2024 Volume 71 Issue 6 Pages 547-559
2024 Volume 71 Issue 6 Pages 547-559
The pituitary gland is endocrine tissue composed of two distinct parts with different origins: the adenohypophysis (adenohypophyseal placode origin) and the neurohypophysis (neuroectoderm origin). Differentiation of endocrine cells in the pituitary gland leads to hormone synthesis, secretion into the capillary network, and transportation to target organs. In 1988, the discovery of the pituitary transcription factor PIT1 sparked research on endocrine cell differentiation. In the twenty-first century, the discovery that SOX2-positive stem/progenitor cells give rise to all types of pituitary endocrine cells advanced research on differentiation processes using diverse marker molecules. Lineage tracing using specific marker genes from early embryos revealed that during construction of the anterior pituitary from the adenohypophyseal placodal cells the developing anterior pituitary incorporates diverse cell types originating from the neural crest-derived and ectodermal-derived cells. Consequently, the postnatal anterior pituitary becomes a mosaic of terminally differentiated cells of different origin and with different life histories. It has also been revealed that most of the postnatal stem/progenitor cells form at least solid clusters in the parenchyma. Moreover, the classification and role of S100β-positive cells had been ambiguous, but now they are identified as a major component of postnatal stem/progenitor cells. This paper provides an updated overview of pituitary development.
The pituitary gland is the major endocrine organ located below the brain and produces many hormones and secretes them into diverse target organs to maintain vital homeostasis in all vertebrates (Fig. 1). This gland is small, even in adult humans, making up around one-hundred thousandth of the body weight, and is composed of two distinct entities with different origins: the adenohypophysis (anterior gland, comprised of the rostral tip, anterior lobe, and intermediate lobe originating from the adenohypophyseal placode) and the neurohypophysis (comprised of the pituitary stalk and posterior lobe originating from the neuroectoderm). The pituitary gland is regulated by several physiological demands from the hypothalamus and target organs.

The pituitary gland. The posterior lobe (PL) secretes peptide hormones, VP (vasopressin), and OT (oxytocin) that are produced in the hypothalamus. The intermediate lobe (IL) produces peptide hormones, MSH (melanocyte-stimulating hormone), and others in the melanotropes. The anterior lobe (AL) produces PRL (prolactin) in the mammotropes; GH (growth hormone) in the somatotropes; FSH (follicle-stimulating hormone) and LH (luteinizing hormone) in the gonadotropes; TSH (thyroid-stimulating hormone) in the thyrotropes; and, peptide hormones, ACTH (adrenocorticotropic hormone), and others in the corticotropes. LH, FSH, and TSH are glycoprotein hormones. ACTH and MSH are peptide hormones processed from the same product of the proopiomelanocortin gene (Pomc), with different processing enzymes.
The existence of the pituitary primordium (Rathke’s pouch) was first reported in 1838 by Rathke [1] as a small, irregularly rounded depression deep in the oral cavity of amphibian, avian, and mammalian embryos. He could not find it in further developed embryos. Rathke’s pouch significantly expands, altering in shape, and gives rise to various endocrine cells during development by the time of birth. Many studies have been conducted and summarized in detail regarding pituitary gland organogenesis, including the differentiation of endocrine cells based on the spatiotemporal expression of many transcription factors [2-4].
Between 2008 and 2009, important reports regarding pituitary development and maintenance were published. The reports showed that stem/progenitor cells of the pituitary gland are SOX2 (sex determining region Y-box 2)-positive and can differentiate into all types of endocrine cells [5, 6], facilitating the analysis of stem/progenitor cells in this tissue. Another significant advancement was the generation of genetically modified rats (S100β-promoter/GFP (green fluorescence protein)-transgenic (S100β-TG) rats) to make it possible to visualize S100β-positive cells [7], providing important information about S100β-positive cells, which have been ambiguously defined until now [8, 9]. By utilizing SOX2 and S100β as marker molecules, new insights into the properties and roles of pituitary stem/progenitor cells have been progressively unveiled [10, 11]. In this paper, we will describe recent findings primarily from rats and mice concurrently to supplement the existing knowledge on pituitary gland development. Before delving into the subject, we would like to provide an explanation of the issue concerning S100β-positive cells in the pituitary gland.
S100β was initially discovered as a brain-specific acidic protein that is soluble in a 100% ammonium sulfate solution (S100), and at that time it was believed to be associated with memory substances [12]. The late Professor Keiichi Uyemura initiated the research for S100β during his studies in France [13]. After returning to Japan, Uyemura’s group also discovered the myelin basic protein P0 (P0) in the peripheral nervous system [14, 15], which is currently used to trace neural crest cells (NCCs) and neural crest derived cells (NCDCs) in P0-Cre/EGFP (enhanced GFP) mice.
We collaborated with him on investigating S100β in chicken and bovine brains [16] and on generating an antibody against bovine S100β. Using this S100β antibody, Nakajima et al. first reported S100β-positive signals in the follicular type and stellate type cells with distinct cell morphologies in the rat anterior lobes [17]. At that time, both cell types were collectively referred to as folliculo-stellate cells (often abbreviated as FS cells). For this reason, S100β was considered a suitable marker molecule for folliculo-stellate cells.
