2024 Volume 71 Issue 6 Pages 623-633
2024 Volume 71 Issue 6 Pages 623-633
Interleukin 17A (IL-17A) is a key cytokine promoting osteoblast formation, which contributes to osteogenesis. IL-17A functions in autophagy inhibition within osteoblasts. Metallothionein-2 (MT-2), as an important reactive oxygen species (ROS)-scavenging molecule, prevents oxidative stress from damaging osteoblast formation. The relationship between IL-17A-regulated autophagy and MT-2 production under oxidative stress deserves further exploration. In this study, we first investigated the roles of IL-17A in osteoblastic differentiation and ROS production in osteoblast precursors in the presence of hydrogen peroxide (H2O2). Next, we explored the effects of IL-17A on autophagic activity and MT-2 protein expression in osteoblast precursors in the presence of H2O2. Ultimately, by using autophagic pharmacological agonist (rapamycin) and lentiviral transduction technology, the relationship between autophagy, IL-17A-regulated MT-2 protein expression and IL-17A-regulated ROS production was further elucidated. Our results showed that in the presence of H2O2, IL-17A promoted osteoblastic differentiation and inhibited ROS production. Moreover, in the presence of H2O2, IL-17A inhibited autophagic activity and promoted MT-2 protein expression in osteoblast precursors. Importantly, IL-17A-promoted MT-2 protein levels and -inhibited ROS production were reversed by autophagy activation with rapamycin. Furthermore, IL-17A-inhibited ROS production were blocked by MT-2 silencing. In conclusion, IL-17A promotes ROS clearance by inhibiting autophagic degradation of MT-2, thereby protecting osteoblast formation from oxidative stress.
BONE INTEGRITY depends on the dynamic balance between bone formation dominated by osteoblasts and bone absorption dominated by osteoclasts. Osteoblasts are the cytological basis of osteogenesis. A decrease in the number or function of osteoblasts can lead to impaired osteogenic ability, ultimately leading to osteoporosis. Reactive oxygen species (ROS) can cause severe oxidative damage to osteoblasts, resulting in significantly reduced osteogenesis [1-4]. As a representative type of ROS, hydrogen peroxide (H2O2) causes significant oxidative stress, resulting in protein damage and DNA breakage, and a subsequent decline in osteoclast formation [5-8]. Remarkably, adding H2O2 can construct the classic in vitro cell model for oxidative stress, especially osteoporosis-associated oxidative stress. H2O2-related cell models have been used in various in vitro studies of osteoporosis, including postmenopausal and senile osteoporosis [9-11], making it representative in exploring the solutions to oxidative stress-induced bone loss. Therefore, finding effective strategies to resist the damage of H2O2 is crucial for establishing a good osteogenic environment.
IL-17A is an important pro-osteogenic factor. Previous study showed that IL-17A can promote osteoblastic differentiation, and bone regeneration and remodeling [9]. IL-17A can also exhibit strong osteogenic effects when directly intervening with bone marrow mesenchymal stem cells (MSCs) [10]. In patients with ankylosing spondylitis, IL-17A can induce osteoblastic differentiation by activating JAK2/STAT3 signaling [11]. Similar results were also revealed in other studies [12, 13]. However, the underlying mechanism regarding IL-17A-regulated osteoblast formation remains unclear. In addition, IL-17A is an important autophagy-regulating factor. The production of IL-17A exacerbates the pulmonary inflammation and fibrosis induced by fine particulate matter by inhibiting PI3K/Akt/mTOR/autophagy activation pathway [14]. Similarly, IL-17A can inhibit autophagy in lung epithelial cells [15]. Additionally, IL-17A can repress autophagic death of hepatocellular carcinoma cells by inhibiting BCL2 degradation [16]. Importantly, autophagy inhibition caused by IL-17A is inherently related to its maintenance in osteoblast formation. Chen et al. and Wang et al. have demonstrated that high levels of IL-17A not only inhibit autophagy of osteoblast precursors, but also facilitate osteoblast formation [12, 13]. Therefore, several studies have recognized the negative effects of IL-17A on autophagy; it may contribute to osteoblast formation. Autophagy can serve a series of biological processes as a molecular degradation mechanism. IL-17A can prevent autophagic degradation of various molecules. IL-17A has an effective role in repressing autophagic degradation of BCL2; it suppresses BCL2-Beclin1 signaling and subsequent autophagy activation [15, 16]. Remarkably, IL-17A enhances RANKL expression in primary osteoblasts via autophagy inhibition [13]. Chen et al. also clarified that IL-17A can suppress autophagic degradation of RANKL in osteoblasts by inhibiting BCL2-Beclin-autophagy activation pathway [12]. As a free radical scavenger, metallothionein can effectively prevent the accumulation of free radicals under various stress conditions; it plays a beneficial role in maintaining the body’s redox balance [17-19]. Metallothionein-2 (MT-2), as a subtype, is involved in the fighting against H2O2-inhibited osteoblast formation, and its downregulation is an important cause for impaired osteogenesis under oxidative stress [8]. As an important molecule for the regulation of autophagic degradation, the role of IL-17A in the degradation of MT-2 protein deserves further exploration. The resolution of the above issue may help elucidate the promoting effect of IL-17A on osteoblast formation.
This study is the first to describe the biological mechanism underlying IL-17A-enhanced osteogenesis from the perspectives of autophagy and oxidative stress through a series of in vitro studies.
