.0–1,265.5; n = 13) and 100.4 (21.0–1,102.9; n = 11) mm3 in Asian patients and 177.5 (18.0–6,112.9; n = 24) and 139.0 (13.7–3,638.0; n = 12) mm3 in non-Asian patients.
A similar proportion of Asian and non-Asian patients with evaluable assessments had either a ≥20% decrease or a ≥20% increase in tumor volume during the study period (Fig. 6).

Fig. 6
Proportion of patients with a ≥20% decrease, no change, or a ≥20% increase in tumor volume during the study period
PK analyses performed in Asian and non-Asian patients enrolled in LINC 3 showed that mean osilodrostat maximal plasma concentration (Cmax) and trough plasma concentration (Ctrough) were numerically higher in Asian patients than in non-Asian patients at the same osilodrostat dose (Fig. 7).

Fig. 7
Summary of Cmax and Ctrough after a 2 mg incident osilodrostat dose in patients of Asian and non-Asian origin
Furthermore, PK analyses in a Phase I study of healthy Japanese and Caucasian subjects (study A2102) showed that osilodrostat bioavailability (Cmax and AUC) was also numerically higher in Japanese subjects than in Caucasian subjects at osilodrostat doses ranging from 0.5 to 2.0 mg/day (Supplementary Table 1A). Similar results were observed when the data were adjusted for body weight (Supplementary Table 1B).
Discussion
To our knowledge, this is the only study to date evaluating the effects of a medical treatment for Cushing’s disease separately in patients of Asian and non-Asian origin. Data from this pooled analysis of LINC 3 and LINC 4 show that osilodrostat provided rapid and sustained reductions in mUFC alongside improvements in clinical parameters of hypercortisolism in patients of Asian and non-Asian origin. Osilodrostat was generally well tolerated in both patient groups, with efficacy and safety outcomes consistent with those from the parent studies [8-11]. However, there were some differences between the two groups.
Overall, Asian patients generally required numerically lower osilodrostat doses to achieve mUFC control than non-Asian patients. One possible reason for this could be the numerically higher relative osilodrostat bioavailability in patients of Asian origin than for other ethnicities, as shown in both LINC 3 and study A2102. Data show that body weight is not a major determinant of the differences in osilodrostat bioavailability between patients of Asian origin and other ethnicities. In view of the numerically higher osilodrostat bioavailability in patients of Asian origin than for other ethnicities, a lower starting dose of 1 mg bid in Asian patients is recommended in the manufacturer’s label [16]. Another difference between Asian and non-Asian patients was the frequency of some AEs; for example, adrenal insufficiency was reported in a numerically higher proportion of Asian than non-Asian patients (44.6% vs. 22.1%), whereas fatigue and arthralgia were reported in more non-Asian than Asian patients (40.9% vs. 19.6% and 33.8% vs. 17.9%, respectively). Furthermore, the incidence of macroadenomas at baseline was numerically higher in Asian patients than in non-Asian patients. This may explain, in part, the numerically higher baseline UFC levels in Asian patients, which may have resulted in more pronounced symptoms of glucocorticoid withdrawal as cortisol levels decreased during treatment. There may also have been differences between countries in the use of terminology to report AEs relating to hypocortisolism, including the preferred term of adrenal insufficiency, for which there was no protocol-mandated requirement for clinical diagnosis. It is possible that some of the cases reported as adrenal insufficiency were, in fact, glucocorticoid withdrawal syndrome; however, clinical data to establish a diagnosis (e.g., morning serum cortisol levels) were not available for most patients, so it was not possible to differentiate between adrenal insufficiency and glucocorticoid withdrawal syndrome. Findings from a Phase II study of osilodrostat in Japanese patients with non-pituitary Cushing’s syndrome also suggest that Asian patients could be at higher risk of hypocortisolism-related events, such as adrenal insufficiency and glucocorticoid withdrawal syndrome, than non-Asian patients [17]. Further understanding of these differences will allow more informed decisions on management when considering patients of different racial backgrounds.
