Abstract
Agranulocytosis is a serious adverse effect of methimazole (MMI) and propylthiouracil (PTU), and although there have been reports suggesting a dose-dependent incidence in relation to both drugs, the evidence has not been conclusive. The objective of our study was to determine whether the incidences of agranulocytosis induced by MMI and PTU exhibit dose-dependency. The subjects were 27,784 patients with untreated Graves’ disease, 22,993 of whom were on an antithyroid drug treatment regimen for more than 90 days. Within this subset, 18,259 patients had been treated with MMI, and 4,734 had been treated with PTU. The incidence of agranulocytosis according to dose in the MMI group was 0.13% at 10 mg/day, 0.20% at 15 mg/day, 0.32% at 20 mg/day, and 0.47% at 30 mg/day, revealing a significant dose-dependent increase. In the PTU group, there were 0 cases of agranulocytosis at doses of 125 mg/day and below, 0.33% at 150 mg/day, 0.31% at 200 mg/day, and 0.81% at 300 mg/day, also revealing a significant dose-dependent increase. The incidence of agranulocytosis at MMI 15 mg and PTU 300 mg, i.e., at the same potency in terms of hormone synthesis inhibition, was 0.20% and 0.81%, respectively, and significantly higher in the PTU group. Our findings confirm a dose-dependent increase in the incidence of agranulocytosis with both drugs, but that at comparable thyroid hormone synthesis inhibitory doses PTU has a considerably higher propensity to induce agranulocytosis than MMI does.
METHIMAZOLE (MMI) AND PROPYLTHIOURACIL (PTU) are antithyroid drugs (ATDs) that are widely used to treat hyperthyroidism and Graves’ disease. Despite their efficacy, both drugs are associated with significant adverse events, notably agranulocytosis, a critical reduction in granulocytes [1]. Because of the prevalence of Graves’ disease, it is important to accurately assess the risk of agranulocytosis after starting a patient on an ATD. Historically, by 1946, thiourea, which was first used to treat hyperthyroidism in 1942, had been reported to induce agranulocytosis in 1.9% of those treated and result in a 26% fatality rate [2]. An association between MMI with an agranulocytosis-related death was first documented in 1952 [3]. Despite the long-term use of these ATDs, the epidemiology, risk determinants, and pathogenesis of agranulocytosis remain insufficiently understood, largely due to its rare occurrence.
A previous study based on data from the Japan Medical Data Center reported ATD-induced agranulocytosis rates of between 0.1% and 0.15% [4]. However, a study of a cohort of 15,398 Graves’ disease patients revealed that agranulocytosis developed in 0.31% of the patients treated with MMI and 0.55% of the patients treated with PTU [5], and the difference in incidence may have been due to differences between the doses of MMI and PTU. The fact that the incidence of agranulocytosis has been found to increase with the dose of MMI [6-9], from 0.0% at 15–25 mg/day to 1.0% at MMI 30–60 mg/day, has suggested a dose-response relationship [10]. Moreover, during a year-long observation period of untreated Graves’ patients Takata et al. observed a higher agranulocytosis rate at MMI 30 mg/day (0.814%) than at MMI 15 mg/day (0.219%) [11]. By contrast, no clear dose-response pattern in agranulocytosis was found in patients treated with PTU [5, 8]. Several studies have indicated a higher risk of agranulocytosis during treatment with PTU than during treatment with MMI [6]. However, PTU is commonly prescribed at higher doses than MMI, and no studies have directly compared the incidence of agranulocytosis at equivalent doses in terms of their inhibitory effects on thyroid hormone synthesis.
To address this knowledge gap, we investigated the dose-dependency of the incidence of agranulocytosis and both MMI and PTU based on data obtained from nearly 30,000 Graves’ disease patients. We also contrasted the risks of agranulocytosis associated with equivalent thyroid hormone synthesis inhibition by the two drugs.
Subjects and Methods
The initial study population consisted of 28,494 patients with untreated Graves’ disease diagnosed at our clinic between January 1, 2005 and December 31, 2022, 24,896 of whom were treated with an ATD: 20,727 had been started on MMI and 4,169 on PTU. This final cohort comprised 4,431 males and 20,465 females, and their median age was 38 years (range: 4 to 92 years). The remaining 3,598 patients were initiated on treatment with inorganic iodine. During the course of the study, 210 patients were switched from MMI to PTU and 782 from PTU to MMI, and thus a total of 21,509 patients had been treated with MMI and 6,275 with PTU. There were 22,993 (82.8%) patients in the ATD-treated group who had been treated with an ATD for more than 90 days. MMI had been prescribed to treat 18,259 of them, 3,898 males and 14,361 females, median age 40 years (range: 4–91 years), median treatment duration 665 days (range: 90–6,698 days), and PTU had been prescribed to treat the other 4,734 of them, 128 males and 4,606 females, median age 32 years (range: 7–85 years), median treatment duration 653 days (range: 90–6,524 days). Treatment duration was estimated to have been the total number of days covered by prescriptions, which included the numbers of days between clinic visits.