Primary osteoblast precursors were prepared from the calvaria of newborn mice [20]. Briefly, the skulls of mice (24 hours after birth) were dissected, rinsed with PBS, and digested in fresh 0.1 mg/mL collagenase type II in α-MEM at 37°C for 20 minutes (repeated 5 times). After digestion, the supernatant was mixed and centrifuged to obtain cell pellets. The cells were then maintained in α-MEM containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 mg/mL streptomycin sulfate, at 37°C with 5% CO2. The medium was replaced with α-MEM containing 1% bovine serum albumin (BSA), and the cells were cultured for 16 hours before preparing for subsequent experiments. Animal study was approved by the Animal Care and Use Committee of Women and Children’s Hospital Qingdao University. In all assays, osteoblast precursors were treated with the osteoblastic induction medium, containing 2 mM β-glycerophosphate, 60 μg/mL ascorbic acid and 10 nM dexamethasone (Sigma-Aldrich, St. Louis, MO, USA), and the Intervention of H2O2.
Osteoblastic induction and ALP stainingOsteoblast precursors were seeded onto a 12-well plate at an initial number of 5 × 104 cells per well (in all assays). After 7 days of osteoblastic induction, the formation of differentiated osteoblasts was confirmed via the corresponding Alkaline phosphatase (ALP) staining kit according to manufacturer’s protocols (Beyotime, Jiangsu, China).
Alkaline phosphatase (ALP) activity analysesAfter 7 days of induction, ALP activity was evaluated with the corresponding kit in accordance with manufacturer’s protocols (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China).
Analyses regarding mineralization capacityAfter 14 days of induction, the mineralization capacity was evaluated with Alizarin red staining. The indicated cells were fixed using ice-cold 70% ethanol, and stained using Alizarin red S according to manufacturer’s protocols (Sigma-Aldrich). The percentages of positive areas were detected to quantitatively evaluate mineralized areas using ImageJ 1.47 software.
Lentiviral transductionRecombinant lentivirus encoding shRNA targeting Mt2 (Top Strand: 5'-CACCG*TCTATAAAGCATGTAACTGACAATACGAATATTGTCAGTTACATGCTTTATAGA-3'; Bottom Strand: 5'-AAAATCTATAAAGCATGTAACTGACAATATTCGTATTGTCAGTTACATGCTTTATAGAC*-3') was constructed by homologous recombination between the expression vector (pEX-Puro-Lv105) and shRNA in 293 cells using the lentivirus construction kit according to manufacturer’s protocols. The control vector (Top Strand: 5'-CACCG*TCTGAAAGTACCAATCAGTATAATACGAATATTATACTGATTGGTACTTTCAGA-3'; Bottom Strand: 5'-AAAATCTGAAAGTACCAATCAGTATAATATTCGTATTATACTGATTGGTACTTTCAGAC*-3') was constructed and packaged using the same method. After 2 days, the supernatants were harvested, and cells were incubated in complete DMEM containing lentivirus and 5 μg/mL polybrene at a multiplicity of infection (MOI) of 40 for 2 days. The infected cells were selected using puromycin (10 μg/mL). The silencing efficiency of lentiviral gene was detected using qRT-PCR and Western Blotting assays.
Quantitative real-time PCR (qRT-PCR) assaysThe total RNA from the indicated cells was extracted and purified by Trizol methods. cDNA synthesis and quantitative real-time PCR (qRT-PCR) assays were performed according to manufacturer’s protocols (Takara, Tokyo, Japan). The pre-designed primer sequences are presented in Table 1.
Specific primer sequences for qRT-PCR
| Gene | Forward (5'-3') | Reverse (5'-3') |
|---|---|---|
| Osteocalcin (Ocn) | AGCAGCTTGGCCCAGACCTA | TAGCGCCGGAGTCTGTTCACTAC |
| Collagen type I (Col1) | AGAACAGCGTGGCCT | TCCGGTGTGACTCGT |
| Osterix (Osx) | ATGGCGTCCTCTCTGCTTG | TGAAAGGTCAGCGTATGGCTT |
| Mt2 | TGCTGACGGGATTTCTGGGAGAG | TAGGCGAGCCACTATCTCAAGGAC |
| Gapdh | ACCACAGTCCATGCCATCAC | TCCACCACCCTGTTGCTGTA |
Following the reaction, the melting curve and amplification curve regarding qRT-PCR were identified. The melting curve conformed to the characteristic of a narrow single peak at 80–90°C. Furthermore, the primer dimer curve peaks were absent. Accordingly, the specificity of each primer could be qualified. Then, the relative quantitative analyses were used to obtain C(t) values, and the housekeeping gene (GAPDH) served as internal parameter to calculate C(t) values. Finally, the relative ratio of target gene content in the experimental sample and the control sample was calculated using the control sample as a reference.
Measurement of intracellular ROSThe treated cells were seeded onto 24-well plates. Next, the intracellular ROS were assessed by using DCFH-DA fluorescent probe (Sigma-Aldrich). The cells were rinsed 3 times with PBS, and then incubated with using DCFH-DA fluorescent probe for 25 min. Following rinsing with PBS for 3 times, the fluorescence intensity was quantified at 488 nm excitation wavelength and 525 nm emission wavelength using the fluorescent plate reader.