Individual patient mUFC data show that 39.3% of Asian and 20.1% of non-Asian patients had severe disease at baseline (mUFC >5 × ULN). However, mean mUFC decreased to within the normal range in both groups, and reductions were sustained over long-term treatment. Furthermore, morning serum and salivary cortisol levels normalized and LNSC levels decreased from high baseline levels in Asian and non-Asian patients. Reasons why mean LNSC levels remained above the ULN in both Asian and non-Asian patients, but morning salivary cortisol levels normalized, are unknown and require further investigation; one possible explanation could be because dose-titration and -adjustment decisions in the LINC clinical trials were aimed at mUFC normalization rather than LNSC normalization. However, in clinical practice, osilodrostat dosing is flexible [1, 5, 7, 18], which may help achieve normalization of LNSC as well as mUFC. Furthermore, the number of patients with available LNSC assessments was limited, particularly during the extension phases; therefore, the data should be interpreted with caution. Overall, osilodrostat treatment can lead to reduction and/or normalization of mUFC, regardless of disease severity. LNSC reflects normal circadian rhythm of corticotropin-releasing hormone (CRH), ACTH, and cortisol pulses and is a more sensitive test for detecting recurrence of Cushing’s syndrome than mUFC. Increased LNSC levels are detectable in patients with disease recurrence, even if mUFC levels are normal [7, 18, 19]; conversely, it is assumed that during medical therapy, mUFC normalization is achieved before that of LNSC. Thus, if mUFC but not LNSC is normalized, normal circadian rhythm, with the lowest cortisol levels at night, is not yet restored. Data from previous studies of patients with Cushing’s disease treated with medical therapy, including a pooled analysis of patients from LINC 3 and LINC 4 treated with osilodrostat, show that treatment outcomes may be better in patients in whom both mUFC and LNSC are controlled, compared with patients who have mUFC control alone [19-21]. Further studies are needed to evaluate the importance of normalizing both mUFC and LNSC and restoring normal circadian rhythm, and to determine whether there are any differences in dual mUFC and LNSC normalization rates and the resultant effects on treatment outcomes between Asian and non-Asian patients.
Improvements in cardiovascular and metabolic-related parameters and physical manifestations of hypercortisolism that were observed during the core phase were maintained during long-term treatment in both groups. Improvements in muscle atrophy and hirsutism in females appeared to be observed later in Asian patients than in non-Asian patients. Although the mechanisms behind this are unknown, there are several factors that may explain the differences between the two groups, including: differences in duration of persistent hypercortisolism prior to medical therapy; severity of clinical and physical manifestations of hypercortisolism at baseline [22, 23]; diet (including protein intake) [24, 25]; exercise [26]; regional differences in healthcare practices (access to gyms, physical therapy); and differences in how photographs of physical manifestations of hypercortisolism were assessed locally, given that this assessment was subjective. However, further investigation is warranted to better understand the differences between the two groups. In addition, quality-of-life scores also improved in both Asian and non-Asian patients during osilodrostat treatment, demonstrating that osilodrostat can reduce the burden of disease in patients with Cushing’s disease across different races.
Osilodrostat was generally well tolerated in Asian and non-Asian patients. AEs of special interest were less common during long-term treatment (week 48 onwards) than during dose-titration periods and were mostly manageable without treatment discontinuation.
Although treatment discontinuation because of AEs was low, it appeared that a numerically higher proportion of Asian patients discontinued treatment because of an AE than non-Asian patients, possibly because these AEs were reported in more Asian than non-Asian patients. However, there is no further explanation as to why there were differences in discontinuation rates between the two patient groups.
