Abstract
A 67-year-old man with type 1 diabetes, Cronkhite-Canada syndrome, and membranous nephropathy who received insulin therapy was admitted to our hospital with right hemiplegia and dysarthria. Brain magnetic resonance imaging revealed a lesion with a high diffusion-weighted imaging signal and low apparent diffusion coefficient signal in the posterior limb of the left internal capsule. He was hypoglycemic with a blood glucose level of 56 mg/dL (3.1 mmol/L). Following glucose administration, the patient’s symptoms resolved within several hours. The patient experienced similar transient hypoglycemic hemiplegia at midnight, three times within 10 days. In a literature review of 170 cases of hypoglycemic hemiplegia, 26 cases of recurrent hemiplegia were investigated. Recurrent hypoglycemic hemiplegia occurs more frequently on the right side than on the left side, and most recurrences occur within approximately a week, almost exclusively at midnight and in the early morning. We speculate that hypoglycemia-associated autonomic failure may be involved in the nocturnal recurrence of episodes. In our patient, depleted endogenous insulin secretion and lipodystrophy at the injection site, may have acted as additional factors, leading to severe hypoglycemia despite the absence of apparent autonomic neuropathy. Clinically, it is important to recognize hypoglycemia as a cause of hemiplegia to avoid unnecessary intervention and to maintain an appropriate blood glucose level at midnight and early in the morning to prevent recurrent hypoglycemic hemiplegia.
IN 1928, Ravid reported that hypoglycemia causes transient hemiplegia as a neurological symptom [1]. Hypoglycemic hemiplegia has been documented in patients with diabetes, insulinoma, and other conditions; however, it is sometimes overlooked, leading to unnecessary interventions such as antiplatelet therapy [2]. Diffusion-weighted magnetic resonance imaging (DWI) of the head has been reported to show high signals in the posterior limb of the internal capsule and corpus callosum [2]. Selective vulnerability of lesions to hypoglycemia, cerebral vasospasm, and underlying cerebrovascular disease are among the potential mechanisms underlying the disease [3]. Repeated hemiplegia due to hypoglycemia has also been reported; however, the characteristics of these recurrent cases have not been thoroughly investigated. Here, we present the case of a patient with type 1 diabetes who experienced repeated nocturnal hypoglycemic hemiplegia three times within 10 days. We also reviewed the literature on recurrent hypoglycemic hemiplegia.
Case Report
A 67-year-old man sought medical advice at our hospital for incomplete right hemiplegia and dysarthria. He had a history of ileal pseudotumor, Cronkhite-Canada syndrome (CCS), and membranous nephropathy (MN). The patient had no family history of diabetes mellitus. He was diagnosed with diabetes at the age of 30 years and was started on insulin therapy by a general practitioner. At 60 years of age, he consulted a diabetes specialist and was found to have a high anti-glutamic acid decarboxylase (GAD) antibody titer and was diagnosed with slowly progressive insulin-dependent (type 1) diabetes mellitus. At the age of 63 years, marked hyperglycemia and hypoglycemia became prominent, and glycated hemoglobin (HbA1c) remained in the 8–9% range, with a total daily insulin dosage of 79 units (15 units of long-acting insulin and 64 units of rapid-acting insulin). The patient regularly monitored his blood glucose levels and adjusted insulin dose accordingly. His blood glucose levels exhibited significant fluctuations, including episodes of both hypoglycemia and hyperglycemia, with readings as high as 500 mg/dL (27.8 mmol/L). He occasionally had hypoglycemic episodes following light exertion while shopping; however, hypoglycemia occurred most often without specific triggers. He usually had symptoms, such as sweating, during hypoglycemia and did not experience loss of consciousness due to hypoglycemia. The presence of decreased vibration sensation and absence of the Achilles tendon reflex indicated that he had diabetic neuropathy. However, there was no apparent autonomic dysfunction, as evidenced by the coefficient of variation of R-R intervals measured at 11.04% and the absence of orthostatic hypotension during the simple Schellong test. The patient did not present diabetic retinopathy or nephropathy.