Western Blotting assaysThe extraction of total proteins from indicated cells relied on RIPA buffer (Beyotime), and protein quantification relied on BCA protein assay kit (Beyotime). A 15% SDS-PAGE gel was used to load and electrophorese the lysates. After the transfer to polyvinylidene fluoride membranes (PVDF), the separated proteins were incubated with primary antibodies, including rabbit anti-MT-2 (PA5-102549, 1:1000; ThermoFisher Scientific, Waltham, MA, USA), and rabbit anti-LC3B (ab192890, 1:2000), Catalase (ab76110, 1:1000), p47phox (ab308256, 1:1000), p67phox (ab175293, 1:1000) and GAPDH (ab9485, 1:2500) (Abcam, Boston, MA, USA) antibodies at 4°C overnight. After rinsing, the membranes were incubated with a secondary antibody for 60 min at room temperature. The immunoreactive signals were visualized by using an ECL kit (Millipore, MA, USA), and quantified by using a ChemiDoc image analyser (Bio-Rad, Hercules, CA, USA).
Measurement of NADPH oxidase activityNADPH oxidase activity was measured via the enhanced lucigenin chemiluminescence method [8]. Briefly, the treat cells were rinsed 5 times in ice-cold PBS, and scraped from the plate in PBS. The cell pellets were centrifuged at 800 g at 4°C for 10 min, and resuspended in lysis buffer. Cell suspensions were homogenized with 100 strokes in a Dounce homogenizer on ice. Next, 100 μL of homogenate was added into 900 μL of 50 phosphate buffer (pH 7.0) containing 1 mM EGTA, 150 mM sucrose, and 5 μM lucigenin as the electron acceptor and 100 μM NADPH as an electron donor. The light emission in terms of relative light units was detected every minute for 10 min with a plate reader spectrophotometer (SpectraMax190; Molecular Devices, Sunnyvale, Silicon Valley Center, USA). The data representing NADPH oxidase activity were expressed as mean light units (MLU) per minute per milligram of protein.
Immunofluorescence assaysFor LC3-puncta-related immunofluorescence staining, the permeated cells were incubated with anti-LC3B antibody (#2775, 1:200; Cell Signaling Technology, Boston, MA, USA) at 4°C overnight, and then stained with a fluorochrome-labelled secondary antibody (#4413, 1:1000; Cell Signaling Technology) for 1 hour. Next, cell nuclei were counterstained with DAPI for 15 minutes. Finally, the images were acquired under a fluorescent microscopy (Olympus IX71, Tokyo, Japan).
Statistical analysisThe experiments were repeated three or more times. The data are presented as mean ± SEM from three independent assays. Statistical analyses were conducted with SPSS19.0. Shapiro-Wilk test was performed to confirm that all data conform to the normal distribution. For comparisons, Student’s t-test, one-way ANOVA and two-way ANOVA were performed. Tukey test was used for Post-Hoc Multiple Comparisons of one-way ANOVA and two-way ANOVA. Differences were considered significant at a threshold of p < 0.05.
Firstly, we observed the effects of IL-17A on osteoblastic differentiation in the presence of H2O2. As shown in Fig. 1A, C, IL-17A enhanced ALP staining intensity and ALP activity in a concentration-dependent manner. Consistent with this, the results regarding alizarin red staining showed a concentration-dependent increase in mineralized nodules and mineralized areas under IL-17A intervention (Fig. 1B, D). In addition, IL-17A increased the mRNA levels of osteogenesis-related genes (OCN, OSX, and COL1) in a concentration-dependent manner (Fig. 1E–G). These results indicate that IL-17A can enhance osteoblastic differentiation under H2O2 intervention. Additionally, IL-17A also enhanced ALP activity and mRNA levels of osteogenesis-related genes (OCN, OSX, and COL1) in a concentration-dependent manner (Supplementary Fig. S1A–D).

IL-17A promotes osteoblastic differentiation in the presence of H2O2. (A, C) ALP staining and activity in osteoblast precursors treated with 0, 0.5, 5 or 50 ng/mL of IL-17A and 100 μM of H2O2 for 7 days under osteogenic induction. (B, D) Alizarin red staining in osteoblast precursors treated with the indicated reagents and H2O2 for 14 days under osteogenic induction. The histogram in D showing the percentage of positive areas stained. (E–G) The mRNA expression of OCN, OSX and COL1 in osteoblast precursors treated with the indicated reagents and H2O2 for 7 days under osteogenic induction. Data are presented as mean ± SEM from three independent experiments. The demotion in letters (a to b; b to c) indicates a significant decrease with p < 0.05 by one-way ANOVA and Tukey Post-Hoc Multiple Comparisons, and the same letter represents no statistical difference. Cont, control group.
Next, we investigated the effects of IL-17A on ROS production in the presence of H2O2. The promoting effect of H2O2 addition on ROS production in osteoblast precursors was first confirmed (Fig. 2A). In addition, H2O2 caused a decrease in MT-2 mRNA and protein levels (Supplementary Fig. S2A, B), which is consistent with previous work [8]. Moreover, LC3 conversion rate (presented as the ratio of LC3II to LC3I) also increased with H2O2 addition (Supplementary Fig. S2B), suggesting that H2O2-induced transcriptional inhibition and autophagy are both involved in its inhibition on MT-2 production. It was observed that IL-17A reduced ROS production in a concentration-dependent manner (Fig. 2B, C). Additionally, IL-17A decreased NADPH oxidase activity and the protein expression of catalase in a concentration-dependent manner (Fig. 2D–F). Moreover, three concentrations of IL-17A significantly inhibited the protein expression of p47phox and p67phox, the critical cytosolic subunits of NADPH oxidase (Fig. 2E, G, H). Therefore, IL-17A plays a significant inhibitory effect in ROS production in osteoblast precursors in the presence of H2O2.