The term folliculo-stellate cell was proposed to unify two different cell types with distinct morphologies, the follicular type and the stellate type, as “une même famille cellulaire” (the same cell family) based on intracellular structure [18]. However, there is a serious problem as the first observation missed other S100β-positive cells, which are polygonal type cells lining the marginal cell layer (MCL) in the postnatal rat [19, 20] and human [21] anterior lobes as reviewed in [22]. Looking back now, it is evident that categorizing two distinct cell types in terms of cell morphology into a single nomenclature and missing the MCL cell member caused confusion and ambiguous judgment for S100β-positive cells. Several reviews have attempted to reconcile the conflicting S100β-positive cell groups [8, 9], nevertheless, its ambiguity remains.
On the other hand, in the context of tissue renewal, Yoshimura’s group had suggested a relationship between the marginal layer cells and follicular type cells [23, 24]. This theory has not seen significant progress. However, studies on S100β-TG rats that were generated three decades later provide us with much important knowledge about S100β-positive cells lining the MCL and scattered in the parenchyma, as discussed below. Additionally, it has been demonstrated that S100β-positive cells of two distinct origins invade the pituitary gland through distinct pathways in different developmental periods.
Incidentally, S100β was later discovered in various tumors and even considered as a marker molecule for them. However, in the peripheral nervous system, S100β is known to be involved in the development and differentiation of Schwann cells, which originate from NCCs. Below we will discuss this using the terms “follicular type” and “stellate type” cells, avoiding the use of the term “folliculo-stellate cell” as much as possible. We will now move on to the main issue.
The pituitary primordium, Rathke’s pouch, was discovered in the embryonic oral cavity in 1838 [1]. However, a longstanding debate about the origin of the germ line has persisted [25, 26]. Eventually, a partial dissection of a portion of the cranial region led to the conclusion that the origin of Rathke’s pouch is a region within the anterior head ectoderm, which contributes to forming the cranial placode in the early embryo [27, 28] (Fig. 2A). Different origins of Rathke’s pouch, such as the ANR (anterior neural ridge) of the neuroectoderm-derived neural plate, had been reported [25, 29, 30], while another position is postulated at the slight anterior part of the non-neural ectoderm in chickens [31]. It appears that there is still no conclusive agreement on this matter.

Cranial placodes, neural crest cells (NCCs), and early development of the pituitary primordium. A. Adenohypophyseal placode (Ad), olfactory placode (Olf), optic placode (Op), and otic placode (Ot) are indicated. B. Migration of NCCs from the border between the epidermis (EP) and neural fold (NF). NT: neural tube. C. The adenohypophyseal placodal cells migrate to the oral ectoderm and invaginate to form the oral cavity (OC). The embryonic pituitary develops as a result of a large number of signaling factors (blue) from surrounding tissue, especially infundibulum (IF, the prospective posterior lobe), and transcription factors (red) temporarily expressed in the invaginating pituitary primordium. The number at the top of each figure shows the embryonic (E) day of rats (r) and mice (m) in order of rat/mouse.
During the completion of the neural tube closure, a diverse population of NCCs emerges from the neural fold and acquires migratory ability through epithelial-mesenchymal transition (EMT) (Fig. 2B). These cell populations, also known as the fourth germ layer cells, invade various tissues during their development while maintaining stem/progenitor properties [32-34]. Among these cell populations, it has recently been revealed that two cell populations are involved in pituitary development in different time periods via distinct pathways. One is characterized by means of gene tracing analysis using P0-Cre/EGFP reporter mice (P0-Cre/EGFP: P0 protein promoter fused with CRE-recombinase and EGFP reporter) [35], and the other is by immunohistochemical analysis for SOX10 using S100β-TG rats, respectively [36].
At the first stage of anterior pituitary development, cells of the adenohypophyseal placode migrate to an invaginating part of the oral ectoderm (oral cavity), which is close to the diencephalon, suggesting involvement of the diencephalon in pituitary development as was advocated over one hundred years ago [25, 26]. Today, it has been demonstrated that contact with structures such as the hypothalamus, notochord, and primitive gut is necessary for the development of the pituitary primordium [29, 37]. Indeed, a number of signaling factors, such as FGFs, BMPs, SHH, and WNTs, are secreted from the ventral part of the diencephalon (a prospective infundibulum) to regulate the expression of many transcription factors temporarily expressed in the primordium of Rathke’s pouch, thus promoting the development of this organ (Fig. 2C) [3, 38-41].
Simultaneously with the invaginating oral cavity (Fig. 3A), on rat embryonic day 11.5 (rE11.5) corresponding to that of mouse (m)E9.5, positive signals of the pituitary-specific transcription factor PROP1 (prophet of Pit1) first emerge (Fig. 3B) [42]. PROP1 is an exclusive factor that determines the differentiation of endocrine cells [43] and is solely expressed in the anterior pituitary. At the same time, in the mouse on mE9.5, P0-promoter active (CRE-positive) NCCs are observed in the oral ectoderm as CRE-positive/GFP-positive cells and P0-lineage NCDCs emerge as CRE-negative/GFP-positive cells in the early Rathke’s pouch (eRP) [35] (Fig. 3C). More recently, experiments using zebrafish have revealed unexpected results indicating that cells expressing the endodermal marker SOX17 are present in Seessel’s pouch, the endodermal tissue, on 34 hours post fertilization (hpf) (Fig. 3D, upper panel) and then invade the pituitary primordium on 40 hpf (Fig. 3D, lower panel) [44]. However, this invasion has not yet been confirmed in mammals, although Seessel’s pouch has frequently been discussed as a candidate for the origin of the mammalian anterior pituitary for over one hundred years [26]. It has been revealed that the endodermal-lineage cells exist as both stem/progenitor cells and endocrine cells and express Prop1 in the postnatal pituitary of zebrafish [44].