The 2006 guidelines issued by the Japan Thyroid Association for the pharmacological management of Graves’ disease advise that, as a general rule, patients should undergo evaluations every two to three weeks for at least three months after starting them on ATD therapy, primarily to monitor them for potential adverse effects.
Since 2005, our institution has implemented a protocol in which liver function and blood cell counts are assessed every two weeks during the initial three months of ATD therapy and in which patients continued on ATD therapy undergo evaluations of thyroid function, liver function, and blood cell counts at each subsequent clinic visit. Before 2009, the initial dosage of MMI varied from 5 mg/day to 60 mg/day, depending on the severity of the hyperthyroidism, while the initial dosage of PTU was set between 50 mg/day and 500 mg/day, according to the severity of the condition. Since 2010, we have started treatment of mild hyperthyroidism at MMI 15 mg/day and treatment of moderate to severe hyperthyroidism with a combination of MMI 15 mg/day and potassium iodide (KI) 50 mg/day, and we never prescribe MMI at doses greater than 15 mg/day.
In this study, we defined agranulocytosis as a granulocyte count below 500/μL. The incidence rates of agranulocytosis were determined by dividing the number of cases by the number of patients who had been on ATD therapy for more than 90 days.
Statistical Analysis
The data are reported as the mean and standard deviation (SD) for normal distributions and as the median and range for non-normal distributions. We used the Mann-Whitney U-test for between-group comparisons of quantitative variables. We report qualitative variables as absolute counts and percentages, and we compared them by using Pearson’s chi-squared test or Fisher’s exact test, as appropriate. We used the Cochran-Armitage trend test to analyze binomial percentage trends in ordinal variables. We regarded results with a p-value below 0.05 as statistically significant. All our statistical evaluations were performed using the JMP-Pro v17.20 software program.
This study was approved by the Ethics Committee of Ito Hospital (Approval No.: 415).
Results
Patient demographics and diagnosis of agranulocytosis
Agranulocytosis developed in 42 (0.23%) of the 18,259 MMI-treated patients and in 23 (0.49%) of the 4,734 PTU-treated patients. There were no significant differences in age and gender distribution according to whether the patients had developed agranulocytosis (Table 1). The median time to the detection of agranulocytosis in the MMI group was 42 days (range 10–722 days), and it was within 90 days in 39 (92.9%) of the 42 patients, but it was diagnosed on days 122, 293, and 722, respectively, in the other 3 cases. The median time to the diagnosis of agranulocytosis in the PTU group was 28 days (range: 14–2,989 days), and it was within 90 days in 18 patients (78.2%), but it was diagnosed on days 273, 518, 903, 1,134, and 2,989, respectively, in the other 5 cases. In five patients agranulocytosis developed after being initially treated with MMI and then being switched to PTU. The reasons for the switch from MMI to PTU were adverse side effects of MMI in three of these patients and a desire to become pregnant in the other two. By contrast, none of the patients started on PTU and switched to MMI developed agranulocytosis.
Table 1
Demographic profile of patients who received MMI and PTU therapy: analysis of the incidence of agranulocytosis
| ATD |
|
agranulocytosis(–) |
agranulocytosis(+) |
p value |
| MMI |
No. of patients |
18,248 |
42 |
|
| age (years, mean ± SD) |
40.8 ± 14.5 |
39.3 ± 13.3 |
n.s. |
| sex (M:F) |
3,897:14,351 |
4:38 |
p = 0.06* |
| PTU |
No. of patients |
4,725 |
23 |
|
| age (years, mean ± SD) |
33.2 ± 8.4 |
32.7 ± 10.6 |
n.s. |
| sex (M:F) |
128:4,597 |
0:23 |
n.s. |
* Fisher’s Exact Test; MMI, methimazole; PTU, propylthiouracil; n.s., not significant.
Fever, sore throat, and other symptoms of infection were present in 38 of the 65 cases of agranulocytosis and absent in 26 cases, and whether they were present was unknown in 1 case. There were no fatalities among the patients who developed agranulocytosis, and there were no cases of agranulocytosis in the group of patients treated with inorganic iodine.
