2024 Volume 71 Issue 7 Pages 643-650
2024 Volume 71 Issue 7 Pages 643-650
Achondroplasia (ACH) is a representative skeletal disorder characterized by rhizomelic shortened limbs and short stature. ACH is classified as belonging to the fibroblast growth factor receptor 3 (FGFR3) group. The downstream signal transduction of FGFR3 consists of STAT1 and RAS/RAF/MEK/ERK pathways. The mutant FGFR3 found in ACH is continuously phosphorylated and activates downstream signals, resulting in abnormal proliferation and differentiation of chondrocytes in the growth plate and cranial base synchondrosis. A patient registry has been developed and has contributed to revealing the natural history of ACH patients. Concerning the short stature, the adult height of ACH patients ranges between 126.7–135.2 cm for men and 119.9–125.5 cm for women in many countries. Along with severe short stature, foramen magnum stenosis and spinal canal stenosis are major complications: the former leads to sleep apnea, breathing disorders, myelopathy, hydrocephalus, and sudden death, and the latter causes pain in the extremities, numbness, muscle weakness, movement disorders, intermittent claudication, and bladder-rectal disorders. Growth hormone treatment is available for ACH only in Japan. However, the effect of the treatment on adult height is not satisfactory. Recently, the neutral endopeptidase-resistant CNP analogue vosoritide has been approved as a new drug for ACH. Additionally in development are a tyrosine kinase inhibitor, a soluble FGFR3, an antibody against FGFR3, meclizine, and the FGF2-aptamer. New drugs will bring a brighter future for patients with ACH.
Achondroplasia (ACH, MIM #100800) is a representative skeletal disorder characterized by rhizomelic shortened limbs and short stature [1, 2]. The incidence of ACH is reported to be from 3.6 to 6.0 per 100,000 [3-5]. Thus, ACH represents a rare disease, but still is one of the most common congenital skeletal dysplasia. Inheritance is autosomal dominant; however, 80% of patients have a de novo variant [2, 6]. ACH—along with hypochondroplasia (HCH, MIM #146000), thanatopholic dysplasia (TD, MIM #187600 for TD1 and #187601 for TD2), and severe achondroplasia with developmental delay and acanthosis nigricans (SADDAN, MIM #616482)—is classified as belonging to a fibroblast growth factor receptor 3 (FGFR3) chondrodysplasia group in the Nosology of genetic skeletal disorders [7]. A new drug, vosoritide, has been approved to treat short stature in ACH patients in many countries including the USA, Brazil, Australia, and Japan, and in the EU [8]. A new era has begun for ACH in both clinical and research settings. This review summarizes the features of ACH briefly and then focuses on the development of new drugs for ACH.
The FGFR3 gene was revealed to be responsible for ACH in 1994 [9, 10]. The p.Gly380Arg (c.1138G>A in most cases, c.1138G>C in some cases) missense variant of FGFR3 is found to be heterozygous in 99% of ACH cases [11]. This variant is a constitutive active form of FGFR3 [1]. FGFR3 is expressed in the proliferating and prehypertrophic zone of the growth plate, and constitutive activation of FGFR3 leads to abnormal proliferation and differentiation of chondrocytes; that is to say, it leads to an abnormal endochondral ossification process [12] (Fig. 1).