AEs related to hypocortisolism were reported in more Asian than non-Asian patients (58.9% vs. 40.3%, respectively). One potential reason for this could be the high relative bioavailability of osilodrostat in Asian compared with non-Asian patients, as supported by the PK data in LINC 3 (Fig. 7) and study A2102 (Supplementary Table 1). This may have resulted in a rapid decrease in cortisol, leading to symptoms of glucocorticoid withdrawal syndrome that were reported as hypocortisolism-related AEs [27]. It is possible that Asian patients have a different metabolic profile than non-Asian patients that affects the rate of metabolism and/or absorption of osilodrostat. For example, Asian patients may have higher or lower levels of certain enzymes involved in the cortisol synthesis pathway and in the metabolism of osilodrostat. As such, Asian patients may be more sensitive to osilodrostat or have higher osilodrostat bioavailability than non-Asian patients. Furthermore, differences in the steroidogenic enzyme profile in Asian and non-Asian patients could lead to the apparent increased risk of hypocortisolism-related AEs in Asian patients. Additional research is needed to understand the mechanisms of osilodrostat bioavailability in Asian and non-Asian patients. Another potential reason for the larger number of hypocortisolism-related AEs reported in Asian patients than in non-Asian patients could be differences in the reporting of hypocortisolism-related AEs between countries as these AEs were as reported by the investigator.
Similarly, a numerically higher proportion of AEs related to pituitary tumor enlargement were reported in Asian than in non-Asian patients (21.4% vs. 9.1%, respectively). This could be because of the numerically higher incidence of macroadenomas at baseline in Asian than in non-Asian patients. ACTH levels increased over time in Asian and non-Asian patients; however, there were no trends observed in ACTH levels in patients who experienced an AE related to pituitary tumor enlargement. Several studies have reported that tumor volume is not a major determinant of hormone secretion or clinical outcomes in patients with Cushing’s disease [28-30]. The absence of a trend between tumor volume and ACTH could be related to a lack of sensitivity in glucocorticoid feedback mechanisms; macroadenomas tend to show low ACTH bioactivity, whereas microadenomas tend to have very potent ACTH bioactivity [31]. However, further research is needed to elucidate the mechanisms of ACTH bioactivity. ACTH secretion from the anterior pituitary gland is stimulated by release of CRH from the hypothalamus. Although CRH was not evaluated in this analysis, further research on the effects of CRH on tumor volume in patients with Cushing’s disease, including differences between Asian and non-Asian patients, would be of interest.
Despite the apparent greater occurrence of AEs related to pituitary tumor enlargement in Asian patients, a small reduction in median tumor volume was observed in this cohort from baseline (83.0 mm3) to week 72 (69.4 mm3). One possible reason for this could be that most Asian patients (64.3%) had a microadenoma at baseline. This observation is consistent with findings of a study evaluating long-term changes in 177 patients with pituitary microadenomas, in whom 2/3 microadenomas remained unchanged or decreased in size [32]. However, there was a potential trend toward more Asian than non-Asian patients having a ≥20% increase in tumor volume from baseline to week 72 (44.8% vs. 34.6%, respectively), which requires further evaluation.
Accumulation of adrenal hormone precursors is expected based on the mechanism of action of osilodrostat [16]. AEs related to accumulation of adrenal hormone precursors were reported in 53.6% of Asian and 61.7% of non-Asian patients. These AEs were more common during the dose-titration phases than during long-term treatment (48 weeks onwards) and were mostly manageable with additional therapy. Levels of adrenal hormone precursors increased from baseline to week 48 but stabilized during long-term treatment in both Asian and non-Asian patients. AEs related to accumulation of adrenal hormone precursors have also been reported with other steroidogenesis inhibitors; therefore, close monitoring of patients on all adrenal-blocking medications, including osilodrostat, is important to allow early detection of, and prompt intervention to manage, these AEs [7].
Mean testosterone levels in all female patients, regardless of racial background, increased from baseline to week 48 and decreased toward baseline levels during long-term treatment. This observation is consistent with that described in the parent studies [8-11]. The reduction in testosterone levels in females during long-term treatment was accompanied by improvements in hirsutism. Testosterone levels in male patients increased from baseline to week 12 and remained within the normal range thereafter. During the study, testosterone levels were numerically higher in non-Asian male patients than in Asian male patients. Previous studies have shown that testosterone levels in young, healthy males of Asian origin are lower than in healthy males of other racial origins [33, 34]. Future studies on gonadal recovery rates in males following cortisol control may be of interest.
One potential reason for the reductions in testosterone levels and the fact that AEs related to adrenal hormone precursors were less frequent over long-term treatment could be related to osilodrostat targeting multiple steroidogenic enzymes with differing levels of specificity, potentially reducing the action of androgen precursors over time [35]. However, further research is required to understand this mechanism.