Correlations between the initial doses of MMI and PTU and incidence of agranulocytosis
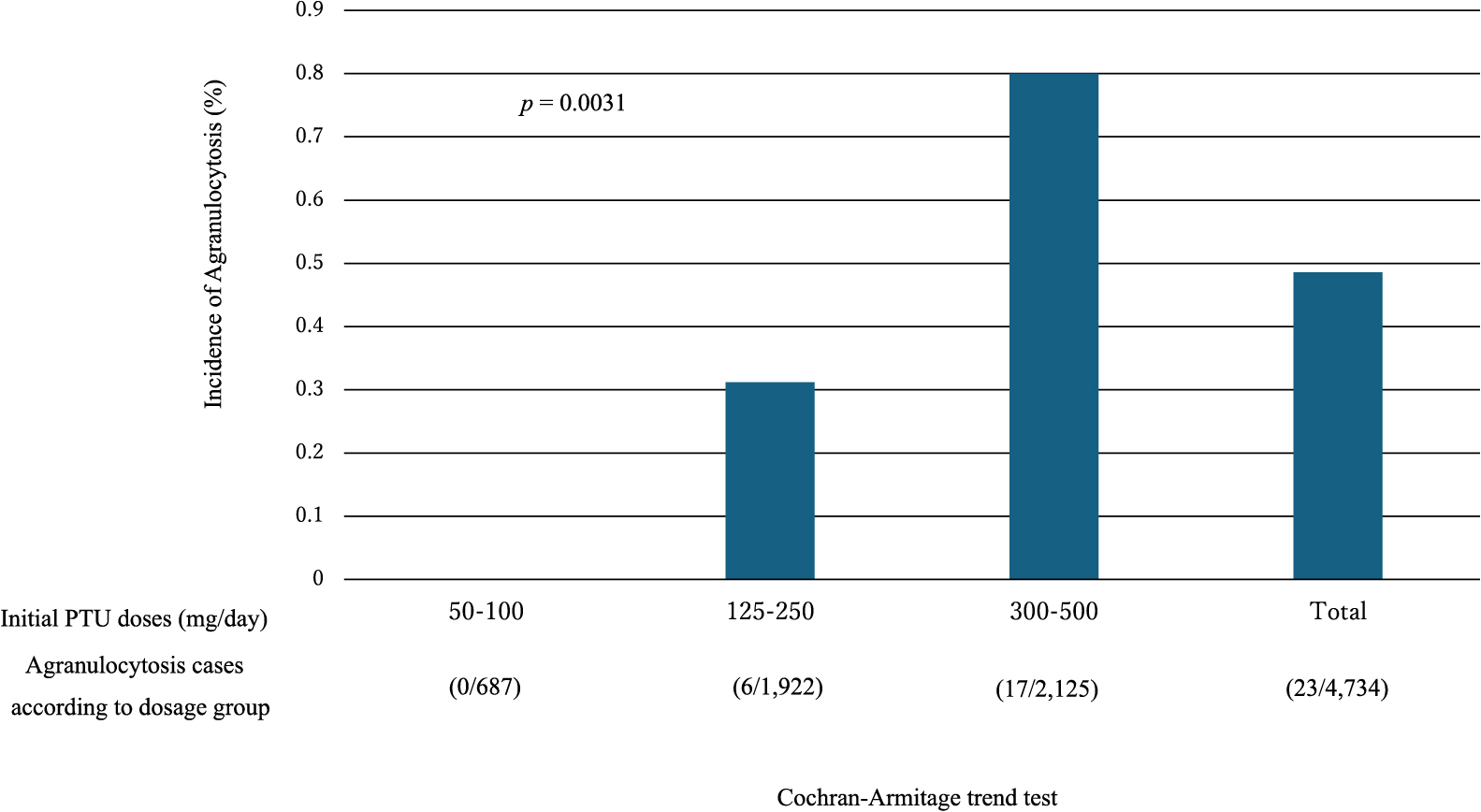
The neutrophil count plummeted to below 100/μL in 40.5% of the agranulocytosis patients treated with MMI and in 47.8% of those treated with PTU, and these low counts were a sign of severe agranulocytosis (Table 2-a). No correlations were found between the initial doses of MMI and PTU and the lowest neutrophil counts (Table 2-b, c). The agranulocytosis developed within 90 days of starting ATD therapy in 92.9% of the patients in the MMI group who developed agranulocytosis and in 78.2% in the PTU group who developed agranulocytosis. According to dose, the incidence of agranulocytosis in the MMI group was 0.13% at 10 mg/day, 0.20% at 15 mg/day, 0.32% at 20 mg/day, and 0.47% at 30 mg/day, and there was a significant dose-dependent increase (Cochran-Armitage trend test, p = 0.006) (Table 3, Fig. 1). In the PTU group, there were 0 cases of agranulocytosis among the 688 patients on a 125 mg/day or lower dose, but the incidence of agranulocytosis was 0.33% at 150 mg/day, 0.31% at 200 mg/day, and 0.81% at 300 mg/day, and there was a significant dose-dependent increase (Cochran-Armitage trend test, p = 0.0031) (Table 4, Fig. 2).