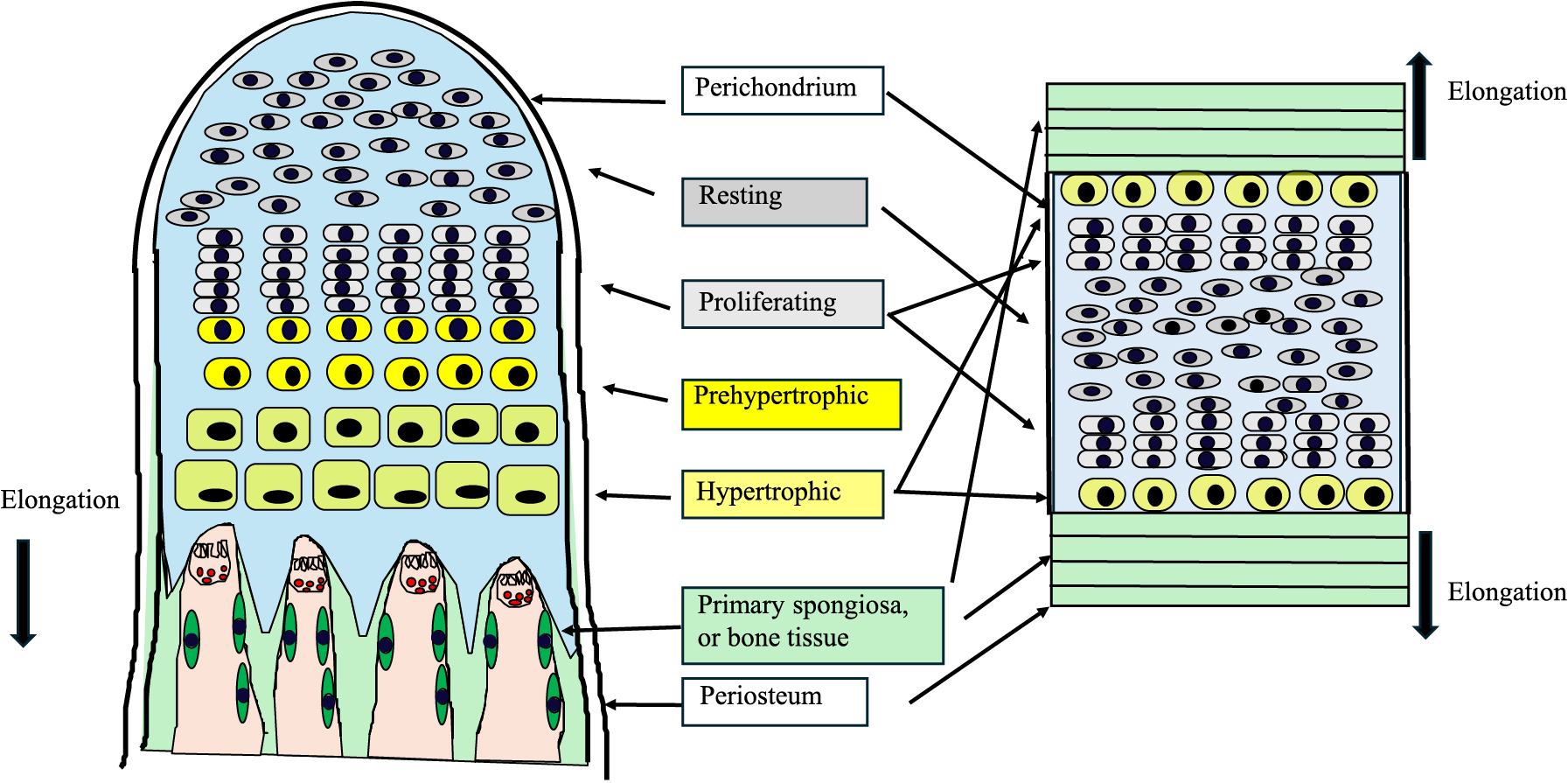
Structure of the growth plate and synchondrosis
The structure of the growth plate and the synchondrosis is illustrated in the left and right panel, respectively. Chondrocytes exhibit proliferation and differentiation in layers consisting of resting, proliferating, prehypertorphic and hypertrophic chondrocytes, and finally are replaced by bone or primary spongiosa. This biological event is called endochondral ossification. Long bones are formed through endochondral ossification, and the elongation of bones is unidirectional. The cranial base bones are enlarged through synchondrosis. Synchondrosis consists of two opposite growth plates sharing a common reserve zone of chondrocytes. The skull base expands bidirectionally. Abnormal development of the growth plate and the synchondrosis accounts for not only short-limbed short stature but also foramen magnum stenosis and stenosis of the spinal canal in ACH.