Early Rathke’s pouch (eRP). A. eRP formed in an invaginating part of the oral ectoderm (oral cavity, red) connecting with endodermal Seessel’s pouch (Se). IF: infundibulum. B. On rat embryonic day 11.5 (rE11.5), PROP1 (red, white arrow) appears in the SOX2-positive cells (not indicated) of eRP. C. On mouse (m) E9.5, invasion of P0-lineage neural crest cells to eRP. Positive signals for CRE-recombinase (CRE, red) and GFP (green) are indicated. D. In the zebrafish, SOX17-lineage endodermal cells (red) are present in Se (red arrow) on 34 hours post fertilization (hpf) of the zebrafish (upper panel) and then invade eRP (white arrow) on 40 hpf (lower panel), but this invasion is not yet confirmed in mammals.
Formation of Rathke’s pouch is nearly completed at rE13.5 (Fig. 4A). At this time, rat Rathke’s pouch is a disk-shaped cell mass, with a diameter of approximately 500 μm and a thickness of 200 μm, with a volume of 20 nL and composed of approximately 6,500 cells (Fig. 4B, Supplementary Fig. S1 and Video S1) [45]. All cells within rat Rathke’s pouch are positive for both SOX2 and PROP1, except in the rostral tip (Fig. 4C) [42]. At this time, P0-lineage NCDCs in mouse Rathke’s pouch become positive for both SOX2 and PROP1 (the first wave of invasion), while a significant number of P0-lineage NCDCs accumulate at the anteroexternal region of Rathke’s pouch (contributing to the second wave of invasion) (Fig. 4D) [35]. Subsequently, the development and differentiation of pituitary endocrine and non-endocrine cells proceed rapidly.

Invasion of extra-adenohypophyseal placode-lineage cells to Rathke’s pouch (RP). A. Invasion of P0-lineage neural crest cells (P0) to RP. RT: rostral tip, IF: infundibulum. B. Microscopic image of isolated rat RP on rE13.5. C. In the rat, all cells within the pouch are positive for both SOX2 (green) and PROP1 (red), except in the rostral tip (RT). D. In mouse RP on mE11.5, GFP-positive P0-lineage cell (green) positive for PROP1 (red) and SOX2 (gray) is observed (white arrow in the insets).
After the formation of Rathke’s pouch, the pouch undergoes extensive expansion in all directions. The dorsal (intermediate lobe) and ventral (anterior lobe) domains become distinct, facing each other across the lumen (known as Rathke’s residual lumen), along with a noticeable expansion of the parenchyma of the anterior lobe. By rE15.5/mE13.5, a large recess known as Atwell’s recess forms between the rostral tip and the anterior lobe to initiate the formation of a capillary network (vasculogenesis) within the anterior lobe [26, 46] (Fig. 5A). Two distinct cell lineages invade through Atwell’s recess. One consists of the NCDCs as the second wave of invasion [35] (Fig. 5B), while the other is comprised of mesenchymal-lineage cells [47] (Fig. 5C).

Invasion of diverse lineage cells through Atwell’s recess (AR). A. Prior to vasculogenesis, AR receives diverse lineage cells. The second wave of P0-lineage cells (P02) and mesenchymal cells (Me) invade the anterior lobe (AL). IL: intermediate lobe, PL: posterior lobe, RT: rostral tip. B. P0-expressing CRE-positive cells (CRE, green) invade the anterior lobe through AR as the second wave of invasion. They are negative for SOX2 (white) and PROP1 (red). C. In the rat, S100β-positive cells (green, white arrow), mainly mesenchyme-lineage, invade through AR. D. Invading cells are all negative for SOX2 (white) and the majority are PRRX1-positive (red). E-I. Cells positive for neurotrophin receptor p75 (E, white) and marker molecules related to vasculogenesis (white): Isolectin B4 (F), NESTIN (G), DESMINE (H) and VIMENTIN (I) are partly positive for S100β (green, white arrow), PRRX1 (red, yellow arrowhead) or both (green arrow).
The former cells are located in front of Rathke’s pouch and differ from cells of the first wave of invasion in that they are negative for both SOX2 and PROP1. Some of the GFP-positive NCDCs are also positive for NG2, a marker for multipotent mesenchymal pericytes [48], while there are also P0-promoter active (CRE-positive)/GFP-positive cells inside the developing anterior pituitary [35]. It is suggested that P0-Cre/EGFP mice display not only NCCs and NCDCs but also non-NCCs which are tissue-resident vascular precursors and are P0-promoter active (CRE-positive) even in the postnatal period [49].