Findings from this pooled analysis are similar to those reported in a Phase II study of osilodrostat in Japanese patients with non-Cushing’s disease Cushing’s syndrome, in which osilodrostat led to reductions in mUFC in all patients, with >80% reduction observed in 6/7 patients at week 12 [17]. Hypocortisolism-related AEs were reported in 7/9 patients, were mostly mild or moderate in severity, and were managed by dose interruption/adjustments and/or glucocorticoid therapy; no patients discontinued treatment because of these AEs [17].
There are limitations to this analysis, including the differences in study designs between LINC 3 and LINC 4. LINC 3 included an 8-week randomized-withdrawal phase after an initial open-label period, whereas LINC 4 included an initial 12-week, double-blind, randomized, placebo-controlled period followed by an open-label period. Therefore, periods during which patients received placebo were excluded from the analysis. Furthermore, not all time points were available in both studies for some of the assessments, limiting data reporting for some parameters. Another limitation of this analysis was the heterogeneous population of Asian patients and the small number of patients enrolled from each country.
Conclusions
Osilodrostat demonstrated beneficial effects in patients of Asian and non-Asian origin in terms of mUFC control and improvements in cardiovascular and metabolic-related parameters, physical manifestations of hypercortisolism, and quality of life. Patients of Asian origin achieved mUFC control at numerically lower doses, which may be explained by the numerically higher bioavailability of osilodrostat in Asian patients than in non-Asian patients. Although AEs related to hypocortisolism and pituitary tumor enlargement were reported in more Asian than non-Asian patients, osilodrostat was generally well tolerated in both patient groups. Numerically lower doses of osilodrostat required to achieve mUFC control and more common reporting of hypocortisolism-related AEs suggest that Asian patients are more sensitive to osilodrostat than non-Asian patients. Improvements in muscle atrophy and hirsutism were observed during later stages of osilodrostat treatment in Asian patients than in non-Asian patients. Osilodrostat is an effective and well tolerated treatment option for patients of Asian and non-Asian origin.
Author Contributions
The studies were designed and funded by Novartis Pharma AG, with endorsement from the LINC 3 and LINC 4 steering committees (BMKB, MF, AS, RP, PJS). Statistical support for these analyses were funded by Recordati. BMKB, MF, RP, EJL, RL, JHK, RW, YY, ZL and PJS enrolled patients into the studies. Data were collected by investigators of the LINC 3 and LINC 4 study groups using the funder’s data management systems. AP and Recordati’s statistical team analyzed the data. A data-sharing and kick-off meeting was held for all authors and an outline prepared by a professional medical writer based on interpretation provided by the authors. Each new draft of the manuscript subsequently prepared by the medical writer was reviewed and revised in line with direction and feedback from all authors. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank all investigators, nurses, study coordinators and patients who participated in the LINC clinical trial program. We thank Beth Harrahill, BSc (Hons), MRes, Mudskipper Business Ltd, for medical editorial assistance with this manuscript. The program was funded by Novartis Pharma AG; however, as of July 12, 2019, osilodrostat is an asset of Recordati.
Disclosures
AS reports serving as a speaker and consultant for Recordati; he was also a member of the LINC 3 steering committee. BMKB reports occasional consulting honoraria from Lundbeck H/S, Neurocrine, Recordati Rare Diseases, Sparrow, and Xeris Pharmaceuticals (Strongbridge); she served on the LINC 3 steering committee and is also a member of the Editorial Board of Endocrine Journal. MF reports grants to her university and occasional scientific consulting fees from Recordati Rare Diseases, Sparrow, and Xeris Pharmaceuticals (Strongbridge); she was a member of the LINC 3 steering committee. RP has received research funding from Recordati, Corcept Therapeutics, Strongbridge Biopharma, and Neurocrine Biosciences and occasional consulting honoraria from Corcept Therapeutics, Recordati, Crinetics Pharmaceuticals, and H Lundbeck A/S. EJL, RL, JHK, RW, YY, and ZL have no conflicts of interest to disclose. AP is an employee of Recordati. AMP was an employee of Recordati when the analyses were conducted. PJS reports consultancy for Teva Pharmaceuticals.