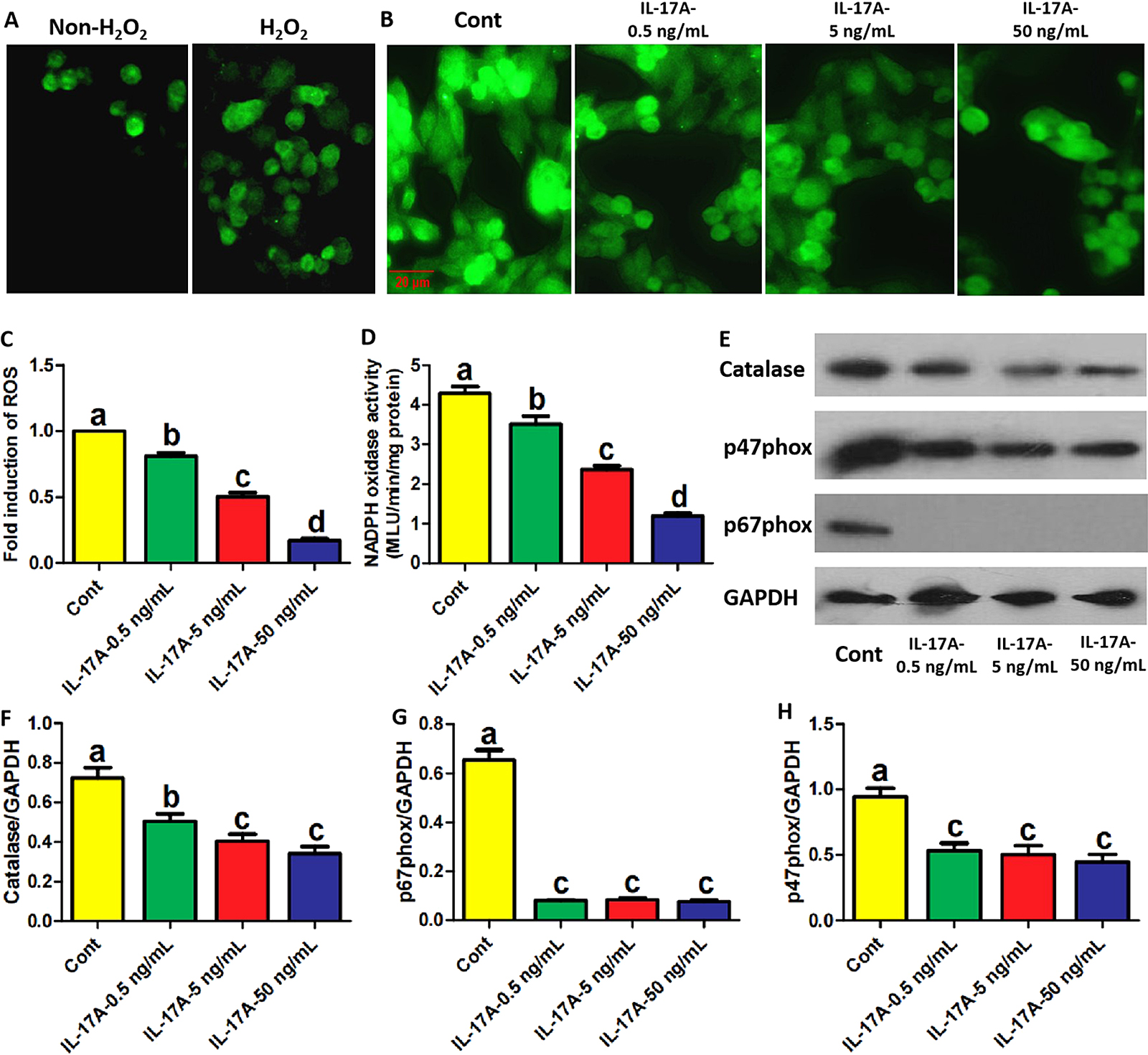
IL-17A inhibits ROS production in osteoblast precursors in the presence of H2O2. (A–C) The fluorescence intensity of indicated osteoblast precursors incubated with DCFH-DA fluorescent probe (representative images shown) following treatment with the indicated reagents and H2O2 for 2 days under osteogenic induction. The images in A identified the impact of H2O2 on ROS production. Scale bar, 20 μm. ROS levels represented as the fold induction by normalizing the fluorescence value of other groups to that of control group. (D) NADPH oxidase activity in osteoblast precursors treated with the indicated reagents and H2O2 for 2 days under osteogenic induction. (E–H) The protein levels of Catalase, p47phox and p67phox in osteoblast precursors treated with the indicated reagents and H2O2 for 2 days under osteogenic induction. Data are presented as mean ± SEM from three independent experiments. The demotion in letters (a to b; b to c; c to d) indicates a significant decrease with p < 0.05 by one-way ANOVA and Tukey Post-Hoc Multiple Comparisons, and the same letter represents no statistical difference. Cont, control group.
