2024 Volume 71 Issue 9 Pages 851-861
2024 Volume 71 Issue 9 Pages 851-861
Interleukin-2-inducible tyrosine kinase (ITK) is a crucial cytoplasmic protein in the T-cell signaling pathway. Here, we aimed to demonstrate the anti-inflammatory effect of the selective IL-2-induced tyrosine kinase inhibitor BMS-509744 (BMS) on Graves’ orbitopathy (GO) in an in vitro model. ITK mRNA expression in orbital tissues from GO and normal controls was compared using real-time polymerase chain reaction (RT-PCR) and immunohistochemistry. Primary cultured orbital fibroblasts from each group were pretreated with BMS and stimulated with interleukin (IL)-1β to induce inflammatory reaction. ITK mRNA expression was evaluated using western blotting, and inflammatory cytokine production and downstream transcription factor expression were analyzed after pretreatment with BMS. ITK mRNA expression in GO tissues was significantly higher than that in normal control tissues. After stimulation with IL-1β, ITK phosphorylation significantly increased in both GO orbital and normal control tissues. BMS inhibited IL-1β-induced IL-8 expression in the GO orbital fibroblasts. BMS pretreatment significantly suppressed NF-κB phosphorylation in both GO and normal controls. The selective ITK inhibitor attenuates proinflammatory cytokine production and proinflammatory transcription factor phosphorylation in in vitro model of GO.
Graves’ orbitopathy (GO), also known as thyroid eye disease (TED), is an autoimmune inflammatory disorder of the orbit and the most common extrathyroidal manifestation of Graves’ disease. The annual incidence of clinically relevant GO is 16 per 100,000 population in women and 2.9 in men, respectively [1]. GO involves the binding of autoantibodies to the thyroid-stimulating hormone receptor (TSH-R) in the thyroid tissue and orbital fibroblasts. This autoimmune condition activates cellular and humoral immune reactions by secreting inflammatory cytokines and chemokines [2]. Consequent infiltration of autoreactive T-cell and B-cell results in orbital inflammation [2]. Additionally, abnormally elevated IGF-1 receptor (IGF-1R) levels in GO orbital fibroblasts contribute to TSH-R activation and autoimmune reaction [3]. Orbital fibroblasts stimulated by immune cells actively proliferate and differentiate into adipocytes and induce massive remodeling of the extracellular matrix in the orbital tissue [4, 5]. These pathological changes cause various ocular complications in GO including periorbital swelling, ocular pain, proptosis, diplopia, and vision-threatening compressive optic neuropathy.
As the major pathogenesis of GO is autoimmune-induced inflammation, the main treatment for GO in the acute phase is intravenous glucocorticoid therapy for immune suppression [6]. In case of inadequate response to glucocorticoids or accompanying severe adverse effects, alternative immunosuppressants such as methotrexate, cyclosporine, azathioprine, and rituximab are available [7-10]. Recently, teprotumumab, which is a human monoclonal IgG1 antibody that selectively binds to insulin-like growth factor 1 receptor (IGF-1R), has been suggested as a novel targeted therapy [11].
Interleukin-2-inducible T-cell kinase (ITK) is an important Tec kinase in T cells and natural killer (NK) cells and plays a key role in signal transduction pathways via T-cell receptors and Fc receptors [12]. During CD4+ T-cell differentiation, ITK is expressed both in T helper type 1 and T helper type 2 cells. Additionally, ITK knockout mice show major defects in T-cell development and function. Thus, dysregulated ITK causes diverse T cell-related disorders such as allergic reactions, inflammation, autoimmune diseases, and T-cell malignancies [13].
BMS-509744 (BMS) is an aminothiazole-based molecular inhibitor of ITK that binds to the ATP-binding site of the ITK kinase domain [14]. BMS showed more than 200-fold selectivity to ITK than to other Tec family tyrosine kinases and inhibited ITK with a half-maximal inhibitory concentration (IC50) of 19 nM [14]. Furthermore, BMS suppressed TCR-induced PLCγ1 phosphorylation, T-cell proliferation, calcium mobilization, and IL-2 production. The suppressive effect of BMS on lung inflammation in a mouse model of allergic asthma has been previously reported [15].
In this study, we evaluated the ITK expression level in GO orbital tissues compared with that in normal orbital tissues and assessed the anti-inflammatory effect of BMS in an in vitro GO model.