The downstream signal transduction of FGFR3 consists of STAT1 and RAS/RAF/MEK/ERK pathways [13]. Mouse studies indicate that FGFR3 binds to the cognitive ligand FGF18 or FGF9 secreted from perichondrium, which leads to its autophosphorylation and then activation of p21 through STAT1. Subsequently, p21 inhibits chondrocyte proliferation. FGFR3 activates FGFR substrate 2α (FRS2α) as well, and then the RAS/RAF/MEK/ERK cascade is induced. This signal transduction cascade finally activates SOX9, a key transcription factor for chondrocyte development, and then inhibits chondrocyte hypertrophy and matrix production [13, 14]. Among FGFs, FGF2 is also a ligand of FGFR3 at least in humans and is generated from chondrocytes in the growth plate [15]. In ACH, the mutant FGFR3 is continuously phosphorylated without its ligand. However, the binding of the ligands FGF2, 9, and 18 further activate FGFR3. Elucidation of the pathogenesis of ACH reveals the molecular targets of new drugs. In fact, several kinds of new drugs are already under development based on the findings of FGFR3 signaling molecules. This is explained in the last part of this review [16, 17].
The key biological event related to ACH is endochondral ossification. The molecules that control endochondral ossification have recently been revealed [18]. Long bones are formed through endochondral ossification. Elongation of bones is dependent on the proliferation and differentiation of chondrocytes in the growth plate, which is a columnar structure at the end of long bones made up of chondrocytes (Fig. 1).
While cranial vault bones are formed in an intramembranous fashion, the cranial base is formed through synchondrosis, a similar biological event to endochondral ossification observed in long bones [19] (Fig. 1). The cranial base is developed from the neural crest cells, partly in conjunction with paraxial mesoderm [19]. There are three synchondroses: spheno-occipital, anterior intraoccipital, and posterior intraoccipital [20]. Synchondrosis, consisting of two opposite growth plates sharing a common reserve zone of chondrocytes, is critical for cranial base expansion and development [20]. Due to this structure, the skull base expands bidirectionally. Synchondrosis is also disturbed in ACH, and early closure of synchondrosis is observed [20, 21]. Abnormal development of endochondral ossification and synchondrosis accounts for not only short-limbed short stature but also for foramen magnum stenosis and stenosis of the spinal canal in ACH patients.
ACH has skeletal characteristics such as marked short stature, prominent forehead, flat nasal bridge, large head, short fingers, limited extension of the elbow joint, and genu varum (bow legs) [1, 2, 22]. In addition, the abdomen and buttocks protrude due to thoracic kyphosis and lumbar lordosis. Furthermore, the trident hand, characterized by excess separation between the third and fourth fingers, is observed as a specific sign. Concerning the short stature, the adult height of ACH patients has been reported from several countries, and it ranges between 126.7–135.2 cm for men and 119.9–125.9 cm for women [23, 24] (Table 1). It is noteworthy that the adult height of ACH patients varies between races with rather small difference. Persistent and recurrent otitis media is also a common symptom in pediatric patients. Obesity is also a frequent complication and weight gain should be monitored [22, 25].
Reported adult height of achondroplasia patients
| Study (first author) | adult height (cm, mean ± SD): | adult height (SDS): | ||
|---|---|---|---|---|
| male | female | male | female | |
| Owen et al. | 135.2 ± 5.6 | 125.9 ± 3.9 | –5.7 | –5.7 |
| Tofts et al. | 134 | 125 | –5.6 | –5.8 |
| Merker et al. | 132.3 ± 4.9 | 124.4 ± 4.6 | –6.1 | –5.9 |
| Wynne-Davies et al. | 132.2 ± 1.6 | 123.9 ± 1.1 | –6.1 | –6.0 |
| Murdoch et al. | 131.6 ± 5.6 | 123.5 ± 6.0 | –6.2 | –6.1 |
| Horton et al. | 130.7 ± 6.5 | 125.2 ± 6.0 | –6.1 | –5.7 |
| Tachibana et al. | 130.4 ± 5.9 | 124.0 ± 5.3 | –6.1 | –5.9 |
| Hoover-Fong et al. | 129.9 ± 6.3 | 122.4 ± 5.9 | n.d. | n.d. |
| Del Pino et al. | 128.3 ± 7.7 | 119.9 ± 7.2 | –6.4 | –6.5 |
| Hoover-Fong et al. | 126.7 ± 10.1 | 119.9 ± 10.4 | –5.8 | –6.2 |
Adult height of male and female patients with achondroplasia is summarized in the Table. The adult height ranges between 126.7–135.2 cm for males and 119.9–125.9 cm for females. References of all data on adult height are included in reference 23 except ref 24 data, which indicated 129.9 cm for male and 122.4 cm for female. n.d.: not determined
A patient registry has been developed and has contributed to revealing the natural history of ACH patients. The largest ACH patient registry is the Achondroplasia Natural History Study (CLARITY) consisting of over 1,300 subjects [26]. The registry reports a surgical burden associated with ACH [27]. Reports regarding the quality of life (QoL) of ACH patients are increasing, and pain and movement limitations, severe short stature (less than 140 cm), and spinal canal stenosis are associated with lower QoL [28-32]. The systematic literature review reported that short stature by any cause may lead to low QoL [33]. Intervention for severe short stature will likely be significance in improving QoL for ACH patients.