Table 2-a
Numbers of patients treated with MMI and PTU and of patients with symptoms of infection according to their lowest neutrophil count
| Lowest neutrophil counts (per μL) |
No. of patients treated with MMI (%) |
No. of patients treated with PTU (%) |
No. of patients with symptoms of infection |
Total numbers of patients |
| 0–<100 |
17 (40.5) |
11 (47.8) |
22 |
28 |
| 100–<200 |
3 (7.1) |
5 (21.7) |
5 |
8 |
| 200–<300 |
5 (11.9) |
3 (13.0) |
3 |
8 |
| 300–<400 |
9 (21.4) |
1 (4.3) |
5 |
10 |
| 400–<500 |
8 (19.0) |
3 (13.0) |
3 |
11 |
| Total |
42 |
23 |
38 |
65 |
%: percentage of patients treated with MMI or PTU; MMI, methimazole; PTU, propylthiouracil.
Table 2-b
Numbers of patients according to their initial dose of MMI (methimazole) and lowest neutrophil count
| Lowest neutrophil count (per μL) |
Initial dose of MMI (mg/day) |
| 10 |
15 |
20 |
30 |
Total numbers of patients |
| 0–<100 |
1 |
8 |
2 |
6 |
17 |
| 100–<200 |
0 |
2 |
0 |
1 |
3 |
| 200–<300 |
0 |
5 |
0 |
0 |
5 |
| 300–<400 |
0 |
6 |
1 |
2 |
9 |
| 400–<500 |
1 |
6 |
0 |
1 |
8 |
| Total |
2 |
27 |
3 |
10 |
42 |
Table 2-c
Numbers of patients according to their initial dose of PTU (propylthiouracil) and lowest neutrophil count
| Lowest neutrophil count (per μL) |
Initial dose of PTU (mg/day) |
| 150 |
200 |
300 |
500 |
Total numbers of patients |
| 0–<100 |
0 |
3 |
8 |
0 |
11 |
| 100–<200 |
1 |
0 |
4 |
0 |
5 |
| 200–<300 |
0 |
0 |
3 |
0 |
3 |
| 300–<400 |
0 |
0 |
0 |
1 |
1 |
| 400–<500 |
1 |
1 |
1 |
0 |
3 |
| Total |
2 |
4 |
16 |
1 |
23 |
Table 3
Details of patients treated with MMI (n = 21,509)
| Initial daily dose of MMI (mg/day) |
5 |
7.5 |
10 |
12.5 |
15 |
17.5 |
20 |
22.5 |
25 |
30 |
40 |
45 |
50 |
60 |
Total |
| No. of patients treated with MMI |
384 |
23 |
1,859 |
24 |
15,342 |
2 |
1,174 |
1 |
13 |
2,679 |
2 |
3 |
1 |
2 |
21,509 |
| No. of patients treated with MMI for at least 90 days |
327 |
21 |
1,579 |
20 |
13,225 |
2 |
950 |
1 |
10 |
2,117 |
2 |
2 |
1 |
2 |
18,259 |
| No. of patients who developed agranulocytosis |
0 |
0 |
2 |
0 |
27 |
0 |
3 |
0 |
0 |
10 |
0 |
0 |
0 |
0 |
42 |
| Incidence of agranulocytosis (%) |
0 |
0 |
0.13 |
0 |
0.20 |
0 |
0.32 |
0 |
0 |
0.47 |
0 |
0 |
0 |
0 |
0.23 |
We excluded the 3,250 patients who dropped out within 90 days after starting treatment.
To determine the incidence of agranulocytosis, the denominator was established as the number of patients who had taken MMI for at least 90 days following the initiation of oral administration (in bold).
MMI, methimazole

Table 4
Details of patients treated with PTU (n = 6,275)
| Initial daily dose of PTU (mg/day) |
50 |
75 |
100 |
125 |
150 |
200 |
250 |
300 |
400 |
450 |
500 |
Total |
| No. of patients treated with PTU |
229 |
5 |
566 |
1 |
767 |
1,689 |
7 |
2,991 |
9 |
10 |
1 |
6,275 |
| No. of patients treated with PTU for at least 90 days |
193 |
4 |
490 |
1 |
603 |
1,311 |
7 |
2,110 |
9 |
5 |
1 |
4,734 |
| No. of patients who developed agranulocytosis |
0 |
0 |
0 |
0 |
2 |
4 |
0 |
17 |
0 |
0 |
0 |
23 |
| Incidence of agranulocytosis (%) |
0 |
0 |
0 |
0 |
0.33 |
0.31 |
0 |
0.81 |
0 |
0 |
0 |
0.49 |
We excluded 1,541 patients who dropped out within 90 days after starting treatment.
To determine the incidence of agranulocytosis, the denominator was established as the number of patients who had taken PTU for at least 90 days following the initiation of oral administration (in bold).