Orbital explants containing adipose connective tissue were harvested during orbital decompression surgery from 11 patients with GO (8 female, 3 male; aged 25–58 years) (Table 1). Normal orbital adipose tissue explants were obtained from 11 healthy controls who were free from thyroid disease during upper or lower lid blepharoplasty (8 female, 3 male; ages 25–67 years). The study protocol was approved by the institutional review board of Soonchunhyang Hospital, Soonchunhyang University College of Medicine (2020-04-006) and Severance Hospital Yonsei University College of Medicine (4-2022-0272). It adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all the participants.
Demographics in this study
| No | Sex/Age | Duration (year) | CAS | Proptosis | Surgery |
|---|---|---|---|---|---|
| Graves’ orbitopathy | |||||
| 1 | F/25 | 1.5 | 0/7 | 20/20 | Decompression |
| 2 | F/28 | 1.3 | 0/7 | 21/21 | Decompression |
| 3 | F/36 | 6.5 | 3/7 | 21/21 | Decompression |
| 4 | M/33 | 11.0 | 2/7 | 20/20 | Decompression |
| 5 | F/44 | 2.5 | 2/7 | 20/20 | Decompression |
| 6 | M/46 | 2.5 | 3/7 | 26/26 | Decompression |
| 7 | F/37 | 7.5 | 2/7 | 26/26 | Decompression |
| 8 | F/41 | 1.6 | 0/7 | 21/21 | Decompression |
| 9 | F/35 | 3.6 | 1/7 | 23/23 | Decompression |
| 10 | F/49 | 4.1 | 2/7 | 23/21 | Decompression |
| 11 | M/58 | 3.9 | 1/7 | 20.5/22 | Decompression |
| Normal healthy control | |||||
| 1 | F/43 | N/A | N/A | N/A | Upper blepharoplasty |
| 2 | F/44 | N/A | N/A | N/A | Upper blepharoplasty |
| 3 | M/67 | N/A | N/A | N/A | Upper blepharoplasty |
| 4 | F/25 | N/A | N/A | N/A | Upper blepharoplasty |
| 5 | F/42 | N/A | N/A | N/A | Upper blepharoplasty |
| 6 | M/24 | N/A | N/A | N/A | Upper blepharoplasty |
| 7 | F/29 | N/A | N/A | N/A | Lower blepharoplasty |
| 8 | F/33 | N/A | N/A | N/A | Lower blepharoplasty |
| 9 | F/41 | N/A | N/A | N/A | Lower blepharoplasty |
| 10 | M/46 | N/A | N/A | N/A | Lower blepharoplasty |
| 11 | F/51 | N/A | N/A | N/A | Lower blepharoplasty |
All 11 patients with GO had achieved stable euthyroidism at the time of orbital decompression operation. None of the patients had received steroid treatment or radiotherapy for at least 3 months before surgery. Clinical information of the patients pertaining to past medical history, family history, smoking history, and the onset of the ocular symptom was collected. Patients who were pregnant, breastfeeding, or had chronic systemic disease for which they were taking medication were excluded.
Reagents and cell cultureThe reagents used in this study are listed in Table 2. Orbital fibroblasts were extracted and cultured as previously described [16]. Orbital explants harvested from the surgery were minced and cultured primarily in plastic culture dishes containing Dulbecco’s modified Eagles’ medium (DMEM):F12 (1:1) supplemented with 20% fetal bovine serum (FBS), 1% penicillin-streptomycin. After verifying the proliferation of orbital fibroblasts, serial passage of monolayers was performed using trypsin/EDTA, and the primary cells were cultured in 10-mm dishes with DMEM:F12 (1:1) supplemented with 10% FBS and antibiotics. The cells from passages 3–7 were analyzed in the study.
The source of the reagents in this study
| Reagents | Source |
|---|---|
| Fetal bovine serum | Gibco, Waltham, MA, USA |
| Dulbecco’s modified Eagle’s medium (DMED) Phosphate buffer saline (PBS) Penicillin and gentamicin |
Welgene, Gyeongsangbuk-do, Gyeongsan-si, South Korea |
| 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) BMS509744 |
Sigma-Aldrich, Inc, St. Louis, MO, USA |
| Rabbit polyclonal antibody against ITK (No. ab32039) | Abcam, Cambridge, UK |
| Recombinant human interleukin (IL)-1β Enzyme-linked immunosorbent assay kit for IL-8 |
R&D systems, Minneapolis, MN, USA |
| Antibodies for phosphorylated (p-) and total (t-) Akt, p-p38 mitogen activated protein kinase (MAPK), t-p38, p-c-Jun N-terminal kinase (JNK), t-JNK, p-extracellular signal-regulated kinase (ERK), t-ERK, p-nuclear factor κ-light-chain-enhancer of activated B-cells (NF-κB), t-NF-κB | Cell Signaling Technology, Danvers, MA, USA |
| β-actin | Santa Cruz Biotechnology, Santa Cruz, CA, USA |
Quantitative real-time PCR (qPCR) was used to quantitatively evaluate ITK gene transcript levels in orbital fibroblasts pretreated with or without BMS in the GO and normal groups. Additionally, orbital fibroblasts from patients with GO and normal orbital fibroblasts were pretreated with BMS (1 μM) for 0, 2, and 24 h, followed by stimulation with IGF-1 (100 ng/mL) for 48 h. Then, the IL-8 mRNA expression levels after BMS treatment and IGF-1 stimulation were quantified using RT-PCR.