Data Sharing Statement
The datasets generated and analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request. Recordati Rare Diseases will share the complete de-identified patient dataset, study protocol, statistical analysis plan, and informed consent form upon request, effective immediately following publication, with no end date.
Supplementary Table 1 (A) PK of osilodrostat (day 14) and (B) PK of osilodrostat (day 14) with body weight adjustment in healthy Japanese and Caucasian subjects
| (A) |
|
Osilodrostat dose
(mg/day) |
Geometric mean |
Geometric mean ratio |
|
|
Japanese |
Caucasian |
Estimate |
90% CI |
| Cmax, ng/mL |
0.5 |
1.31 |
1.03 |
1.27 |
1.01–1.59 |
| 1.0 |
2.45 |
1.93 |
1.27 |
1.01–1.59 |
| 2.0 |
5.56 |
4.63 |
1.20 |
0.97–1.49 |
| AUC0–12h, ng·h/mL |
0.5 |
6.14 |
4.73 |
1.30 |
0.98–1.72 |
| 1.0 |
12.52 |
9.93 |
1.26 |
0.93–1.70 |
| 2.0 |
29.92 |
20.31 |
1.47 |
1.12–1.94 |
| AUC12–24h, ng·h/mL |
0.5 |
5.26 |
4.14 |
1.27 |
0.95–1.69 |
| 1.0 |
11.31 |
8.89 |
1.27 |
0.94–1.72 |
| 2.0 |
28.10 |
16.72 |
1.68 |
1.27–2.22 |
| (B) |
|
Osilodrostat dose
(mg/day) |
Geometric mean |
Geometric mean ratio |
|
|
Japanese |
Caucasian |
Estimate |
90% CI |
| Cmax, ng/mL |
0.5 |
1.29 |
1.04 |
1.25 |
0.99–1.57 |
| 1.0 |
2.45 |
1.94 |
1.26 |
1.01–1.58 |
| 2.0 |
5.45 |
4.71 |
1.16 |
0.92–1.46 |
| AUC0–12h, ng·h/mL |
0.5 |
6.17 |
4.72 |
1.31 |
0.98–1.75 |
| 1.0 |
12.52 |
9.90 |
1.27 |
0.93–1.72 |
| 2.0 |
30.13 |
20.16 |
1.49 |
1.11–2.02 |
| AUC12–24h, ng·h/mL |
0.5 |
5.35 |
4.09 |
1.31 |
0.98–1.75 |
| 1.0 |
11.34 |
8.77 |
1.29 |
0.95–1.75 |
| 2.0 |
28.98 |
16.22 |
1.79 |
1.32–2.41 |
Data are from study A2102, in which 83 healthy subjects (Japanese, n = 43; Caucasian, n = 40) were treated with osilodrostat for 2 weeks. In Japanese subjects, mean (SD) body weight was 65.7 (7.19), 68.8 (7.89) and 64.6 (7.22) kg, respectively, in the 0.5, 1.0 and 2.0 mg/day osilodrostat groups; corresponding values in Caucasian subjects were 70.7 (6.63), 71.3 (5.61) and 74.7 (8.91) kg, respectively. AUC0–12h, area under the concentration–time curve between 0 and 12 hours; AUC12–24h, area under the concentration–time curve between 12 and 24 hours; CI, confidence interval; Cmax, time to maximal plasma concentration; PK, pharmacokinetics; SD, standard deviation

References
- 1 Gadelha M, Gatto F, Wildemberg LE, Fleseriu M (2023) Cushing’s syndrome. Lancet 402: 2237–2252.
- 2 Puglisi S, Perini AME, Botto C, Oliva F, Terzolo M (2024) Long-term consequences of Cushing’s syndrome: a systematic literature review. J Clin Endocrinol Metab 109: e901–e919.
- 3 Reincke M, Fleseriu M (2023) Cushing syndrome: a review. JAMA 330: 170–181.