Then, the roles of IL-17A in osteoblast precursor autophagy and MT-2 protein expression were observed in the presence of H2O2. As shown in Fig. 3A, B, IL-17A inhibited LC3 conversion in a concentration-dependent manner in the presence of H2O2. IL-17A also decreased the number of LC3 puncta-positive cells in a concentration-dependent manner (Fig. 3E, F). Importantly, IL-17A promoted MT-2 protein expression in a concentration-dependent manner, and the alterations in MT-2 protein caused by IL-17A were inversely proportional to the alterations in LC3II protein levels and LC3 puncta-positive cells (Fig. 3A, C). Accordingly, IL-17A can inhibit autophagic responses in osteoblast precursors in the presence of H2O2, which may be involved in IL-17A-enhanced MT-2 protein expression. Additionally, IL-17A at 0.5 ng/mL inhibited LC3 conversion and promoted MT-2 protein expression, while 50 ng/mL of IL-17A showed the opposite pattern (Supplementary Fig. S3A–C), which not only conforms to previous reports, but also once again identifies the effect of IL-17A-regulated autophagy on MT-2 expression. To avoid errors caused by extreme oxidative stress, we retested the corresponding parameters at low concentrations of H2O2 (10 μM). The result still showed that IL-17A inhibited LC3 conversion in a concentration-dependent manner whereas IL-17A promoted MT-2 protein expression in a concentration-dependent manner (Supplementary Fig. S4A–C). And the alterations in MT-2 protein caused by IL-17A were inversely proportional to the alterations in LC3II protein levels. IL-17A also inhibited ROS production and enhanced various osteogenic parameters, including ALP activity and mRNA levels of osteogenic genes in a concentration-dependent manner (Supplementary Fig. S4D–I). Therefore, we believe that this experiment was not extremely affected by H2O2-induced oxidative stress.
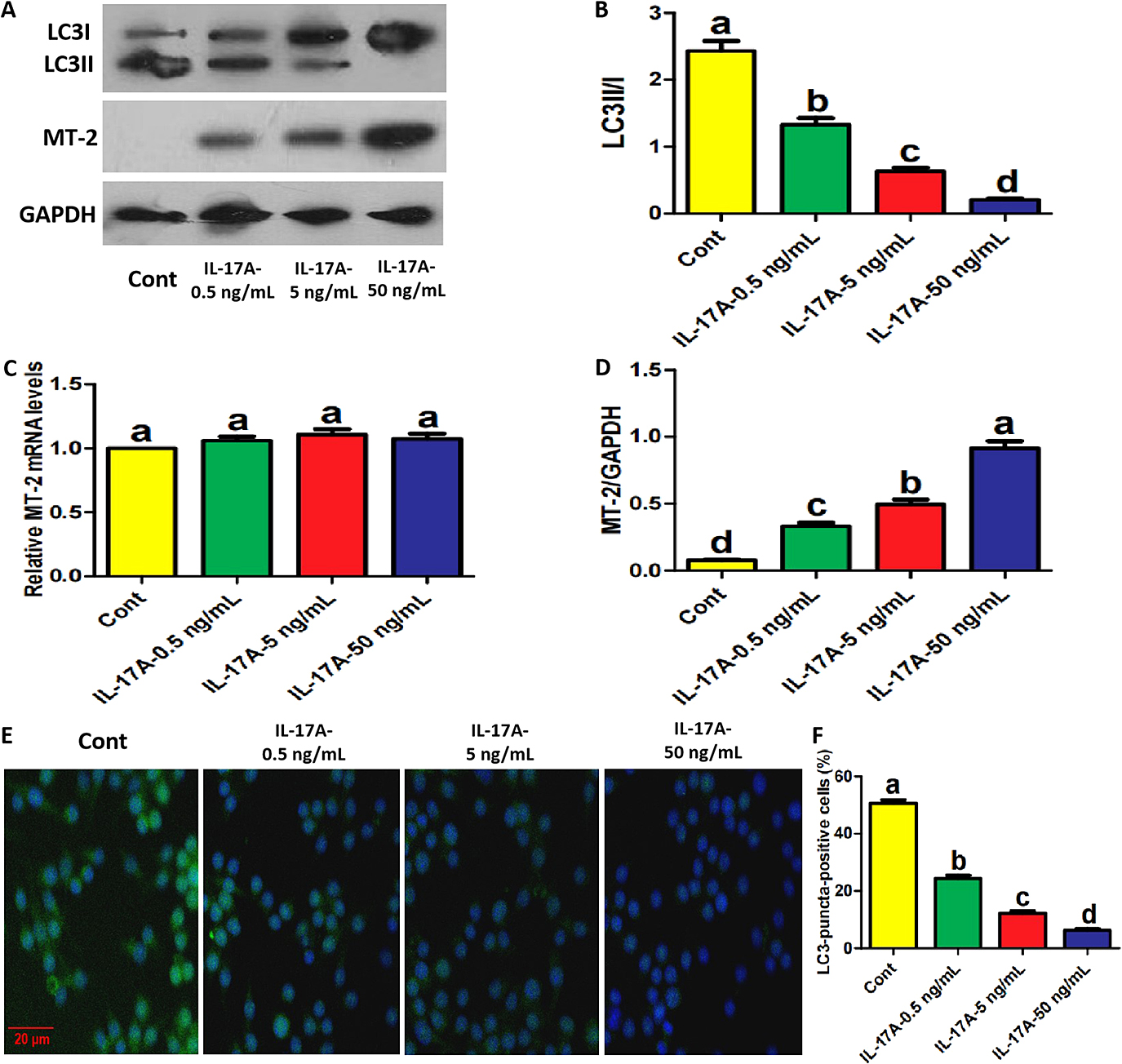
IL-17A inhibited autophagy and promoted MT-2 protein expression in osteoblast precursors in the presence of H2O2. (A–C) The protein levels of LC3 and MT-2 in osteoblast precursors treated with the indicated reagents and H2O2 for 2 days under osteogenic induction. LC3 conversion rate in B is presented as the ratio of LC3II to LC3I. (D) The mRNA expression of MT-2 in osteoblast precursors treated with the indicated reagents and H2O2 for 2 days under osteogenic induction. (E, F) The proportion of LC3-puncta-positive cells treated with the indicated reagents and H2O2 for 2 days under osteogenic induction (≥5 dots, 50 cells/field, n = 5). Scale bar, 20 μm. Data are presented as mean ± SEM from three independent experiments. The demotion in letters (a to b; b to c; c to d) indicates a significant decrease with p < 0.05 by one-way ANOVA and Tukey Post-Hoc Multiple Comparisons, and the same letter represents no statistical difference. Cont, control group.