According to the manufacturer’s protocol, 1 μg of isolated total RNA was acquired from the orbital fibroblasts using Trizol agent (Invitrogen, Carlsbad, CA, USA). After reverse-transcription into complementary DNA (cDNA), the cDNA was amplified and quantified using the TaqMan Universal polymerase chain reaction master mix (Applied Biosystems, Foster City, CA, USA) in a thermocycler (ABI 7300 real-time PCR thermocycler; Applied Biosystems, Carlsbad, CA, USA). The primer sequences for amplification are listed in Table 3. ITK gene expression was calculated using the threshold cycle (Ct) value and 2–ΔΔCt method. Each qPCR experiment was performed in triplicates.
Primer sequence for each gene for real-time polymerase chain reaction (PCR)
| Gene | Forward/Reverse | Sequence |
|---|---|---|
| ITK | Forward | 5'-TGGTGCACAAACCTCAACCT-3' |
| Reverse | 5'-CACATGGTTCTCCACCGTCA-3' | |
| GAPDH | Forward | 5'-ATGGGGAAGGTGAAGGTCG-3' |
| Reverse | 5'-GGGGTCATTGATGGCAACAATA-3' | |
| IL-8 | Forward | 5'-CAATCCTAGTTTGATACTCCC-3' |
| Reverse | 5'-AATTACTAATATTGACTGTGGAG-3' |
Immunohistostaining of GO and normal orbital tissue was performed to evaluate ITK protein expression in the orbital tissue. GO and healthy orbital tissues were fixed in 5% formalin and embedded in paraffin after 24 h. The formalin-fixed paraffin-embedded GO and normal orbital tissue samples were cut into 4-μm sections, deparaffinized, and rehydrated. Immunostaining was performed using the Dako PT Link and Dako Autostainer 48S Link platform (Dako, Glostrup, Denmark). High-temperature antigen retrieval was achieved by heating the samples in FLEX Target Retrieval Solution High pH buffer (Dako k8004) for 20 min at 95°C. Then, the samples were immersed in 3% H2O2 for 10 min to inactivate the endogenous peroxidase and co-incubated for 1 h at room temperature with 1:100 dilution of a primary antibody to ITK (Rabbit polyclonal antibody, ab32039, Abcam, Cambridge, UK). Then, the slides were rinsed with Tris-buffered saline (TBS), incubated with horseradish peroxidase (HRP)-labelled polymer according to the manufacturer’s instructions, and incubated for 20 min at room temperature. Color reaction was developed using 3,3'-diaminobenzidine tetrachloride (DAB) chromogen solution for 5 min. All slides were counterstained with hematoxylin for 10 min. The stained images of each sample were captured using an Olympus BX60 microscope (Olympus, Corp. Melville, NY, USA). To quantify the staining, the ratio of stained area to total area was estimated using Image J software (National Institutes of Health, Bethesda, MD, USA).
Cell viabilityThe non-cytotoxic concentration of BMS on orbital fibroblasts was determined by assessing the proliferation of orbital fibroblasts from GO and control group. Identical concentration of cells (1 × 105 cells/well) from each group was seeding into 24-well plates. The orbital fibroblasts were exposed to serial concentrations of BMS (control, 0.5, 1, 2, 5, 10 μM) for 24 and 48 h, followed by incubation with 5 mg/mL of MTT solution (Sigma-Aldrich, Inc, St. Louis, MO, USA) at 37°C for 3 h. The absorbance of the dye at 630 nm was measured using an enzyme-linked immunosorbent assay (ELISA) microplate reader (EL 340 Bio Kinetics Reader; Bio-Teck Instruments, Winooski, VT, USA). Cell viability is expressed as a percentage relative to that of the control group, which was not treated with BMS.