Six days before admission, the patient experienced pararthria, weakness, and paresthesia in the right upper and lower limbs at 4 am, which resolved spontaneously in the morning (blood glucose levels were not measured). Six days later, he developed weakness in the right upper and lower limbs again at 2 am and visited the emergency department of our hospital. Physical examination revealed dysarthria, weakness (manual muscle test 4/5), hypoesthesia in the right upper and lower limbs, right facial nerve paralysis with positive Barre’s sign, positive Mingazzini sign, and an NIH stroke scale score of 6. His height and body weight were 170.5 cm and 64.6 kg, respectively (body mass index, 22.2 kg/m2). His blood pressure was 181/101 mmHg and heart rate was regular at 69 beats/min, body temperature was 36.4°C, and he had clear consciousness with no abnormal heart or respiratory sounds. Brain computed tomography revealed no obvious hemorrhagic lesions or early signs of cerebral infarction. Magnetic resonance imaging revealed a lesion with a high DWI signal and low apparent diffusion coefficient signal in the posterior limb of the left internal capsule (Fig. 1). Magnetic resonance angiography of the brain revealed no significant stenosis. Carotid echocardiography showed an isoechoic plaque 2.3 mm thickness and 9.2 mm length in the left common carotid artery without significant stenosis. Laboratory tests revealed a blood glucose level of 56 mg/dL (3.1 mmol/L), blood urea nitrogen of 17.5 mg/dL, creatinine of 1.24 mg/dL, estimated glomerular filtration rate (eGFR) of 45.87 mL/min/1.73 m2 and hemoglobin of 12.1 g/dL. His HbA1c was 8.3%, and serum C-peptide level was below detection limit (<0.02 ng/mL). No obvious abnormalities were observed in the coagulation system or in other parameters. Hypoglycemia was immediately corrected by oral glucose administration.
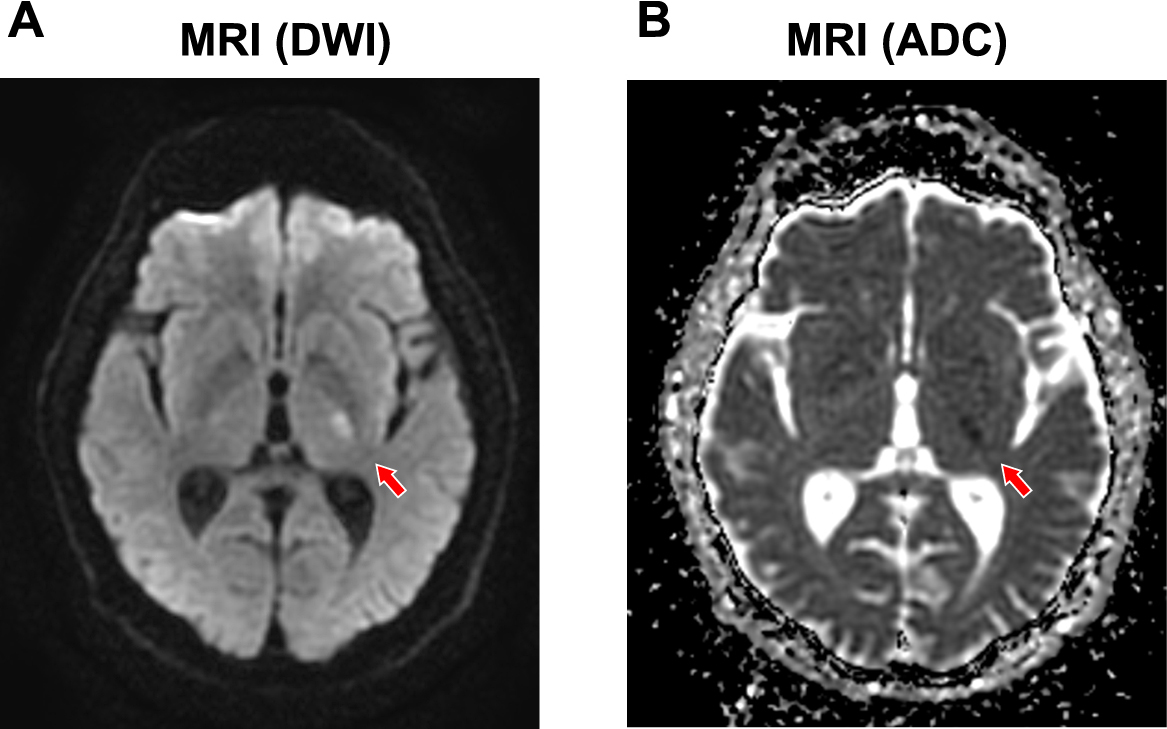
The patient was hospitalized and antiplatelet treatment (ozagrel sodium and cilostazol) was initiated with a diagnosis of lacunar infarction of the posterior limb of the left internal capsule. Basal-bolus insulin therapy was continued in patients with type 1 diabetes. By 11 am, the dysarthria and right incomplete paralysis disappeared.