PTU, propylthiouracil
In the MMI group, the dose of only one (4.8%) of the 27 patients started on an initial oral dose of 15 mg/day had been increased to 40 mg/day at the time agranulocytosis was diagnosed. The MMI dose in one (10%) of the 10 patients started on an initial dose of 30 mg/day had been increased to 45 mg/day at the time of agranulocytosis was diagnosed (Table 5-a). In the PTU group, at the time agranulocytosis was diagnosed the dose of one of the two patients who had initially been prescribed a dose of 150 mg/day had been increased to 200 mg/day, and the dose of one of the four patients who started on 200 mg/day dose had been increased to 350 mg/day (Table 5-b). In addition, the initial dose had been reduced when agranulocytosis was diagnosed in 9 (21.4%) of the patients in the MMI group who developed agranulocytosis and in 6 (26.1%) of the patients in the PTU group who developed agranulocytosis.
Table 5-a
Initial dose of MMI (methimazole) and dose at the time of agranulocytosis was diagnosed
| Initial daily dose of MMI (mg/day) |
No. of patients |
Daily dose of MMI (mg) when agranulocytosis was diagnosed and number of patients who developed agranulocytosis at that dose |
| 10 |
2 |
10 mg, 2 patients |
| 15 |
27 |
2.5 mg, 1 patient; 5 mg, 1 patient; 10 mg, 3 patients; 12.5 mg, 1 patient; 15 mg, 20 patients; 40 mg, 1 patient |
| 20 |
3 |
10 mg, 1 patient; 20 mg, 2 patients |
| 30 |
10 |
10 mg, 1 patient; 15 mg, 1 patient; 30 mg, 7 patients; 45 mg, 1 patient |
Table 5-b
Initial dose of PTU (propylthiouracil) and dose at the time of agranulocytosis was diagnosed
| Initial daily dose of PTU (mg/day) |
No. of patients |
Daily dose of PTU (mg) when agranulocytosis was diagnosed and number of patients who developed agranulocytosis at that dose |
| 150 |
2 |
150 mg, 1 patient; 200 mg, 1 patient |
| 200 |
4 |
100 mg/7 days, 1 patient; 150 mg, 1 patient; 200 mg, 1 patient; 350 mg, 1 patient |
| 300 |
16 |
100 mg, 1 patient; 150 mg, 1 patient; 200 mg, 1 patient; 300 mg, 13 patients |
| 500 |
1 |
100 mg, 1 patient |
Among patients who developed agranulocytosis within 90 days, the median time to diagnosis was 33 days (range: 10–89 days) in the MMI group (n = 39) as opposed to 25.5 days (range: 14–42 days) in the PTU group (n = 18), and the time to diagnosis in the PTU group was significantly shorter than in the MMI group (Mann-Whitney test, p = 0.0026). Among the patients who developed agranulocytosis at starting MMI doses of 15 mg/day and 30 mg/day, the median (range) time to the diagnosis of agranulocytosis was 32.5 days (10–84 days) in the 15 mg/day MMI group (n = 27) and 36.5 days (19–89 days) in the 30 mg/day MMI group (n = 10), and the difference between the two groups was not significant.
Comparison between the cumulative MMI doses in the groups started on MMI 15 mg/day and MMI 30 mg/day who developed agranulocytosis
The median (range) cumulative MMI dose was 485 mg (150–1,260 mg) in the group started on MMI 15 mg/day group (n = 27) that developed agranulocytosis and 863 mg (168–1,990 mg) in the group started on MMI 30 mg/day (n = 10) that developed agranulocytosis, and the difference between the groups was significant (Mann-Whitney U-test, p = 0.0116).
Characteristics of the patients who developed agranulocytosis after 90 days of ATD therapy: presence of clinical symptoms and medication status in the MMI group and PTU group
Three of the patients in the MMI group and 5 of the patients in the PTU group developed agranulocytosis after 90 days of medication, and the ATD dose at the time of diagnosis was higher than the initial dose in 2 of the cases in each group (Table 6). Four patients displayed no symptoms of infection, and the other four patients presented with fever. Four patients were consistent in taking their prescribed doses, and the other four did not take their prescribed doses regularly.
Table 6
Initial dose of ATD (antithyroid drug), dose at diagnosis of agranulocytosis, symptoms of infection, and neutrophil count in patients who developed agranulocytosis during long-term oral ATD therapy
| ATD |
Interval since start of ATD therapy (day) |
Initial dosage (mg/day) |
Dosage at diagnosis (mg/day) |
Medication adherence |
Symptoms |
WBC count at diagnosis (per μL) |
Neutrophil count at diagnosis (per μL) |
| MMI |
122 |
30 |
30 |
irregular |
fever |
1,390 |
28 |
| PTU |
273 |
300 |
100 |
regular |
none |
2,880 |
346 |
| MMI |
293 |
30 |
45 |
irregular |
fever |
1,480 |
385 |
| PTU |
518 |
200 |
350 |
regular |
none |
2,230 |
446 |
| MMI |
722 |
15 |
40 |
regular |
fever |
560 |
22 |
| PTU |
903 |
150 |
200 |
regular |
fever |
2,050 |
267 |
| PTU |
1,134 |
300 |
150 |
irregular |
none |
1,650 |
182 |
| PTU |
2,989 |
200 |
14 |
irregular |
none |
2,170 |
147 |
MMI, methimazole; PTU, propylthiouracil; ATD, antithyroid drug.