On the other hand, in the rat, it has demonstrated that invading cells through Atwell’s recess are composed of diverse cell types positive for several mesenchymal markers, some of which are negative or positive for S100β [47]. These diverse cell types include: 1) those positive for PRRX1 (about 80%) (Fig. 5D), 2) those positive for the neurotrophin receptor (p75) (Fig. 5E), a factor involved in neural crest development [50], and 3) those positive for various markers related to vasculogenesis (Isolectin B4 (Fig. 5F), NESTIN (Fig. 5G), DESMIN (Fig. 5H), and VIMENTIN (Fig. 5I)). Additionally, αSMA-positive cells, which are negative for S100β, are also observed (data not shown). These cells might originate from cell lineages distinct from the adenohypophyseal placode and primarily contribute to the non-endocrine cell populations. It is important to note that all cell types invading from Atwell’s recess are negative for SOX2.
In parallel with the vasculogenesis, the differentiation of endocrine cells commences in the anterior pituitary. By rE16.5, while the PROP1-positive signals in the prospective intermediate lobe decrease markedly, SOX2/PROP1-double positive cells start to scatter in the expanding parenchyma of the anterior lobe. However, SOX2-single positive cells mostly remain in the prospective MCL [42]. Along with this progression, each endocrine cell type appears [51] in accordance with the spatiotemporal expression of various transcription factors essential for differentiation of each one [40, 41, 52, 53]. In rats, lactotropes finally differentiate, and all types of endocrine cells appear by the time of birth.
On rE21.5, the pituitary gland, composed of three lobes, showed a flattened chestnut shape with dimensions (about 500 μm in the dorsoventral axis, 2,500 μm in the left-right axis, and 850 μm in the rostrocaudal axis) and consisted of approximately 113,500, 16,000, and 14,800 cells in the anterior, intermediate, and posterior lobes, respectively (see Supplementary Figs. S2 and S3, and Video S2) [54]. The size of the gland has increased approximately five times in width, 1.66 times in depth, and 2.5 times in height compared to those of Rathke’s pouch on rE13.5 (see Supplementary Table S1). The number of cells in the anterior and intermediate lobes has increased by approximately twenty-two times, but cell volume has decreased by approximately 64%.
Immediately before birth, another invasion is observed in the pituitary gland using immunohistochemical analysis. Cells positive for SOX10, a potent NCC marker, migrate into the posterior lobe through the pituitary stalk [36]. Unlike the P0-lineage NCDCs, SOX10-positive cells are positive for both SOX2 and p75. At that time, S100β-positive pituicytes are located in the posterior lobe, but S100β-positive cells are absent from the intermediate lobe and the anterior lobe. Subsequently, SOX10-positive cells further advance towards the caudal region of the posterior lobe, the intermediate lobe, and the anterior lobe (Fig. 6A). While SOX10-positive cells are initially negative for S100β in the posterior lobe, SOX10/S100β-double positive cells are detected among S100β-single positive cells, suggesting rapid decrease of the Sox10-expression on the way to differentiation (Fig. 6B, C). These SOX10/S100β-positive cells display an elongated cytoplasm containing phalloidin labeled F-actin, exhibiting chase-and-run like migration [55] through the intermediate lobe even on rat postnatal day 60 (rP60) (Fig. 6D). In the anterior lobe, SOX10/S100β/PROP1-triple positive cells are barely detectable on rP3, indicating the generation of postnatal stem/progenitor cells from Sox10-expressing cells (Fig. 6E).

Invasion of the SOX10-positive cell population. A. Schematic diagram. SOX10-positive cells invade through the pituitary stalk (PS) immediately before birth, followed by migration into the caudal region of the posterior lobe (PL), then intermediate lobe (IL), and finally anterior lobe (AL). Immunohistochemistry for SOX10 (red) and S100β (green) in the PL, IL, and AL on rP30 (B) and rP60 (C). D. Phalloidin labelled F-actin (green) that exhibits the feature of migratory activity and immunohistochemistry for SOX10 (red). E. In the anterior lobe, SOX10 (red)/S100β(green)-double positive cells become positive for PROP1 (white, white arrow indicates positivity for SOX10, PROP1, and S100β in the insets).
During the developmental expansion of the embryonic anterior pituitary, some of the SOX2-positive cells scatter within the parenchyma, while those lining the MCL are retained. Characteristic of the postnatal maturation of the anterior lobe is the formation of clusters of stem/progenitor cells in the parenchyma by EMT [56, 57]. In the embryonic anterior pituitary, the coxsackievirus and adenovirus receptor (CAR) localizes in the apical surface of the SOX2-positive cells in the single cell layer facing the oral cavity (Fig. 7A), the cleft of Rathke’s pouch (Fig. 7B), and the MCL (Fig. 7C). After birth, cells positive for CAR on the lateral membrane surface begin to form multiple cell layers beneath the MCL. They then migrate by EMT into the parenchyma of the anterior lobe, forming additional niches by clustering the cells positive for CAR on the apical membrane surface (Fig. 7D–G). Eventually, SOX2/CAR-double positive stem/progenitor cells construct two types of niche, the MCL-niche lining the MCL and the parenchymal-niche forming clusters in the parenchyma of the postnatal anterior lobe. Even in this transition, SOX2-positive cells continue to account for 89.6% of the cells lining the MCL of the anterior lobe on P60, and for 43.8% even on P600 (Fig. 8A). In contrast, PROP1-positive cells lining the MCL display a significant decrease from 68.6% on P5 to 1.2% on P600 (Fig. 8A) [10], indicating a continuous supply of stem/progenitor cells from the MCL niche to the parenchymal niche. Interestingly, the PROP1-positive cells still account for 3.9% on P600, representing 34.1% of the SOX2-positive cells in the parenchyma (Fig. 8A). The migration of stem/progenitor cells from the MCL to the parenchyma reminds one of the mechanism of the pituitary cell renewal system previously proposed by Yoshimura [23, 24]. This supports the hypothesis that marginal layer cells supply parenchymal cells, often referred to as follicular type cells, with differentiation abilities.