Blood glucose levels after admission fluctuated significantly, ranging from 135 to 552 mg/dL (7.5 to 30.7 mmol/L). At 1 am on day 4 of admission, right incomplete hemiplegia recurred. Because the patient was hypoglycemic, with a blood glucose level of 49 mg/dL (2.7 mmol/L), glucose was immediately administered orally. The glucose level increased to 94 mg/dL (5.2 mmol/L), and the symptoms resolved within a few minutes. Based on this episode, we concluded that the patient’s symptoms were caused by hypoglycemic hemiplegia, and antiplatelet therapy was discontinued.
The daily glycemic profile and insulin regimen during hospitalization are shown in Fig. 2. Managing blood glucose levels is difficult because of significant fluctuations presumably caused by depleted insulin secretion. The patient required a high insulin dose, and investigation of the causes of insulin resistance, including malignancy and anti-insulin antibodies, yielded negative results. However, it was later discovered that the patient had lipodystrophy at the insulin injection sites in the abdomen (see Discussion section). Although hypoglycemia was occasionally observed, the patient did not exhibit any further neurological symptoms. Intermittently scanned continuous glucose monitoring (isCGM) was initiated to confirm the glycemic profile on day 23, and adjustments were made to the insulin formulation and correction of insulin for hyperglycemia. This approach led to improved blood glucose management without hypoglycemic episodes, and the patient was discharged on day 33 (Fig. 2).
Literature Review and Discussion
Hypoglycemic hemiplegia occurs in patients with diabetes receiving insulin or hypoglycemic agents, and in patients with insulinoma and insulin autoimmune syndrome. It is characterized by transient symptoms that recover after the correction of hypoglycemia [3]. Foster et al. previously reported that insulin treatment was the primary cause of hypoglycemic hemiplegia and that right hemiplegia was far more common than left hemiplegia (72% vs. 28%) in a clinical review of 29 cases [3]. Yoshino et al. conducted a comprehensive review of 201 patients with hypoglycemic hemiplegia [2] and reported that right hemiplegia was more common than left hemiplegia (66% vs. 34%). Recurrent hypoglycemic hemiplegia reportedly occurred in 78% of 29 patients in Foster’s study [3] and in 6 of 10 (60%) patients in a review reported in a Japanese publication [4]. The characteristics of recurrent hypoglycemic hemiplegia have not been thoroughly investigated. We conducted a comprehensive literature search focusing on patients with recurrent hypoglycemic hemiplegia.
We searched for cases with recurrent episodes of hypoglycemic hemiplegia in the case reports identified in Yoshino’s review (to December 2010) [2] as well as studies registered in PubMed from January 2011 to August 2022 and in Ichushi, the largest database of medical articles in Japan, from January 1980 to August 2022 using the same search criterion that Yoshino et al. used, including keywords “hypoglycemic hemiplegia,” “hypoglycemic hemiparesis,” combined with the keyword “hypoglycemia,” “hemiplegia,” or “hemiparesis” [2]. The reference lists of these articles were also reviewed. Of the 170 patients with hypoglycemic hemiplegia described in the articles whose abstracts and/or full texts were accessible, 26 (including the one in this report) had recurrent hypoglycemic hemiplegia (Table 1). Patient ages ranged from 2 to 80 years and blood glucose levels at the time of hemiplegia ranged from 17 to 74 mg/dL (0.94 to 4.1 mmol/L). Causes include insulin injections in patients with insulin-dependent diabetes mellitus (IDDM), non-insulin-dependent diabetes mellitus, insulin autoimmune syndrome, insulinoma, and the use of oral hypoglycemic agents. Among them, IDDM was the most frequent disease (17 of 26, 65%). Most patients had repeated hemiplegia on the same side, either right (15 of 23, 65%) or left (4 of 23, 17%), with a preference for right as in cases with a single episode of hypoglycemic hemiplegia, but four patients had the attacks on both sides in an alternate manner (4 of 23, 17%) [4-7] (Table 1). Recurrence occurred almost exclusively at midnight or in the early morning, and within a day or week in most cases (Table 1).