Since an MMI:PTU dose ratio of 20:1 is advocated based on their drug efficacy [12], we compared the incidence of agranulocytosis in the MMI 15 mg/day group and PTU 300 mg/day group. The results showed that agranulocytosis had developed in 27 (0.20%) of the 13,225 patients on the 15 mg/day MMI regimen and in 17 (0.81%) of the 2,110 patients on the 300 mg/day PTU regimen, and the incidence of agranulocytosis was significantly higher in the PTU 300 mg/day group (p < 0.0001, Pearson chi-square test).
Discussion
The results of this study of a population of nearly 30,000 patients with untreated Graves’ disease revealed a dose-response correlation between the initial doses of the ATDs and the incidence of agranulocytosis. It was noteworthy that the incidence of agranulocytosis was higher during treatment with PTU than with MMI when doses with equivalent inhibitory effectiveness on thyroid hormone synthesis were compared.
The incidences of agranulocytosis while on ATD therapy have differed across studies and ranged from 0.1% to 5% [13]. An incidence of agranulocytosis of 0.31% during treatment with MMI and 0.55% during treatment with PTU has been reported in a study of 15,398 subjects [5], and a dose-specific analysis in a cohort of 5,950 subjects revealed an incidence of agranulocytosis of 0.219% among patients on an MMI dose of 15 mg/day and 0.814% on a dose of 30 mg/day [11]. The differences in incidences may have been attributable to differences in observation periods, post-medication follow-up rates, and initial dose differences. One study reported that 84.6% of the patients who developed agranulocytosis did so within the initial 90 days of the start of treatment [4], while another study [14] reported that 88.5% developed agranulocytosis within the same period. One study [13] reported adverse skin reactions in 4%–6% of the subjects treated with an ATD, while another [15] documented such skin reactions in 5.6% of patients on MMI 15 mg/day and 11.4% on MMI 30 mg/day. Such adverse reactions may lead to a discontinuation of the ATD before agranulocytosis develops, thereby potentially shortening the follow-up period to less than 90 days and resulting in an underestimated incidence of this serious outcome. Our study addressed this issue by focusing exclusively on patients who continued ATD treatment for 90 days or more, thereby providing a more accurate estimation of the agranulocytosis incidence rate.
The historical literature highlights the dose-dependent risk of MMI-induced agranulocytosis. In 1954, McGavack et al. recorded a 1.0% incidence of agranulocytosis in patients on MMI dosages of 30–60 mg/day and no cases among those on a 15–25 mg/day dosage [10]. Wiberg et al., in 1972, observed the development of agranulocytosis in patients treated with MMI at 120 mg/day [9], and by 1983, Cooper et al. had identified an 8.6-fold increase in the risk of agranulocytosis at MMI dosages of ≥40 mg/day [8]. By contrast, PTU has never been associated with such a clear dose-related risk [8]. Our findings in the present study were consistent with this historical pattern: the incidence of agranulocytosis was 0.13% on MMI 10 mg/day and increased to 0.47% at 30 mg/day, while the incidence of agranulocytosis was 0% at PTU doses below 100 mg/day, but rose to 0.81% at 300 mg/day. Interestingly, our analysis revealed that the PTU 300 mg/day group experienced agranulocytosis at a rate approximately four times higher than the MMI 15 mg/day group. It is important to note that, on a molar basis, a dose of 300 mg of PTU contains approximately 13.4 times more molecules than a dose of 15 mg of MMI, when calculated on a molar basis. However, this does not necessarily translate to a proportionately higher risk of agranulocytosis per molecule of PTU.
We found no association between the severity of agranulocytosis and the initial ATD dosage. Age and gender may play roles in the development of agranulocytosis. The results of some studies have suggested that patients over 40 years of age are at a 6.4-fold higher risk of developing agranulocytosis [8] and have indicated a higher incidence among older adults [5], but our data showed no age-specific trends. Gender disparities in the incidence of agranulocytosis have been reported in the literature. Some studies have pointed to an increased risk in women [5], and others have reported no gender differences [11]. Our own findings hint at a slightly higher risk in females. It should be noted that the ATD dosages when agranulocytosis was diagnosed were often lower than the initial dosage, suggesting that a reduction in dosage may not be sufficient to prevent the onset of agranulocytosis once the process has begun.
While most cases of agranulocytosis associated with an ATD are diagnosed within the first 90 days, several studies have documented later occurrences and post-90-day incidence rates of 15.4% [4] and 11.5% [14]. Our own data showed later occurrences in 7.1% in the MMI group and 21.8% in the PTU group. Tamai et al. [16] documented a late occurrence of agranulocytosis at between 12 weeks and one year in 5 of 12 MMI-related cases. Another study reported that agranulocytosis was detected later in patients treated with PTU than in those treated with MMI [17]. In our own cohort, 7.1% of the MMI patients and 18.2% of the PTU patients developed agranulocytosis after the typical 90-day window.