Subsequently, we applied rapamycin, the pharmacological activator of autophagy, to clarify the relationship between MT-2 protein expression, ROS production and autophagic activity regulated by IL-17A in the presence of H2O2. First, it was observed that all concentrations of IL-17A did not affect MT-2 mRNA expression (Fig. 3D). As shown in Fig. 4A–C, the increased MT-2 protein expression and reduced LC3 conversion caused by IL-17A were partially blocked by rapamycin administration in the presence of H2O2. Additionally, in the presence of H2O2, a decrease in ROS production caused by IL-17A was also restored by rapamycin (Fig. 4D, F). Importantly, in the presence of H2O2, IL-17A-enhanced ALP activity, mineralized nodules, mineralized areas and mRNA levels of osteogenic genes (OCN, OSX, and COL1) were partially blocked by rapamycin (Fig. 4E, G–K). These data indicate that IL-17A-changed autophagy is involved in its regulatory effects on MT-2 protein levels, ROS production and osteoblastic differentiation. Additionally, IL-17A pharmacological inhibitor, secukinumab, also reversed the promoting effects of IL-17A on MT-2 protein expression, ALP activity and mRNA expression of various osteogenic genes (Supplementary Fig. S5A–G) while partially recovering IL-17A-inhibited LC3 conversion, once again identifying the above inference.
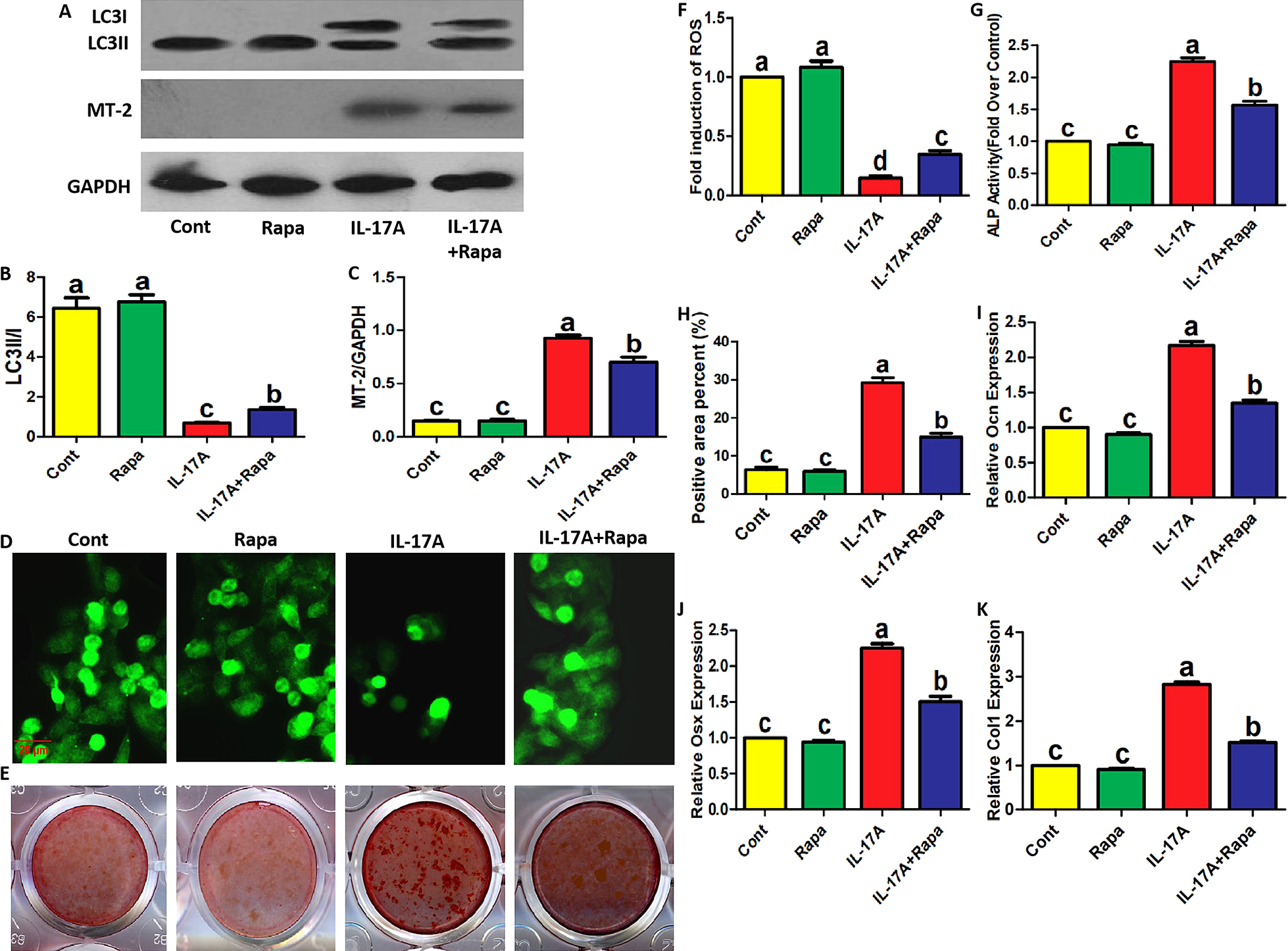
IL-17A-promoted MT-2 protein expression and -inhibited ROS production in osteoblast precursors were reversed by rapamycin. (A–C) The protein levels of LC3 and MT-2 in osteoblast precursors treated with 50 ng/mL of IL-17A and/or 10 nM of Rapamycin in the presence of H2O2 for 2 days under osteogenic induction. LC3 conversion rate in B is presented as the ratio of LC3II to LC3I. (D, F) The fluorescence intensity of indicated osteoblast precursors incubated with DCFH-DA fluorescent probe (representative images shown) following treatment with the indicated reagents and H2O2 for 2 days under osteogenic induction. Scale bar, 20 μm. ROS levels represented as the fold induction by normalizing the fluorescence value of other groups to that of control group. (E, G, H) ALP activity and Alizarin red staining in osteoblast precursors treated with the indicated reagents and H2O2 for 7 or 14 days under osteogenic induction. The histogram in I showing the percentage of positive areas stained. (I–K) The mRNA expression of OCN, OSX and COL1 in osteoblast precursors treated with the indicated reagents and H2O2 for 7 days under osteogenic induction. Data are presented as mean ± SEM from three independent experiments. The demotion in letters (a to b; b to c) indicates a significant decrease with p < 0.05 by one-way ANOVA and Tukey Post-Hoc Multiple Comparisons, and the same letter represents no statistical difference. Cont, control group.