Diagnosis of ACH is made based on the above clinical symptoms and characteristic bone X-ray findings. X-ray findings include shortening of the skull base, a narrow thorax, platyspondyly, caudal interpediculate narrowing, iliac hypoplasia with a trident appearance of the acetabulum, proximal femoral scooping, and stubby tubular bones with metaphyseal cupping, flaring, and corner spur [34]. The diagnostic criteria of ACH are published by the Japan Intractable Diseases Information Center [35]. Genetic tests are useful for definitive diagnosis, and the costs are covered by medical insurance in Japan.
In addition to bone deformity and short stature, spinal cord compression at the cervicomedullary junction due to foramen magnum stenosis can be a serious complication in ACH [22, 36]. Foramen magnum stenosis leads to sleep apnea, breathing disorders, myelopathy, hydrocephalus, and sudden death. Thus, it is very important to diagnose foramen magnum stenosis as an early symptom of ACH. MRI is useful in determining this [37-39]. Infants with foramen magnum stenosis may have irritability, bulging fontanel, headache, vomiting, papillary stasis, abducens nerve palsy, hemiplegia, impaired consciousness, increased blood pressure, and brachycardia. Sleep apnea is not only central but also has strong obstructive elements [36, 40]. Polysomnography is the golden standard in diagnosing sleep-disordered breathing [40]. Skull base hypoplasia also causes narrowing of the jugular foramen, leading to ventricular enlargement [41]. Ventricular enlargement alone does not require a specific intervention; however, a ventricular-peritoneal shunt may be performed when enlargement worsens and is associated with neurological symptoms [35].
Spinal canal stenosis tends to occur at the thoracolumbar level [42]. Spinal canal stenosis is particularly common in older children and adult patients, and can cause pain in the extremities, numbness, muscle weakness, movement disorders, intermittent claudication, and bladder-rectal disorders [27]. To manage all the complications appropriately in ACH, we must understand that the frequency of complications differs according to age [22].
Clinical guidelines have been established by experience and data of the natural history in many countries such as USA and Japan, and those in Europe and Latin America [35, 43-45]. However, there are still many unmet needs, for example, in preventing foramen magnum stenosis and spinal canal stenosis in ACH patients.
Although some favorable effects of growth hormone (GH) therapy on height have been reported, GH therapy for ACH was approved only in Japan [35, 46, 47]. This GH therapy has a history longer than twenty-five years, as it was approved in 1997. Thus, some patients with ACH who received GH therapy have become adults, and their adult heights were reported; however, the improvement was not satisfactory in ACH by GH treatment [48]. In addition, GH treatment may have an side effect, deteriorating foramen magnum stenosis and obstructive sleep apnea. Thus, GH treatment is recommended in ACH patients older than three, and careful monitoring is necessary during the treatment. Although short stature at or below –3 standard deviations (SD) in comparison with the same age and sex standard is also the criteria for GH treatment, almost all ACH patients show short stature greater than –3 SD after the age of three [23].