In our own study, 59% (38/65) of the patients diagnosed with agranulocytosis presented with symptoms of an infection, while 40% (26/65) were asymptomatic, and it was unknown whether one patient had presented with symptoms. Importantly, 21% (6/28) of the severe cases, i.e., cases with neutrophil counts under 100/μL, were asymptomatic. In view of having been able to detect agranulocytosis in patients without symptoms on the basis of blood tests, as evidenced by these data and corroborated by findings in two other studies [5, 18], we advocate regular blood count monitoring as a means of early detection of agranulocytosis.
The pathogenesis of ATD-induced agranulocytosis remains unclear. While it has frequently been proposed that an immune mechanism involving neutrophil destruction by hapten (drug)-specific antibodies and drug-induced autoantibodies plays a key role in idiosyncratic drug-induced neutropenia, there is a lack of strong evidence to support this hypothesis [19]. Evidence supporting an immune mechanism for the development of ATD-induced agranulocytosis has included reports that HLA Class 1 and 2 genes are associated with its development. Tamai et al. [20] found an association between MMI induced agranulocytosis and HLA DRB1*08032, Chen et al. [21] found an association between ATD-induced agranulocytosis and HLA-B*38:02 and HLA DRB1*08:03, and Cheung et al. [22] identified an association between HLA-B*38:02:01 and agranulocytosis induced by carbimazole and MMI. In the present study we observed a significantly higher cumulative dosage of MMI in the 30 mg/day group compared to the 15 mg/day group, although there was no significant difference between the two groups in the time to detection of agranulocytosis. These findings suggest that a threshold level of MMI exposure, both in terms of dosage and duration, is required for a patient on MMI therapy to develop agranulocytosis, particularly in patients with a genetic predisposition. The increased incidence of agranulocytosis in the MMI 30 mg/day group compared to the MMI 15 mg/day group indicates that the strength of genetic predispositions to agranulocytosis may vary, meaning that patients with a stronger predisposition may develop agranulocytosis following lower levels of MMI exposure, whereas those with a weaker predisposition may require higher levels of exposure to trigger the condition.
This study had several limitations. One major limitation of our study was the variation in initial doses of MMI used over different time periods. Specifically, between 2005 and 2009, the initially prescribed MMI doses ranged from 5 mg/day to 60 mg/day, whereas since 2010 the initial doses have been restricted to 15 mg/day or less. This variation in dosing practice over time may have introduced a time-period bias and affected our ability to compare and interpret our data across different years. A second limitation of our study was that the selection criteria for PTU predominantly targeted patients desiring to become pregnant in the near future, which resulted in a higher prevalence of younger women in the PTU cohort. and as a result, there was a significantly higher proportion of young women in the PTU group than in the MMI group. The third major limitation of our study was the observational nature, which means we cannot completely exclude the possibility of unmeasured confounding factors such as viral infections and cyclic neutropenia affecting our results. A fourth limitation was geographical constraints; some patients might have sought emergency services closer to their homes and not returned to our clinic, introducing selection bias and possibly leading to an underestimation of the incidence of agranulocytosis. In conclusion, the results of our study have demonstrated a dose-dependent increase in the incidence of agranulocytosis in patients being treated with ATDs, and the comparison between MMI and PTU at dosages with equivalent inhibitory effects on thyroid hormone synthesis revealed a significantly higher incidence of agranulocytosis among the patients treated with PTU. Based on these findings, we recommend initiating treatment with lower doses of both MMI and PTU to potentially minimize the risk of developing agranulocytosis. Additionally, we advise starting the treatment of patients with moderate to severe hyperthyroidism with a low dose of MMI, supplemented with temporary inorganic iodine, until MMI achieves its full therapeutic effect [15]. This approach may provide safer and more effective management of hyperthyroidism.
Acknowledgments
We would like to express our deep gratitude to the entire staff of Ito Hospital for their unwavering dedication to patient care and diligent data collection. We also wish to thank the patients for their cooperation and consistent engagement throughout the study.
Author Contributions
Conceptualization: J.Y.N.
Data curation: N.S., T.I., M.F., M.M., S.H., H.I., M.I., M.K., A.S., and R.H.
Formal analysis: J.Y.N., K. Inoue
Project administration: J.Y.N., A.Y., and N.W.
Supervision: K.S., and K. Ito.
Writing of the original draft: J.Y.N.
Author Disclosure Statement
The authors declare that they have no conflicts of interest to report in regard to this study.
Natsuko Watanabe and Kiminori Sugino are members of Endocrine Journal’s Editorial Board.