- 4 Hsiao HP, Iglesias ML, Keil MF, Boikos S, Robinson-White A, et al. (2007) Differences in cortisol levels and body mass index between East Asians and Caucasians with Cushing’s syndrome: an ‘East Asian’ phenotype for Cushing syndrome. Clin Endocrinol (Oxf) 66: 753–755.
- 5 Fleseriu M, Varlamov EV, Hinojosa-Amaya JM, Langlois F, Melmed S (2023) An individualized approach to the management of Cushing disease. Nat Rev Endocrinol 19: 581–599.
- 6 Tritos NA, Biller BMK (2019) Current management of Cushing’s disease. J Intern Med 286: 526–541.
- 7 Fleseriu M, Auchus R, Bancos I, Ben-Shlomo A, Bertherat J, et al. (2021) Consensus on diagnosis and management of Cushing’s disease: a guideline update. Lancet Diabetes Endocrinol 9: 847–875.
- 8 Fleseriu M, Newell-Price J, Pivonello R, Shimatsu A, Auchus RJ, et al. (2022) Long-term outcomes of osilodrostat in Cushing’s disease: LINC 3 study extension. Eur J Endocrinol 187: 531–541.
- 9 Gadelha M, Bex M, Feelders RA, Heaney AP, Auchus RJ, et al. (2022) Randomized trial of osilodrostat for the treatment of Cushing disease. J Clin Endocrinol Metab 107: e2882–e2895.
- 10 Gadelha M, Snyder PJ, Witek P, Bex M, Belaya Z, et al. (2023) Long-term efficacy and safety of osilodrostat in patients with Cushing’s disease: results from the LINC 4 study extension. Front Endocrinol (Lausanne) 14: 1236465.
- 11 Pivonello R, Fleseriu M, Newell-Price J, Bertagna X, Findling J, et al. (2020) Efficacy and safety of osilodrostat in patients with Cushing’s disease (LINC 3): a multicentre Phase III study with a double-blind, randomised withdrawal phase. Lancet Diabetes Endocrinol 8: 748–761.
- 12 Fleseriu M, Biller BMK, Bertherat J, Young J, Hatipoglu B, et al. (2022) Long-term efficacy and safety of osilodrostat in Cushing’s disease: final results from a Phase II study with an optional extension phase (LINC 2). Pituitary 25: 959–970.
- 13 Fleseriu M, Pivonello R, Elenkova A, Salvatori R, Auchus RJ, et al. (2019) Efficacy and safety of levoketoconazole in the treatment of endogenous Cushing’s syndrome (SONICS): a Phase 3, multicentre, open-label, single-arm trial. Lancet Diabetes Endocrinol 7: 855–865.
- 14 Nieman L, Boscaro M, Scaroni C, Deutschbein T, Mezosi E, et al. (2021) Metyrapone treatment in endogenous Cushing’s syndrome. Long term efficacy and safety results of the extension of the Phase III/IV study PROMPT. Endocrine Abstracts 73: abst OC3.3.
- 15 Fleseriu M, Auchus RJ, Greenman Y, Zacharieva S, Geer EB, et al. (2022) Levoketoconazole treatment in endogenous Cushing’s syndrome: extended evaluation of clinical, biochemical, and radiologic outcomes. Eur J Endocrinol 187: 859–871.
- 16 Pivonello R, Simeoli C, Paola ND, Larocca A, Crescenzo EM, et al. (2024) Osilodrostat: a novel potent inhibitor of 11-beta-hydroxylase for the treatment of Cushing’s syndrome. touchREV Endocrinol 20: 43–51.
- 17 Tanaka T, Satoh F, Ujihara M, Midorikawa S, Kaneko T, et al. (2020) A multicenter, Phase 2 study to evaluate the efficacy and safety of osilodrostat, a new 11β-hydroxylase inhibitor, in Japanese patients with endogenous Cushing’s syndrome other than Cushing’s disease. Endocr J 67: 841–852.
- 18 Fleseriu M, Biller BMK (2022) Treatment of Cushing’s syndrome with osilodrostat: practical applications of recent studies with case examples. Pituitary 25: 795–809.