Table 1
Characteristics of recurrent hypoglycemic hemiplegia reported in the literature
| Case |
Age |
Sex |
# of episodes |
Type of disease |
Glucose (mg/dL) |
Side of hemiplegia |
Period of diabetes (years) |
Location of abnormalities in brain imaging studies |
Recovery time |
Time in a day |
Timing of recurrence |
Sympathomimetic symptoms |
Autonomic failure |
Reference |
| 1 |
54 |
F |
5 |
IDDM |
ND |
Right |
4 |
ND |
ND |
The evening to midnight |
In 1 day |
– |
ND |
[18] |
| 2 |
40 |
M |
7 |
IDDM |
27 |
Right |
16 |
Negative |
In 4 minutes |
Early morning |
ND |
– |
– |
[19] |
| 3 |
74 |
F |
ND |
NICTH |
22, 17 |
Right |
– |
ND |
By the evening |
Midnight to early morning |
ND |
– |
ND |
[20] |
| 4 |
64 |
F |
5 |
IDDM |
30–70 |
Right |
ND |
Negative |
“Rapidly” |
9 am |
After 3 weeks |
– |
ND |
[3] |
| 5 |
49 |
M |
ND |
IDDM |
22–36 |
Right |
31 |
Negative |
“Rapidly” |
ND |
ND |
– |
– |
[21] |
| 6 |
24 |
F |
ND |
IDDM |
23–74 |
Right, left |
ND |
Negative |
In 6 hours |
Early morning |
ND |
– |
ND |
[5] |
| 7 |
37 |
F |
3 |
Insulinoma |
24 |
Right |
– |
ND |
By the evening |
Early morning |
Within 1 week |
– |
ND |
[22] |
| 8 |
69 |
M |
2 |
NIDDM |
25, 34 |
Right, left |
0 |
Negative |
In 10 minutes |
ND |
In 1 day |
– |
ND |
[6] |
| 9 |
12 |
F |
2 |
IDDM |
41 |
Right |
ND |
Negative |
In 25 hours |
After breakfast |
After 10 months |
– |
ND |
[23] |
| 10 |
18 |
F |
3 |
IDDM |
34 |
Right |
4 |
Negative |
In 2 hours |
ND |
In 1 week |
– |
ND |
[24] |
| 11 |
79 |
M |
2 |
NIDDM |
32 |
Right, left |
ND |
ND |
ND |
ND |
Within 1 day |
– |
ND |
[7] |
| 12 |
24 |
F |
2 |
IDDM |
32 |
Left |
ND |
Right internal capsule |
ND |
ND |
ND |
– |
ND |
[25] |
| 13 |
63 |
F |
4 |
NIDDM |
42 |
Right |
ND |
Negative |
“Rapidly” |
Early morning |
Within 1 month |
– |
ND |
[26] |
| 14 |
57 |
F |
3 |
Insulinoma |
30 |
Right |
– |
ND |
“Rapidly” |
Early morning |
In 1 day |
– |
ND |
[27] |
| 15 |
80 |
F |
3 |
IAS |
34, 43 |
Right |
– |
Negative |
In 1–3 hours |
Early morning |
In 1 day |
– |
ND |
[28] |
| 16 |
80 |
F |
2 |
IAS |
30 |
Right |
– |
Negative |
ND |
Early morning |
In 1 day |
– |
ND |
[29] |
| 17 |
2 |
F |
5 |
IDDM |
36–56 |
Right |
0.5 |
Lateral ventricle |
In 10 minutes to 3 hours |
Early morning |
ND |
– |
ND |
[30] |
| 18 |
20 |
M |
9 |
IDDM |
40–70 |
Left |
8 |
Temporal lobe |
In 1–2 hours |
Early morning |
In 1 day |
– |
ND |
[30] |
| 19 |
28 |
F |
3 |
IDDM |
54 |
Left |
15 |
Right basal ganglia and right internal capsule |
In 1 hour |
Midnight to early morning |
In 1–4 days |
– |
+ |
[31] |
| 20 |
20 |
F |
2 |
IDDM |
36, 41 |
Right, left |
8 |
Negative |
In 2–3 hours |
Early morning |
In 1 week |
– |
– |
[4] |
| 21–23 |
2–20 |
– |
2, 9, 10 |
IDDM |
30–60 |
ND |
ND |
ND |
In 0.5–2.5 hours |
Early morning |
ND, 9 consecutive days, in 10 years |
ND |
ND |
[32] |
| 24 |
79 |
M |
2 |
NIDDM |
30, 47 |
Right |
ND |
Left internal capsule |
“Rapidly” |
Midnight to early morning |
After 2 years |
– |
ND |
[33] |
| 25 |
35 |
F |
2 |
IDDM |
42 |
Left |
17 |
Splenium of the corpus callosum |
In 1 hour |
Early morning |
In 2 days |
– |
ND |
[34] |
| 26 |
67 |
M |
3 |
IDDM |
56, 49 |
Right |
37 |
Posterior limb of the left internal capsule |
In 9 hours |
Midnight to early morning |
In 1 week |
– |
– |
This report |
Abbreviations: M, male; F, female; IDDM, insulin-dependent diabetes mellitus, NIDDM, non insulin-dependent diabetes mellitus; NICTH, non-islet cell tumour hypoglycaemia; IAS, insulin autoimmune syndrome; ND, not determined