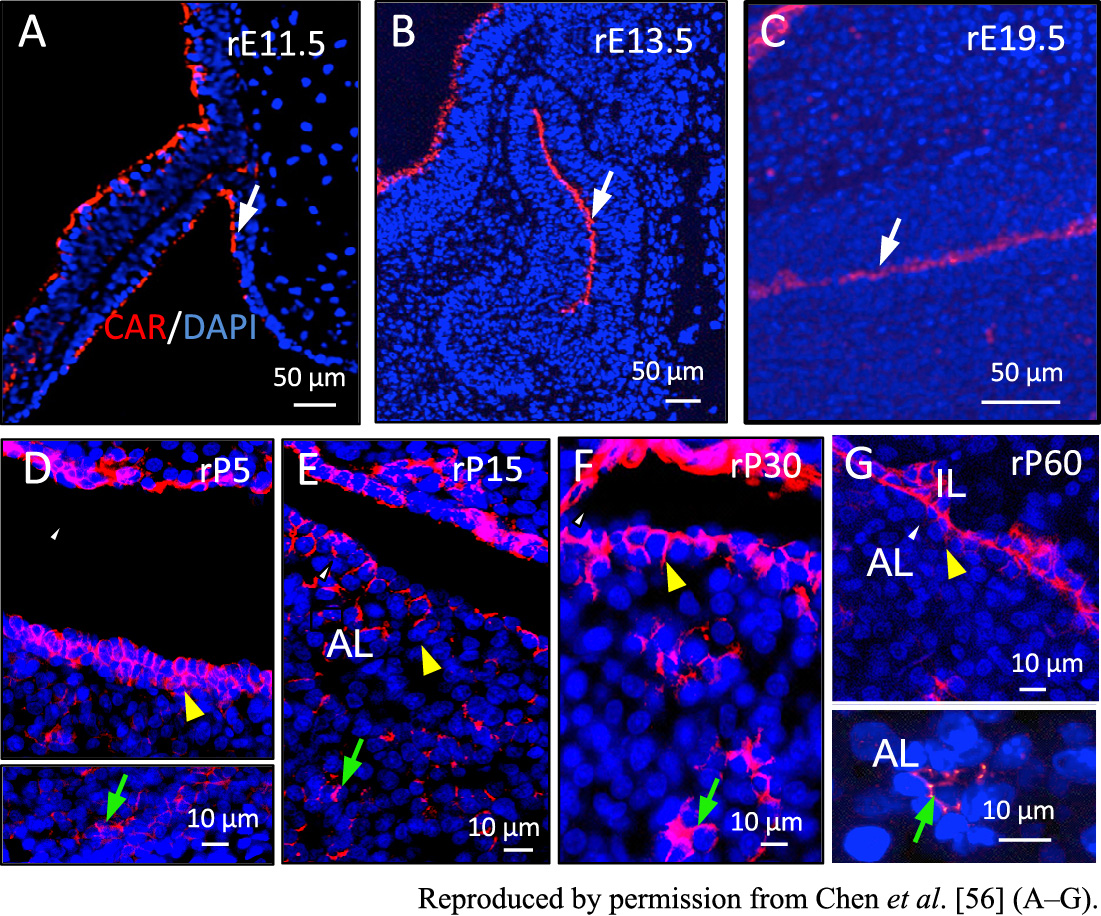
Migration of stem/progenitor cells lining the marginal cell layer (MCL) by epithelial-mesenchymal transition (EMT). Immunohistochemistry for coxsackievirus and adenovirus receptor (CAR) during rat pituitary development is shown. CAR-positive signals (red) are observed in the cells lining the invaginating oral cavity on rE11.5 (A), the cleft of Rathke’s pouch on rE13.5 (B), and the MCL on rE19.5 (C) (white arrow). After birth, CAR, which localizes in the apical membranes during the embryonic period, appears in the lateral membranes (D–G) (yellow arrowhead). CAR-positive cells migrate by EMT and reconstruct the apical surface localization of CAR to form cell clusters in the parenchyma (green arrow).

Population of SOX2-, PROP1-, and S100β-positive cells. A. Cells positive for PROP1 and SOX2 in the MCL and parenchyma during postnatal development. NC: not counted. Immunohistochemistry of positive cells for SOX2, PROP1, and/or S100β on the MCL (B) and in the parenchyma (C). D. Five types of positive cells were observed and their proportions (%) are indicated for each group of the SOX2-positives (four types in the upper table) and S100β-positives (three types in the lower table) by refiguring the data in [10]. DAPI: nuclei number by DAPI staining.