Funding Information
This study was conducted without any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1 Watanabe N, Narimatsu H, Noh JY, Yamaguchi T, Kobayashi K, et al. (2012) Antithyroid drug-induced hematopoietic damage: a retrospective cohort study of agranulocytosis and pancytopenia involving 50,385 patients with Graves’ disease. J Clin Endocrinol Metab 97: E49–E53.
- 2 Moore FD (1946) Toxic manifestations of thiouracil therapy. J Am Med Assoc 130: 315–319.
- 3 Specht NW, Boehme EJ (1952) Death due to agranulocytosis induced by methimazoke therapy. JAMA 149: 1010–1011.
- 4 Nakamura H, Miyauchi A, Miyawaki N, Imagawa J (2013) Analysis of 754 cases of antithyroid drug-induced agranulocytosis over 30 years in Japan. J Clin Endocrinol Metab 98: 4776–4783.
- 5 Tajiri J, Noguchi S, Murakami T, Murakami N (1990) Antithyroid drug-induced agranulocytosis. The usefulness of routine white blood cell count monitoring. Arch Intern Med 150: 621–624.
- 6 Pearce SH (2004) Spontaneous reporting of adverse reactions to carbimazole and propylthiouracil in the UK. Clin Endocrinol (Oxf) 61: 589–594.
- 7 Meyer-Gessner M, Benker G, Lederbogen S, Olbricht T, Reinwein D (1994) Antithyroid drug-induced agranulocytosis: clinical experience with ten patients treated at one institution and review of the literature. J Endocrinol Invest 17: 29–36.
- 8 Cooper DS, Goldminz D, Levin AA, Ladenson PW, Daniels GH, et al. (1983) Agranulocytosis associated with antithyroid drugs. Effects of patient age and drug dose. Ann Intern Med 98: 26–29.
- 9 Wiberg JJ, Nuttall FQ (1972) Methimazole toxicity from high doses. Ann Intern Med 77: 414–416.
- 10 McGavack TH, Chevalley J (1954) Untoward hematologic responses to the antithyroid compounds. Am J Med 17: 36–40.
- 11 Takata K, Kubota S, Fukata S, Kudo T, Nishihara E, et al. (2009) Methimazole-induced agranulocytosis in patients with Graves’ disease is more frequent with an initial dose of 30 mg daily than with 15 mg daily. Thyroid 19: 559–563.
- 12 Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, et al. (2017) 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 27: 315–389.
- 13 Cooper DS (1999) The side effects of antithyroid drugs. The Endocrinologist 9: 457–467.
- 14 Yang J, Zhu YJ, Zhong JJ, Zhang J, Weng WW, et al. (2016) Characteristics of antithyroid drug-induced agranulocytosis in patients with hyperthyroidism: a retrospective analysis of 114 cases in a single institution in China involving 9690 patients referred for radioiodine treatment over 15 years. Thyroid 26: 627–633.
- 15 Sato S, Noh JY, Sato S, Suzuki M, Yasuda S, et al. (2015) Comparison of efficacy and adverse effects between methimazole 15 mg+inorganic iodine 38 mg/day and methimazole 30 mg/day as initial therapy for Graves’ disease patients with moderate to severe hyperthyroidism. Thyroid 25: 43–50.
- 16 Tamai H, Takaichi Y, Morita T, Komaki G, Matsubayashi S, et al. (1989) Methimazole-induced agranulocytosis in Japanese patients with Graves’ disease. Clin Endocrinol (Oxf) 30: 525–530.
- 17 Mutharasan P, Oatis W, Kwaan H, Molitch M (2012) Delayed anithyroid drug-induced agranulocytosis. Endocr Pract 18: e69–e72.
- 18 Nakamura H, Ide A, Kudo T, Nishihara E, Ito M, et al. (2016) Periodic granulocyte count measuring is useful for detecting asymptomatic agranulocytosis in antithyroid drug-treated patients with Graves’ disease. Eur Thyroid J 5: 253–260.
- 19 Curtis BR (2017) Non-chemotherapy drug-induced neutropenia: key points to manage the challenges. Hematology Am Soc Hematol Educ Program 2017: 187–193.
- 20 Tamai H, Sudo T, Kimura A, Mukuta T, Matsubayashi S, et al. (1996) Association between the DRB1*08032 histocompatibility antigen and methimazole-induced agranulocytosis in Japanese patients with Graves disease. Ann Intern Med 124: 490–494.
- 21 Chen PL, Shih SR, Wang PW, Lin YC, Chu CC, et al. (2015) Genetic determinants of antithyroid drug-induced agranulocytosis by human leukocyte antigen genotyping and genome-wide association study. Nat Commun 6: 7633.
- 22 Cheung CL, Sing CW, Tang CS, Cheng VK, Pirmohamed M, et al. (2016) HLA-B*38:02:01 predicts carbimazole/methimazole-induced agranulocytosis. Clin Pharmacol Ther 99: 555–561.
 https://orcid.org/0000-0001-7340-8603
https://orcid.org/0000-0001-7340-8603