Western blotting assayTo evaluate the anti-inflammatory effect of BMS, orbital fibroblasts from patients with GO were pretreated with 1 μM BMS for 2 and 24 h, followed by stimulation with 10 ng/mL of IL-1β for 48 h. After pretreatment, the orbital fibroblasts were washed with cold phosphate-buffered saline (PBS) and incubated for 30 min on ice in lysis buffer containing 1 mM phenylmethylsulfonyl fluoride (PMSF), 50 mM NaF, 10% glycerol, 20 mM HEPES (pH 7.2), 0.1 mM dithiothreitol, 1 mg/mL pepstatin, 1 mg/mL leupeptin, 10 mM Na3VO4, and 1% Triton X-100. The lysates were centrifuged for 10 min at 12,000 × g. Protein concentrations were determined using the Quick Start Bradford Protein Assay Kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Equal quantities of centrifuged cell lysates (50 μg) were boiled in sample buffer and resolved on 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) gels. The obtained proteins were transferred onto a nitrocellulose membrane (Millipore Corp., Billerica, MA, USA) and incubated overnight at 4°C with primary antibodies in Tris-buffered saline Tween-20 (TBST), followed by washing three times with TBST. After treatment with HRP-conjugated secondary antibodies, the immunoreactive bands were detected using chemiluminescence (Amersham Pharmacia Biotech, Inc., Piscataway, NJ, USA). The intensity of each protein was quantified using densitometry and normalized to that of β-actin. The extent of activation of each protein was measured based on the fraction of phosphorylated protein level relative to that of the total protein level.
Enzyme-linked immunosorbent assayELISA was used to determine the suppressive action of BMS on inflammation. Orbital fibroblasts from GO and normal groups were pretreated with different concentration of BMS (0.5 and 1 μM) for 2 h. Then, the cells were exposed to 10 ng/mL of IL-1β for 48 h. IL-8 protein expression was quantified using an ELISA kit (R&D Systems, Minneapolis, MN, USA). Additionally, orbital fibroblasts from GO and normal orbital fibroblasts were pretreated with BMS (1 μM) for 0, 2, and 24 h, followed by stimulation with 100 ng/mL of IGF-1 for 48 h. Then, the level of IL-8 protein produced was quantified using ELISA.
Statistical analysisOrbital fibroblasts were derived from three or more different participants and analyzed in triplicate for all experiments. Mann-Whitney U and Kruskal-Wallis tests were applied for nonparametric data. Kolmogorov-Smirnov test was applied for variables without a normal distribution. SPSS Statistics 22 (IBM Corp., Armonk, NY, USA) was used for statistical analyses. The results with p-value less than 0.05 (p < 0.05) were considered statistically significant.
ITK transcription levels were compared between the GO and normal control groups using RT-PCR based on mRNA extracted from the orbital tissue in each group. RT-PCR result showed that the ITK gene expression levels were significantly higher in GO tissues compared with that in normal tissues (p < 0.05, Fig. 1A). The immunohistochemical experiment using antibodies against ITK showed that ITK was overexpressed in the GO orbital tissue, whereas it was barely stained in the normal orbital tissue (Fig. 1B). Quantitative analysis of the immunostaining results showed that ITK expression was significantly higher in the GO tissue than in normal adipose tissue (p < 0.05, Fig. 1C).

Gene and protein expression of interleukin-2-inducible T cell kinase (ITK) in Graves’ orbitopathy (GO) and normal control orbital tissues. (A) ITK mRNAs extracted from GO (n = 11) and normal control (n = 11) groups were evaluated using real-time polymerase chain reaction. A single dot represents the value obtained from the experiment of a single donor, and the results are presented as the median and interquartile range. (B) The immunostaining intensity in GO (n = 2) and orbital (n = 3) tissue were quantified as the ratio of the stained area to total area (p < 0.05). (C) ITK protein expression was evaluated using immunohistochemical staining with antibodies against ITK. Representative staining results from GO and normal orbital tissue are shown (magnification, 100 times).
After proinflammatory stimulation with IL-1β (10 ng/mL), western blotting assay was used to measure ITK expression levels in protein extracted from GO and normal orbital tissues at various time periods. The relative density ratio of phosphorylated ITK to total ITK was significantly increased in both the GO and normal tissue groups (Fig. 2).