Finally, we evaluated the significance of MT-2 for IL-17A-regulated ROS production and osteogenic parameters using lentiviral transduction technology. MT-2 silencing efficiency by shRNA was identified through qRT-PCR and Western Blotting assays (Fig. 5A, B). MT-2 silencing did not affect LC3 conversion (Fig. 5B). As shown in Fig. 5C, D, in the presence of H2O2, a decrease in ROS production caused by IL-17A was partially reversed by MT-2 silencing. Notably, in the presence of H2O2, IL-17A-enhanced ALP activity and mRNA levels of osteogenic genes (OCN, OSX, and COL1) were partially blocked by MT-2 silencing (Fig. 5E–H). These results supported that IL-17A-enhanced MT-2 expression is involved in its regulatory effects on ROS production and osteoblastic differentiation.

IL-17A-inhibited ROS production in osteoblast precursors was reversed by MT-2 silencing. (A) MT-2 mRNA expression in lentiviruses-transduced osteoblast precursors. (B) MT-2 an LC3 protein expression in lentiviruses-transduced osteoblast precursors. LC3 conversion rate in B is presented as the ratio of LC3II to LC3I. (C, D) The fluorescence intensity of lentiviruses-transduced osteoblast precursors incubated with DCFH-DA fluorescent probe (representative images shown) following treatment with 50 ng/mL of IL-17A and H2O2 for 2 days under osteogenic induction. Scale bar, 20 μm. ROS levels represented as the fold induction by normalizing the fluorescence value of other groups to that of control group. (E) ALP activity in lentiviruses-transduced osteoblast precursors treated with 50 ng/mL of IL-17A and H2O2 for 7 days under osteogenic induction. (F–H) The mRNA expression of OCN, OSX and COL1 in lentiviruses-transduced osteoblast precursors treated with 50 ng/mL of IL-17A and H2O2 for 7 days under osteogenic induction. Data are presented as mean ± SEM from three independent experiments. The demotion in letters (a to b; b to c) indicates a significant decrease with p < 0.05 by one-way ANOVA and Tukey Post-Hoc Multiple Comparisons, and the same letter represents no statistical difference. ***p < 0.001 by Student’s t-test. LV-sh-Cont, lentivirus with control-shRNA; LV-sh-MT-2, lentivirus with MT-2-shRNA.
IL-17A is a classic pro-osteogenic cytokine [9-13]. In addition, IL-17A has the ability to inhibit autophagy; it is involved in the pro-osteogenic effect [12, 13]. As an important subtype of metallothionein, MT-2 can counteract H2O2-induced damage to osteoblast formation [8]; it raises an important scientific question: whether IL-17A-inhibited autophagy can protect MT-2 from degradation and prevent ROS from damaging osteoblast formation. Our experimental evidences revealed the specific mechanism underlying IL-17A-protected osteoblast formation from oxidative stress damage from autophagic perspective.