In terms of positivity for SOX2, PROP1, and S100β, five distinct immunophenotypes (Fig. 8B, C) are present in the anterior lobe of S100β-TG rats on P60. There are four types for SOX2 and three types for S100β. The cell populations for each type indicate that the majority of SOX2-positive cells are S100β-positive (MCL%/parenchyma%, 64.1/82.2), and conversely, most S100β-positive cells are SOX2-positive (86.0/84.8) [10] (Fig. 8D). This highlights the importance of S100β-positive cells as the predominant population of adult stem/progenitor cells. Furthermore, there is a significant difference in the proportion of SOX2/PROP1-double and SOX2/PROP1/S100β-triple positive cells between the MCL (1.5% and 13.4%, respectively) and the parenchyma (10.2% and 68.5%, respectively), which raises compelling questions regarding qualitative differences among them such as the differentiation potential, cell types to be generated, and/or conditions for differentiation.
It has been demonstrated that SOX2-positive stem/progenitor cells form distinct dense cell clusters in the parenchyma of the postnatal anterior lobe [11]. They are resistant to dispersion even under stringent conditions involving higher protease concentrations and more rigorous physical treatments than the typical dispersion methods (Fig. 9A). These cell clusters can be manually isolated and referred to as PS-clusters (parenchymal stem/progenitor cell clusters). The collected PS-clusters from the anterior lobe of adult S100β-TG rats are categorized into three types based on S100β/GFP-expression (Fig. 9B). These are Type A: GFP clusters (comprising SOX2/S100β-double positive cells; 46.8%); Type B: mix-GFP clusters (a mixture of SOX2/S100β-double positive cells and SOX2-single positive cells, 37.0%); and Type C: null-GFP clusters (all composed of SOX2-positive/S100β-negative cells; 16.2%) [11]. The number of clusters containing GFP-positive cells is approximately five times greater than that of null-GFP clusters. Additionally, GFP clusters show the subtle but noticeable capacity to differentiate into endocrine cells under three-dimensional (3D) culture conditions (Fig. 9C). In the case of GH cells, the differentiation capacity is up to 3.1% [11]. Under 2D culture conditions, differentiation into non-endocrine cells, including SOX17-positive cells, is observed (Fig. 9D) [58]. GFP clusters seem to possess the potential to differentiate into various cell types depending on the culture conditions, while null-clusters are unable to differentiate until now.

Parenchymal stem/progenitor cell clusters (PS-clusters). A. In the protease-dispersed S100β-TG rat pituitary (rP60) under stringent conditions, a number of cell clumps (PS-clusters, boxed) remain in the suspension. B. Three types of SOX2-positive PS-clusters are manually collected. Type A: GFP cluster, composed of all GFP-positive cells, Type B: null-GFP cluster, mixture of GFP-positive and -negative cells, and Type C: null-GFP cluster, all GFP-negative cells. Each proportion (%) is indicated in the figure. C. Cultivation of GFP clusters in the three-dimensional (3D) culture medium. In the SOX2-positive cells (red), the cell reacted with the mixture of antibodies against all types of anterior pituitary hormone as indicated by white arrow. D. Cultivation of GFP clusters in the 2D-culture medium. Some of them differentiate into cells positive for NG2, αSMA, or MYOGENIN (green). The rightmost panel shows that the SOX17-positive cell (green) is positive for SOX2 (red) in the insets.
Of note, SOX2-positive cells within PS clusters, which typically consist of an average of twelve cells per cluster, make up approximately 8% of the total cell in the anterior lobe [11]. Importantly, PS clusters have also been observed in the mouse anterior lobe (Kato Y, Ueharu H, Yoshida S, and Kato T, unpublished observations). An especially intriguing area of research involves the establishment in the near future of differentiation conditions for null-GFP clusters.
Until now, pituitary stem/progenitor cells have been analyzed using only singly-dispersed cells prepared from enzymatically dispersed suspension. However, this method covers only about 0.05% [5] and 0.68% [6] of the anterior lobe cells, which is significantly less than that calculated for PS-clusters (8%). Recently, single-cell PCR of pituitary stem/progenitor cells using a similar cell dispersion method has been conducted [59]. However, these studies may lack information on the major population, PS-clusters, due to technical challenges. The development of methods for profiling gene expression in PS clusters is of the utmost importance, as these clusters represent a significant component of postnatal pituitary stem/progenitor cells, making it an urgent research priority.
Based on these findings, it becomes evident that postnatal pituitary stem/progenitor cells, characterized by SOX2 positivity, predominantly comprise S100β-positive cells. These cells are primarily located in the MCL and form clusters in the parenchyma. When studying S100β-positive cells, it has been observed that the majority of them are also SOX2-positive, although there is a small subset that are SOX2-negative. Given these observations, we have attempted to classify non-endocrine cells, as outlined in Table 1. Firstly, non-endocrine cells are divided into two categories based on their immunophenotype for SOX2 (positive or negative). Subsequently, these two categories are further divided into subtypes based on their immunophenotype for S100β (SOX2S100β, SOX2nonS100β, nonSOX2S100β, and nonSOX2nonS100β). In the case of SOX2-positive cells, PROP1 is a useful marker for a third immunophenotype. These subtypes can be more precisely defined using various molecular markers (as indicated in the lower part of Table 1).