- 19 Biller BMK, Fleseriu M, Pivonello R, Feelders R, Gadelha M, et al. (2023) Clinical improvements in patients with Cushing’s disease treated with osilodrostat according to urinary and late-night salivary cortisol levels: pooled analysis from LINC 3 and LINC 4. J Endocr Soc 7: THU041.
- 20 Findling JW, Fleseriu M, Newell-Price J, Petersenn S, Pivonello R, et al. (2016) Late-night salivary cortisol may be valuable for assessing treatment response in patients with Cushing’s disease: 12-month, Phase III pasireotide study. Endocrine 54: 516–523.
- 21 Newell-Price J, Fleseriu M, Pivonello R, Feelders R, Gadelha M, et al. (2023) Pooled analysis from two osilodrostat Phase III studies in Cushing’s disease (LINC 3 and LINC 4): clinical improvements according to urinary and late-night salivary cortisol levels. Endocrine Abstracts 90: P408.
- 22 Misra A, Khurana L (2009) The metabolic syndrome in South Asians: epidemiology, determinants, and prevention. Metab Syndr Relat Disord 7: 497–514.
- 23 Deurenberg P, Deurenberg-Yap M, Guricci S (2002) Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev 3: 141–146.
- 24 El-Salhy M, Patcharatrakul T, Gonlachanvit S (2021) The role of diet in the pathophysiology and management of irritable bowel syndrome. Indian J Gastroenterol 40: 111–119.
- 25 LeCroy MN, Stevens J (2017) Dietary intake and habits of South Asian immigrants living in Western countries. Nutr Rev 75: 391–404.
- 26 Shen L, Meng X, Zhang Z, Wang T (2018) Physical exercise for muscle atrophy. Adv Exp Med Biol 1088: 529–545.
- 27 He X, Findling JW, Auchus RJ (2022) Glucocorticoid withdrawal syndrome following treatment of endogenous Cushing syndrome. Pituitary 25: 393–403.
- 28 Akirov A, Shimon I, Fleseriu M, Dotan I, Manisterski Y, et al. (2022) Clinical study and systematic review of pituitary microadenomas vs. macroadenomas in Cushing’s disease: does size matter? J Clin Med 11: 1558.
- 29 Hwang YC, Chung JH, Min YK, Lee MS, Lee MK, et al. (2009) Comparisons between macroadenomas and microadenomas in Cushing’s disease: characteristics of hormone secretion and clinical outcomes. J Korean Med Sci 24: 46–51.
- 30 Machado MC, Alcantara AE, Pereira AC, Cescato VA, Castro Musolino NR, et al. (2016) Negative correlation between tumour size and cortisol/ACTH ratios in patients with Cushing’s disease harbouring microadenomas or macroadenomas. J Endocrinol Invest 39: 1401–1409.
- 31 Gibson S, Ray DW, Crosby SR, Dornan TL, Jennings AM, et al. (1996) Impaired processing of proopiomelanocortin in corticotroph macroadenomas. J Clin Endocrinol Metab 81: 497–502.
- 32 Hordejuk D, Cheung YM, Wang W, Smith T, Laws E, et al. (2023) Long-term changes in the size of pituitary microadenomas. Ann Intern Med 176: 298–302.
- 33 Biswas M, Hampton D, Turkes A, Newcombe RG, Aled Rees D (2010) Reduced total testosterone concentrations in young healthy South Asian men are partly explained by increased insulin resistance but not by altered adiposity. Clin Endocrinol (Oxf) 73: 457–462.
- 34 Xu L, Au Yeung SL, Kavikondala S, Leung GM, Schooling CM (2014) Testosterone concentrations in young healthy US versus Chinese men. Am J Hum Biol 26: 99–102.
- 35 Bonnet-Serrano F, Poirier J, Vaczlavik A, Laguillier-Morizot C, Blanchet B, et al. (2022) Differences in the spectrum of steroidogenic enzyme inhibition between osilodrostat and metyrapone in ACTH-dependent Cushing syndrome patients. Eur J Endocrinol 187: 315–322.