The fact that hypoglycemic hemiplegia often recurs within a very short time suggests that there may be an additional temporal vulnerability to recurrence once hypoglycemic hemiplegia occurs. A temporarily weakened sympathoadrenal response due to prior hypoglycemia (hypoglycemia-associated autonomic failure) can lead to severe recurrent hypoglycemia without autonomic warning symptoms [8]. Hypoglycemia-associated autonomic failure is distinct from classic diabetic autonomic neuropathy in that it can be induced by prior hypoglycemia and reversed by avoiding hypoglycemia, whereas diabetic autonomic neuropathy is a more stable pathological condition. Interestingly, hypoglycemia-associated autonomic failure can be attributed not only to recent hypoglycemia but also to exercise and sleep [8]. This phenomenon may underlie the frequent occurrence of repeated hypoglycemic hemiplegia at midnight or early in the morning.
The patient presented in this study experienced transient hypoglycemic hemiplegia three times at midnight within 10 days. The characteristics, that is, the type of disease (type 1 diabetes), side of hemiplegia (right), involvement of the left internal capsule, recovery time of the symptoms (in hours), midnight onset of recurrence, and clustering of episodes, were typical of recurrent hypoglycemic hemiplegia.
Concerning the relatively high insulin dose, it was later discovered that the patient developed lipodystrophy at the injection sites in the abdomen. Indeed, after changing the injection site to the thigh, the patient managed to reduce his insulin dose by more than half. It is possible that the effect of insulin was strongly enhanced when injected into a site without lipodystrophy, leading to severe hypoglycemia. We speculate that multiple factors, including lipodystrophy at the injection sites, depletion of endogenous insulin secretion, and the onset of hypoglycemia at night, may have collectively contributed to the development of severe hypoglycemia and subsequent hypoglycemic hemiplegia in this case.
Another intriguing aspect of the case of this patient was that he did not exhibit any signs of autonomic dysfunction and had not previously experienced unconscious or nocturnal hypoglycemia. It is widely recognized that fixed autonomic neuropathy poses a risk for unconscious hypoglycemia and recurrent severe hypoglycemia by attenuating the epinephrine response to low blood sugar levels [9-11]. However, the role of autonomic neuropathy in hypoglycemic hemiplegia has not been well documented in literature. Interestingly, the frequency of autonomic neuropathy seems to be relatively low, at least in cases with recurrent hypoglycemic hemiplegia in which autonomic dysfunction was documented (four out of five patients, see Table 1). Previous studies have demonstrated that even individuals without autonomic neuropathy exhibit a reduced epinephrine response to hypoglycemia at night or after preceding hypoglycemia [12, 13]. While this may elucidate some aspects of the pathophysiology of recurrent nocturnal hypoglycemic hemiplegia in the absence of autonomic neuropathy, as observed in the present case, the rarity of hypoglycemic hemiplegia, compared to the large number of people with diabetes treated with insulin, suggests that additional factors may be necessary for its development. In the present case, the patient had lipodystrophy at the insulin injection site and received exceptionally high insulin doses. This could potentially serve as an additional factor contributing to the occurrence of hypoglycemic hemiplegia.