Phenotypic classification of non-endocrine cells in the adult anterior pituitary
| Population | Phenotype | Signage | Origin | ||
|---|---|---|---|---|---|
| First | Second | Third | |||
| SOX2 | S100β | PROP1 | |||
| Majority | |||||
| + | + | + | SOX2S100β/PROP1 | SOX10 | |
| + | – | + | SOX2nonS100β/PROP1 | P0* | |
| + | + | – | SOX2S100β/nonPROP1 | SOX10 | |
| + | – | – | SOX2nonS100β/nonPROP1 | ||
| Minority | |||||
| – | + | nonSOX2S100β | mesenchymal | ||
| – | – | nonSOX2nonS100β | mesenchymal, P0** | ||
P0* and P0**: P0-lineage cells of the 1st and 2nd waves of invasion, respectively. +: Positive, –: Negative
To further characterize, phenotypes such as PRRX1, PRRX2, SOX9, SOX10, SOX17, NESTIN, CXCL10, CD9, and RALDH3 for SOX2-positive cells, and PRRX1, PRRX2, NG2, Isolectin B4, NESTIN, DESMIN, and VIMENTIN for SOX2-negative cells are useful.
In the anterior lobe, stem/progenitor cells continuously supply endocrine cells throughout life to maintain pituitary function. This raises the question of whether pituitary stem/progenitor cells can support such a large endocrine cell population. In young adult male rats, all anterior lobe cells are renewed approximately every 60–70 days, indicating an average cell turnover rate of 1.55% per day [60]. This suggests that around 1.55% of the stem/progenitor cells must divide daily. SOX2-positive cells present in the parenchyma account for 14.9% of cells in P60 rats and even in 11.3% of P600 rats (Fig. 8A) [10]. In the case of P600 rats, it is estimated to be over seven times the required number of stem/progenitor cells. If each of them can divide once a week, the renewal of anterior lobe cells is conceivable.
During embryonic development, the stem/progenitor cells primarily contribute to differentiation and development. However, after maturation, they provide cells that constitute adult tissue, responding to continuous physiological demands. Understanding how the MCL-niche and the parenchymal niche, which comprises PS-clusters, respond to various demands is a crucial issue.
As mentioned earlier, various lineage cells, distinct from the adenohypophyseal placodal cells, participate in pituitary development. In Table 2, we outline the distribution of these various lineage cells using cell types described in Table 1. Adenohypophyseal placodal cells primarily contribute to embryonic pituitary development. However, due to the technical challenges inherent in tracking these cells, it has not yet been possible to determine proportions and roles in postnatal stem/progenitor cells. Of particular interest is whether SOX2/S100β-positive cells have their origins in adenohypophyseal placodal cells and/or other lineage cells, as they emerge only in the postnatal stem/progenitor cell population.
Cell lineages contributing to the pituitary development
| 1. Adenohypophyseal placodal cells | |
| Before birth | : Mainly contribute to the development and differentiation of endocrine cells as stem/progenitor cells. |
| After birth | : Relationship to SOX2S100β cells and nonSOX2S100β cells in the adult pituitary is unclear. |
| 2. P0-lineage neural crest-derived cells (the 1st wave of invasion) | |
| Before birth | : Transform into SOX2nonS100β/PROP1 cells in Rathke’s pouch, and partially contribute to the development along with adenohypophyseal placodal cells. |
| After birth | : Contribute to differentiation of all endocrine cells (ranging 6–13%) and persist as adult stem/progenitor cells (approximately 5%). |
| 3. SOX17-endodermal cells: unverified in mammals | |
| Before birth | : Partially contribute to development along with adenohypophyseal placodal cells. |
| After birth | : Contribute to the differentiation of endocrine cells (ranging 1.3–4.5%), along with residing as PROP1-positive cells (approximately 2.3%) but unclear for the expression of Sox2 or S100β. |
| 4. P0-lineage cells accumulated outside the pouch (the 2nd wave of invasion) | |
| Before birth | : After invasion of P0-promoter active cells (CRE/GFP-positive, nonS0X2nonS100β), one keeps P0-promoter active to become tissue-resident vascular precursors. The other becomes to be P0-promoter inactive and turns to be NG2-positive pericytes. |
| After birth | : Whether contribute to a renewal of non-endocrine cells is unclear. |
| 5. Mesenchymal cells; several types expressing PRRX1, p75, and S100β of the nonS0X2S100β, or αSMA of the nonS0X2nonS100β | |
| Before birth | : Contribute to the development of the capillary network. |
| After birth | : Contribute to the maintenance of the capillary network, but whether contribute to a renewal of non-endocrine cells is unclear. |
| 6. SOX10-lineage cells | |
| From rE21.5 | : After invasion through the pituitary stalk around birth and advancement to the anterior lobe, transform into SOX2S100β/PROP1 cells, followed by the decline of Sox10 expression. |
| Beyond rP60 | : Contribute to the development of presumed progeny of S0X2S100β cells that constitute the major populations of postnatal stem/progenitor cells. |
Currently, two lineages of S100β-positive cells have been identified: the SOX10-lineage (SOX2S100β) and the mesenchymal lineage (nonSOX2S100β). They are characterized by their phenotype for SOX2 and their invasion pathway and period. The fate of SOX10-positive cells cannot be traced after the decline of Sox10-expression. However, it is evident that their progeny has already acquired the potential to express Prop1 (Fig. 6E), indicating their role as postnatal stem/progenitor cells. Furthermore it is interesting to note that SOX10-positive cells expressing S100β continue to migrate in the intermediate lobe from the boundary area between the posterior and intermediate lobes at least until rP60 (Fig. 6D). Postnatal S100β-expression in the anterior lobe increases approximately four times (rP30) and seven times (rP60) compared to that on rP5 [10]. Clarifying whether adenohypophyseal placodal cells give rise to S100β-positive cells is an essential question, especially considering that the major population of postnatal stem/progenitor cells is made up of the SOX2S100β cells.