First, in the presence of H2O2, IL-17A not only enhanced osteoblastic differentiation but also hinders ROS production in osteoblast precursors. Upon oxidative stress, ROS harms osteoblast formation through various mechanisms. Previous study showed that dexamethasone can induce ROS production, thereby promoting osteoblast precursor apoptosis through endoplasmic reticulum stress and autophagic responses [2]. NAC significantly weakens osteoblast precursor apoptosis and osteoblast dysfunction by clearing ROS, and improves bone ingrowth within porous titanium implants [3]. The similar results were reported in other studies [1, 4, 5]. Our data suggested that the maintenance of IL-17A on osteoblast formation is associated with its elimination effect on ROS. As a ROS eliminator, MT-2 can participate in the protection of osteoblasts under oxidative stress; it is beneficial for osteogenesis [8]. In the presence of H2O2, IL-17A can enhance MT-2 protein expression in osteoblast precursors. Based on this, it was inferred that the elimination effect of IL-17A on ROS is related to its promoting effect on MT-2 production. Current data and previous studies have demonstrated that IL-17A can inhibit autophagic activity [12-16]. Given the molecular degradation property of autophagy, the inhibitory or protective effect of H2O2 or IL-17A on MT-2 protein may be related to their regulatory effects on autophagy. We have validated that IL-17A prevents MT-2 protein degradation upon oxidative stress by inhibiting autophagy, thereby inhibiting ROS production in osteoblast precursors. Moreover, autophagy enhancement with rapamycin also reversed the promoting effect of IL-17A on osteoblast formation. Accordingly, our study depicted a biological process that IL-17A prevents autophagic degradation of MT-2 that can eliminate ROS, thereby preventing the destruction of osteoblast formation under oxidative stress. Remarkably, the pharmacological antagonist of IL-17A reversed the enhancement of IL-17A on MT-2 protein expression and osteogenic parameters by partially improving harmed autophagic activity and MT-2 silencing rescued IL-17A’s roles in ROS production and osteoblast formation, which further confirms the above theory. The working model of this study is presented in Fig. 6. It should be noted that unlike previous literature showing that IL-17A only inhibits osteoblast autophagy at high concentrations (50 ng/mL) upon normal environment [12], which was also confirmed in our additional data, this study demonstrated that all concentrations of IL-17A inhibited osteoblast autophagy under the intervention of H2O2. ROS can lead to sharply enhanced autophagy [21], which was also reflected in our research. Under extreme condition, the regulatory function of corresponding cytokines may also change, causing the biological phenomena different from conventional environments, which requires further research in the future. However, according to current data, our study elucidated the contribution of IL-17A to osteogenic maintenance upon oxidative stress, which is based on its autophagy-inhibiting function. In the common environment, the changes in autophagy and MT-2 proteins were inconsistent with the changes in osteogenic parameters under IL-17A’s influence, which indicated that the low levels of ROS under normal condition results in a very limited effect of MT-2, making it non-critical for IL-17A-regulated osteoblast formation. Moreover, ROS production does indeed affect MT-2 expression, which was confirmed in both current and previous studies [20]. This study focused on the pathway of IL-17A-autophagy-MT-2 protein levels-ROS production. The pathway of IL-17A-ROS production-MT-2 expression should also be described in the future. Additionally, autophagy is definitely not just a function for protein molecule degradation. However, we chose the autophagic degradation of MT-2 protein as a breakthrough point to elucidate the impact of autophagy on osteoblast formation under oxidative stress. Rapamycin, as an autophagy activator, was only used as a research tool to explore the relationship between IL-17A, autophagic degradation and MT-2 levels in the rescue assays.

The working model diagram regarding IL-17A-prevented ROS’s damage on osteogenesis via inhibiting autophagic degradation of MT-2. Briefly, MT-2 can scavenge ROS, which is beneficial for the differentiation of osteoblast precursors into mature osteoblasts. IL-17A can inhibit autophagic degradation of MT-2, thereby promoting MT-2 protein expression and enabling more MT-2 molecules to act on ROS, which is conducive to the inhibition of ROS production and enhanced osteogenesis.
In summary, the current research was based on the bone biology related to oxidative stress, and presented a detailed explanation for the intrinsic mechanism of IL-17A-promoted osteogenesis. Our findings not only uncovered a meaningful osteogenic signaling pathway: IL-17A-autophagy inhibition-increased MT-2-ROS elimination-osteoblastic differentiation, but also provided more potential clues for the improvement of osteoporosis treatment.
IL-17A, Interleukin 17A; ROS, Reactive oxygen species; MT, Metallothionein; MT-2, Metallothionein-2; H2O2, hydrogen peroxide; MSCs, mesenchymal stem cells; FBS, fetal bovine serum; BSA, bovine serum albumin; ALP, Alkaline phosphatase; MOI, multiplicity of infection; PVDF, polyvinylidene fluoride membranes; qRT-PCR, Quantitative real-time PCR; MLU, mean light units; OCN, Osteocalcin; COL1, Collagen type I; OSX, Osterix; cDNA, complementary DNA; DCFH-DA, 2',7'-Dichlorodihydrofluorescein Diacetate; SDS-PAGE, sodium dodecyl sulphate-polyacrylamide gel electrophoresis; NADPH, Nicotinamide Adenine Dinucleotide Phosphate Oxidase; EGTA, Ethylene glycol bis(2-aminoethyl ether)tetraacetic acid
This work was supported by the Project of Qingdao Municipal Health Commission (2020-WJZD278).
The authors have no relevant financial or non-financial interests to disclose. The authors have no conflicts of interest to declare that are relevant to the content of this article.