The present case was complicated with CCS, MN, and type 1 diabetes. The association between CCS and various autoimmune diseases, including MN, systemic lupus erythematosus, rheumatoid arthritis, scleroderma, and hypothyroidism, is well-recognized [14]. However, complications involving CCS, MN, and type 1 diabetes are rare, with only one case reported to date [15]. Given that all these diseases may be associated with autoimmunity, their complications may be interrelated.
Hypoglycemic hemiplegia is often misdiagnosed as cerebrovascular disease [1, 2]. There is no definitive imaging technique for distinguishing acute stroke from hypoglycemic hemiplegia [16]. Therefore, it is crucial to consider the possibility of hypoglycemic hemiplegia when observing patients with hemiplegia. The measurement of blood glucose levels and timely intervention are essential. As described above, we speculate that hypoglycemia-associated autonomic failure might be involved in the clustering of episodes, especially at night. It has been reported that by avoiding hypoglycemia, restoration of autonomic symptoms of hypoglycemia occurs within 2 weeks, and complete reversal of hypoglycemia unawareness is achieved within 3 months [17]. The identification and treatment of nocturnal and insensible hypoglycemia using continuous glucose monitoring and other devices may be useful in preventing hypoglycemic hemiplegia. Maintaining appropriate blood glucose levels at midnight and early in the morning may be important for preventing recurrent hypoglycemic hemiplegia.
Acknowledgements
We thank all the members and secretaries of our department for their valuable support and input in this study.
Disclosure
The authors declare that there is no conflict of interest. Hironori Waki is a member of Endocrine Journal’s Editorial Board.
Ethical Approval
All procedures were performed in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and the Declaration of Helsinki of 1964 and later versions. This case report was reviewed and approved by the Ethics Committee of the Akita University Graduate School of Medicine (approval# 2984, May 26, 2023).
Informed Consent
Written informed consent was obtained from the patients for publication of this case report.
References
- 1 Ravid JM (1928) Transient insulin hypoglycemic hemiplegias. Am J Med Sci 175: 756–769.
- 2 Yoshino T, Meguro S, Soeda Y, Itoh A, Kawai T, et al. (2012) A case of hypoglycemic hemiparesis and literature review. Ups J Med Sci 117: 347–351.
- 3 Foster JW, Hart RG (1987) Hypoglycemic hemiplegia: two cases and a clinical review. Stroke 18: 944–946.
- 4 Ariyasu H, Murakami N, Higashi N, Kato S (2000) A case of repeated hypoglycemic hemiparesis in a patient with type-1 diabetes mellitus. Tonyobyo 43: 443–448 (In Japanese).
- 5 Schmidt BJ, Pillay N (1993) Paroxysmal dyskinesia associated with hypoglycemia. Can J Neurol Sci 20: 151–153.
- 6 Wattoo MA, Liu HH (1999) Alternating transient dense hemiplegia due to episodes of hypoglycemia. West J Med 170: 170–171.
- 7 Webber AP, Benjamin C (2004) Wandering hemiparesis. J R Soc Med 97: 26–27.
- 8 Cryer PE (2013) Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med 369: 362–372.
- 9 Hoeldtke RD, Boden G, Shuman CR, Owen OE (1982) Reduced epinephrine secretion and hypoglycemia unawareness in diabetic autonomic neuropathy. Ann Intern Med 96: 459–462.
- 10 Flatt AJS, Little SA, Speight J, Leelarathna L, Walkinshaw E, et al. (2020) Predictors of recurrent severe hypoglycemia in adults with type 1 diabetes and impaired awareness of hypoglycemia during the HypoCOMPaSS study. Diabetes Care 43: 44–52.
- 11 Heller SR, Macdonald IA, Herbert M, Tattersall RB (1987) Influence of sympathetic nervous system on hypoglycaemic warning symptoms. Lancet 2: 359–363.
- 12 Jones TW, Porter P, Sherwin RS, Davis EA, O’Leary P, et al. (1998) Decreased epinephrine responses to hypoglycemia during sleep. N Engl J Med 338: 1657–1662.
- 13 Heller SR, Cryer PE (1991) Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes 40: 223–226.
- 14 Kopáčová M, Urban O, Cyrany J, Laco J, Bureš J, et al. (2013) Cronkhite-Canada syndrome: review of the literature. Gastroenterol Res Pract 2013: 856873.
- 15 Vernia P, Marcheggiano A, Marinaro V, Morabito S, Guzzo I, et al. (2005) Is Cronkhite-Canada syndrome necessarily a late-onset disease? Eur J Gastroenterol Hepatol 17: 1139–1141.