The contribution of multiple extra-adenohypophyseal placode-lineage cells to the maintenance of the adult anterior lobe has been confirmed. Specifically, populations of postnatal stem/progenitor cells originating from extra-adenohypophyseal placode-lineage cells account for approximately 5% in the adult rat anterior lobe as P0-lineage cells [35] and approximately 2.3% in the adult zebrafish pituitary as SOX17-lineage cells [44]. However, it remains unclear whether they possess lineage-specific plasticity and cellular characteristics that distinguish them from adenohypophyseal placode-lineage cells, presenting an intriguing issue for further investigation.
Maintaining the capillary network is essential for pituitary function, and the cells responsible for this maintenance are indispensable. Potential candidates include P0-lineage NG2-positive cells and mesenchymal cells. Pericytes, which are known to be positive for NG2, contribute to capillary formation with multipotency [48]. However, it has not yet been confirmed whether their progeny with plasticity reside in the postnatal anterior pituitary. Additionally, it remains unclear whether there are adenohypophyseal placode-lineage cells and/or other cell lineages that generate nonSOX2S100β and nonSOX2nonS100β cells.
Moreover, various invading cell types, including those originating from the neural crest cells, exhibit inherent differentiation potential, as described previously [22, 61]. An earlier study conducted with P0-Cre/EGFP mice demonstrated that experimentally induced damage to the olfactory tissue prompted P0-lineage cells within the olfactory epithelium to actively engage in cellular regeneration to repair damaged regions [62]. Variations in the abilities of stem/progenitor cells with different origins in the postnatal anterior pituitary could provide versatility for tissue maintenance through diverse responses. These variations may also be related to the causes of tumors and developmental abnormalities discussed in the review [61]. It is noteworthy that congenital syphilis is the most common dysplasia of neural crest-related pituitary defects pointed out by Cerrizuela et al. [63]. Further research in this area is eagerly anticipated.
Gleiberman et al. demonstrated that some GFP-positive NESTIN-lineage cells (NESTIN-positive stem cells) had already settled in Rathke’s pouch, undergone postnatal expansion, and produced differentiated progeny [64]. They discussed how NESTIN-positive stem cells colonized an organ that initially consisted of entirely differentiated cells derived from embryonic precursors, and described the pituitary gland as a mosaic of differentiated cells with similar phenotypes but of different origins [64]. Research conducted over the following fifteen years demonstrated that the postnatal anterior lobe is colonized by diverse stem/progenitor cells originating from different lineages with difference in the invading pathways at different developmental periods [35, 36, 44, 47]. Notably, colonization by SOX10-positive cells persists, even during sexual maturation [36]. When considering this, along with other recent studies, it is clear that the pituitary gland is a mosaic of differentiated cells, all sharing similar phenotypes but originating from different lineages of stem/progenitor cells.
Even today, nearly two hundred years after Rathke’s initial report, research on the pituitary gland continues to evolve. Recent studies have explored the functionality and pathogenesis of the pituitary gland using organoids [65] and the subcutaneous transplantation of pituitary organoids derived from human ES cells [66]. Despite these cutting-edge investigations, the process of pituitary formation, as summarized above, still requires further examination and verification, as described in the recent review [61]. We will provide a brief overview of these issues below.
Several matters require verification and further exploration: 1) the existence of cells positive for S100β that originated from adenohypophyseal placodal cells and other lineages in the postnatal anterior pituitary; 2) the roles and potential plasticity of extra-adenohypophyseal placodal cells such as NCDCs, endodermal lineage cells, SOX10-positive cells, and cells that invaded through Atwell’s recess; 3) the conditions to differentiate the PS-clusters; 4) the roles and molecular mechanisms of PROP1 and S100β; and, 5) the situation of stem/progenitor cells in the functional pituitary organoid.
The authors would like to express our deep gratitude to everyone who belonged to the Laboratory of Molecular Biology and Gene Regulation, Department of Life Sciences, School of Agriculture, Meiji University, and to Ms. Kyoko Tomizawa and many collaborators of other research institutions for the research results that provide the background of this paper.
The authors declare no conflicts of interest.