C-type natriuretic peptide (CNP) is a member of the natriuretic peptide family and has a growth-promoting effect [49]. ANP and BNP, other members of the family, have natriuretic function. The difference in their receptors accounts for the difference of their function: NPRA for ANP and BNP, and NPRB for CNP [50]. NPRA and NPRB have guanylin cyclase activity, and generate cGMP as a second messenger. The other receptor, NPRC, has no guanylin cyclase activity and works as a clearance receptor [50].
In 2004, Yasoda et al. reported the effect of CNP on the body length of ACH model mice [14]. In humans, the loss of NPRB function results in short stature with short fingers [51]. Conversely, gaining NPRB function results in tall stature with long great toes [52]. Therefore, CNP is a growth-promoting factor both in humans and mice. However, the short half-life of CNP due to the rapid degradation by the neutral endopeptidase is an obstacle for the development of CNP as a drug. Vosoritide is a CNP analogue resistant to the neutral endopeptidase by the modification of both the N-terminal and C-terminal end that has a longer half-life [53]. As a mechanism, CNP or CNP analogues activate NPRB, and increase cGMP in chondrocytes, endothelial cells, and the brain [49]. Then, cGMP-dependent protein kinase is activated. The CNP signal pathway crosstalks with the RAS/RAF/MEK/ERK pathway and inhibits signal transduction (Fig. 2). Because the RAS/RAF/MEK/ERK pathway is constitutively activated in ACH, a CNP or CNP analogue is likely to be an effective drug for ACH. Besides the crosstalk, our group and that of Dr. Yasoda reported that the CNP exerts its own effect on chondrocyte proliferation and differentiation through protein kinase A (PKA) and/or cAMP responsive element binding protein (CREB) [54, 55]. Clinical trials were conducted, and vosoritide has been approved as a drug for ACH in many countries [8, 56-58]. Vosoritide is the first drug for ACH in most countries, except for Japan where GH is already used [35]. Through the use of vosoritide, clinical practice for ACH is changing [8, 59, 60]. CNP analogues with a longer half-life are now in the drug development pipeline [61, 62].

Development of new drugs for achondroplasia by modifying the FGFR3 signal
The downstream signal transduction of FGFR3 consists of STAT1 and RAS/RAF/MEK/ERK pathways. When FGFR3 binds to the cognitive ligand FGF2, FGF18, or FGF9 secreted from perichondrium, it exerts autophosphorylation by its own tyrosine kinase and then activates STAT1 and RAS/RAF/MEK/ERK pathways. NPRB activated by CNP or CNP analogues (vosoritide and transcon CNP) generated cGMP from GTP. cGMP activates PKGII (cGMP-dependent protein kinase II), and then PKGII inhibits the MEK/ERK pathway that is constitutively activated in chondrocytes in ACH. In hypertrophic chondrocytes where FGFR3 is not expressed, CNP has a direct effect on chondrocytes through PKA and phosphorylated CREB (cAMP response element binding protein). New drugs including the CNP analogue, soluble FGFR, anti-FGFR3 antibody, FGF2 aptamer, TKI (tyrosine kinase inhibitor), and meclizine are recently approved or are in clinical trials. The mechanism for the alleviation of the hyperactive FGFR is described in the text.
More drugs are being developed for ACH with different molecular targets (Fig. 2) [16, 17]. A soluble FGFR3 has been developed, and a clinical study was conducted [63]. However, the trial was halted for unknown reasons. Anti-FGFR3 antibody, SAR-442501 is under clinical development.
An FGFR kinase inhibitor was developed for cancer with hyperactive FGFR. BGJ398, otherwise known as infigratinib, for example, is an orally bioactive panspecific FGFR inhibitor. Infigratinib inhibits FGFR1, FGFR2, and FGFR3, and is less active against FGFR4 [64]. First, infigratinib was examined for its effect on malignant tumors such as cholangiocarcinoma and bladder cancer associated with hyperactive FGFR. Interestingly, FGF23-related rickets/osteomalacia model mice are also a target of treatment because FGF23 exerts its effect through FGFR and αKlotho [65]. Infigratinib was effective in these models, but was often associated with gastrointestinal problems and hyperphosphatemia. However, the dose of infigratinib is reduced by 10–100 times in the treatment of ACH patients [66]. Now, a clinical trial is being conducted, with the expectation of a reduction in adverse events [66].