- 16 Chuang KI, Hsieh KL, Chen CY (2019) Hypoglycemic encephalopathy mimicking acute ischemic stroke in clinical presentation and magnetic resonance imaging: a case report. BMC Med Imaging 19: 11.
- 17 Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo-Jack S, et al. (2013) Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and The Endocrine Society. Diabetes Care 36: 1384–1395.
- 18 Criscitiello M, Messer ER (1931) Neurologic phenomena in the course of the treatment of diabetes. N Engl J Med 205: 1246–1248.
- 19 Silas JH, Grant DS, Maddocks JL (1981) Transient hemiparetic attacks due to unrecognised nocturnal hypoglycaemia. Br Med J (Clin Res Ed) 282: 132–133.
- 20 Penman WA, Johnson JK (1982) Recurrent hemiplegia due to hypoglycaemia. Postgrad Med J 58: 501–502.
- 21 Pell AC, Frier BM (1990) Restoration of perception of hypoglycaemia after hemiparesis in an insulin dependent diabetic patient. BMJ 300: 369–370.
- 22 Shanmugam V, Zimnowodzki S, Curtin J, Gorelick PB (1997) Hypoglycemic hemiplegia: insulinoma masquerading as stroke. J Stroke Cerebrovasc Dis 6: 368–369.
- 23 Kossoff EH, Ichord RN, Bergin AM (2001) Recurrent hypoglycemic hemiparesis and aphasia in an adolescent patient. Pediatr Neurol 24: 385–386.
- 24 Carter F, Taylor C (2002) Transient hypoglycemic hemiparesis. J Natl Med Assoc 94: 999–1001.
- 25 Cordonnier C, Oppenheim C, Lamy C, Meder JF, Mas JL (2005) Serial diffusion and perfusion-weighted MR in transient hypoglycemia. Neurology 65: 175.
- 26 Nannapaneni N, Elkhider A, Steinberg J (2014) Hypoglycemic hemiparesis: the stroke masquerader. Am J Med 127: e13.
- 27 Hasuo Y, Nakamura Y, Fujishima F (1985) A case of insulinoma with transient hemiplegia as the main complaint. Rinsho To Kenkyu 62: 3237–3241 (In Japanese).
- 28 Sasaki J, Gotoh T, Watanabe K, Ogayama H, Sasanuma J (1990) Transient hypoglycemic hemiplegia due to insulin autoimmune syndrome—a case report. No To Shinkei 42: 95–98 (In Japanese).
- 29 Hayashi A, Mizuno K, Tani M (1991) A case of elderly-onset insulin autoimmune syndrome with right hemiplegia during hypoglycemic attack. Horumon To Rinsho 39: 63–66 (In Japanese).
- 30 Miyamoto S, Sasaki N, Tanabe Y (1992) Two cases of hypoglycemic hemiplegia. Horumon To Rinsho 40 (extra issue: Endocrinology Cases of Interest 18): 152–153 (In Japanese).
- 31 Tei H, Uchiyama S, Shibagaki Y, Mochizuki A, Maruyama S (1992) A case of hypoglycemic hemiplegia. Nosotchu 14: 499–504 (In Japanese).
- 32 Miyamoto S, Someya T, Tanabe Y, Sato H, Sasaki N, et al. (2003) [Neurological disorders] Transient localized neurological symptoms associated with hypoglycemia in type 1 diabetes mellitus. Shonika Rinsho 56: 1079–1082 (In Japanese).
- 33 Kubota S, Hirata J, Kojima M, Kunii M, Tomita A, et al. (2014) A case of repeated hypoglycemic hemiparesis due to posterior limb of internal capsule lesion. Nosotchu 6: 370–373 (In Japanese).
- 34 Kakiba T, Yamamoto Y, Yamamoto K, Yoshioka K, Sato T (2020) A case of hypoglycemic hemiplegia with reversible high-signal area in the cerebral corpus callosum on MRI diffusion-weighted imaging. Shimaneigaku 40: 43–46 (In Japanese).
 https://orcid.org/0000-0003-1880-849X
https://orcid.org/0000-0003-1880-849X
 https://orcid.org/0000-0003-0404-605X
https://orcid.org/0000-0003-0404-605X
 https://orcid.org/0000-0002-5302-9793
https://orcid.org/0000-0002-5302-9793