Meclizine is also an orally active drug and was picked up after a screening of approved drugs based on inhibitory activity toward the RAS/RAF/MEK/ERK pathway. When meclizine was administered to ACH model mice, a growth-promoting effect was observed [67]. A clinical trial has started.
We reported the effect of RBM-007, an RNA aptamer developed to neutralize the FGFR3 cognate ligand FGF2 (FGF2 aptamer), in in vitro and in vivo ACH models in 2021 [68]. Aptamer is a single-stranded nucleic acid molecule that binds protein with high affinity and high specificity like an antibody [69]. Aptamer has many advantages over antibodies such as strong chemical stability and rapid bulk production capability. Thus, aptamer technology is being applied in developing a substantial number of new drugs.
As an in vitro differentiation of chondrocytes experiment, Yamashita A, a member of Prof. Tsumaki’s group, has reported a differentiation system using human-induced pluripotent stem (iPS) cells [70]. In this culture system, iPSCs derived from ACH patients do not differentiate into chondrocytes. However, FGF2-aptamer rescued the proliferation arrest, degradation of cartilaginous extracellular matrix, premature senescence, and impaired hypertrophic change. 100 nM FGF2 aptamer promoted the chondrogenic differentiation of Ach-iPSCs with characteristic safranin-O-positive matrix formation and improved the expression of chondrocyte marker genes such as SOX9, COL2A1, and ACAN.
Then, in collaboration with Prof. Tsumaki, we developed a Xenograft model to recapitulate the human growth plate in which human iPS cell-derived cartilaginous pellets were implanted in the subcutaneous spaces of immunocompromised SCID mice for four weeks [71]. iPS cell-derived cartilage formed a growth plate-like structure with stratified differentiated chondrocytes including hypertrophic chondrocytes. In this Xenograft model, the size of hypertrophic chondrocytes is correlated with the severity of FGFR3-related disorders. FGF2 aptamer rescued impaired chondrocyte differentiation and maturation in the growth plate cartilage. Finally, we examined the effect of FGF2 aptamer on ACH model mice where mutated FGFR3 (G380R) is expressed in chondrocytes under a type II collagen promoter (Fgfr3Ach). The administration of 10 mg/kg FGF2 aptamer once every two days for three weeks significantly improved body and femur length. However, the treatment did not normalize the length of the body and the femur in Fgfr3Ach. The first phase of the FGF2 aptamer clinical trial has finished, and the phase two study is ongoing.
As described in the opening sentence above, short stature is associated with shortened limbs in ACH. It remains unclear whether growth-promoting drugs improve the proportions of ACH patients. As an alternative or additional method to improve short stature, limb lengthening has been performed for many years [72]. Lower limb lengthening is typically two-segment: bilateral tibial lengthening followed by single-level bilateral femoral lengthening. However, some ACH patients undergo tibial lengthening only. The mean length gain in the tibias was 9 cm (6–12 cm) and in the femurs the gain was shorter than in the tibias [72]. Commonly observed complications arising from limb lengthening include infection, stiff ankles, ankle valgus, knee valgus, and nerve paralysis [72]. Internationally, limb lengthening is often performed in Spain and Italy, while rare in USA [27]. Hopefully, height and limb length gains by way of new drugs will be sufficient to make limb lengthening obsolete.
New drugs will bring a brighter future for patients with ACH. It is ideal to take advantage of the combination of new drugs with distinct mechanisms. However, we still do not know the extent to which we can manage the complications associated with ACH. It is a challenge because prenatal treatment may be a necessary preventative measure. Moreover, long-term follow-up is required to evaluate the effect of new drugs on adult height, surgical burden, and QoL. For an accurate evaluation and appropriate management, a multidisciplinary team approach should be established.
COI: K. Ozono received the lecture fees from Kyowa Kirin and Alexion Pharma. K. Ozono and T. Michigami are members of Endocrine Journal’s Board.