Effect of IL-1β (10 ng/mL) treatment on interleukin-2-inducible T cell kinase (ITK) phosphorylation in Graves’ orbitopathy (GO) (n = 3) and normal healthy (n = 5) fibroblasts at different time periods (0–48 h). Representative bands are shown. (A) Total (t-) and phosphorylated (p-) ITK expression levels were evaluated using western blotting of the total protein extracts from both GO and normal fibroblasts. (B) Relative density ratio of ITK (p-level/t-level) are presented as means ± standard deviation. (p < 0.05)
To identify the nontoxic concentration of BMS for orbital fibroblasts, the orbital fibroblasts from GO patients were treated with various concentration of BMS (0, 0.5, 1, 2, 5, 10 μM) for 24 and 48 h. Compared with that of the control group, the cell viability after treatment with BMS at the 0–10 μM concentration range was >95% at 24 and 48 h (Fig. 3). Based on this result, the maximal nontoxic concentration of BMS was determined to be 10 μM for 48 h in orbital fibroblasts, and 1 μM of BMS was applied for the subsequent experiments.

Effect of BMS on the viability of orbital fibroblasts. Orbital fibroblasts from patients with Graves’ orbitopathy (GO) (n = 3) were seeded in 24-well culture plates and various concentration of BMS (0–10 μM) were applied for 24 and 48 h, and MTT assay was performed in duplicate. Results are presented as percentages of untreated control values and are presented as means ± standard deviation.
To evaluate the effect of BMS on proinflammatory cytokine expression, IL-8 production was assessed using western blotting and ELISA. After pretreatment of 1 μM BMS for 0, 2, and 24 h, the orbital fibroblasts from GO patients were stimulated with IL-1β (10 ng/mL) for 48 h. Western blotting analyses showed that IL-8 expression, which was enhanced by IL-1β stimulation, was significantly inhibited by BMS treatment (p < 0.05, Fig. 4A). However, no significant difference was detected in the suppressive effect on IL-8 expression in cells without IL-1β stimulation between 2 h and 24 h of BMS exposure. To evaluate the dose-dependent effect of BMS, orbital fibroblasts harvested from the orbital tissues of patients with GO and healthy control groups were pretreated with different BMS concentration (0.5 and 1 μM) for 2 h. After stimulation with IL-1β for 48 h, IL-8 secretion was assessed using ELISA. BMS exerted a suppressive effect on IL-1β-induced IL-8 secretion at both 0.5 and 1 μM concentrations in a dose-dependent manner in both GO (n = 2) and normal (n = 2) orbital fibroblasts (p < 0.05, Fig. 4B). Additionally, we found that IGF-1 (100 ng/mL)-induced IL-8 gene and protein expression was significantly attenuated by BMS treatment (Supplementary Fig. 1).

Effect of BMS on IL-1β-induced IL-8 production. (A) Orbital fibroblasts from patients with Graves’ orbitopathy (GO) (n = 3) were pretreated with BMS (1 μM) for 2 and 24 h, followed by stimulation with IL-1β (10 ng/mL) for 48 h. IL-8 expression was evaluated using western blotting analysis. Three samples from each individual were assayed in duplicate. Representative bands are shown (p < 0.05). (B) Orbital fibroblasts from patients with GO (n = 2) and normal individuals (n = 2) were pretreated with different concentration of BMS (0.5 and 1 μM) for 2 h, followed by stimulation with IL-1β for 48 h. IL-8 secretion was evaluated using ELISA. Two samples were assayed from each individual in duplicate.
To evaluate the effect of BMS on intracellular signaling pathways, the expression of various proinflammatory transcription factors including NF-κB, Akt, ERK, p38, and JNK was analyzed using western blotting analysis. Orbital fibroblasts extracted from GO and normal orbital tissue were pretreated with 1 μM BMS for 1 h and stimulated with IL-1β (10 ng/mL) for 1 h. BMS significantly suppressed IL-1β-induced NF-κB phosphorylation in both GO and normal control cells (p < 0.05, Fig. 5). Additionally, BMS exerted an inhibitory effect on IL-1β-induced phosphorylation of ERK, p38, and JNK in both GO and normal orbital fibroblasts (Fig. 6). In contrast, BMS attenuated Akt phosphorylation only in the GO fibroblasts. Moreover, western blotting analysis revealed a mild increase in NF-κB phosphorylation in naive conditions in the absence of IL-1β stimulation. However, this increase was not statistically significant.

Effect of BMS on NF-κB phosphorylation in Graves’ orbitopathy (GO) (n = 3) and normal orbital (n = 3) fibroblasts. The fibroblasts were pretreated with BMS (1 μM) for 1 h prior to IL-1β (10 ng/mL) stimulation for 1 h. The cell lysates were analyzed using western blotting to determine phosphorylated (p-) and total (t-) NF-κB protein expression. Three samples from each individual were assayed in duplicate. Each column in the graph represents a relative density ratio of NF-κB (p-NF-κB/t-NF-κB) and are presented as means ± standard deviation. (p < 0.05)
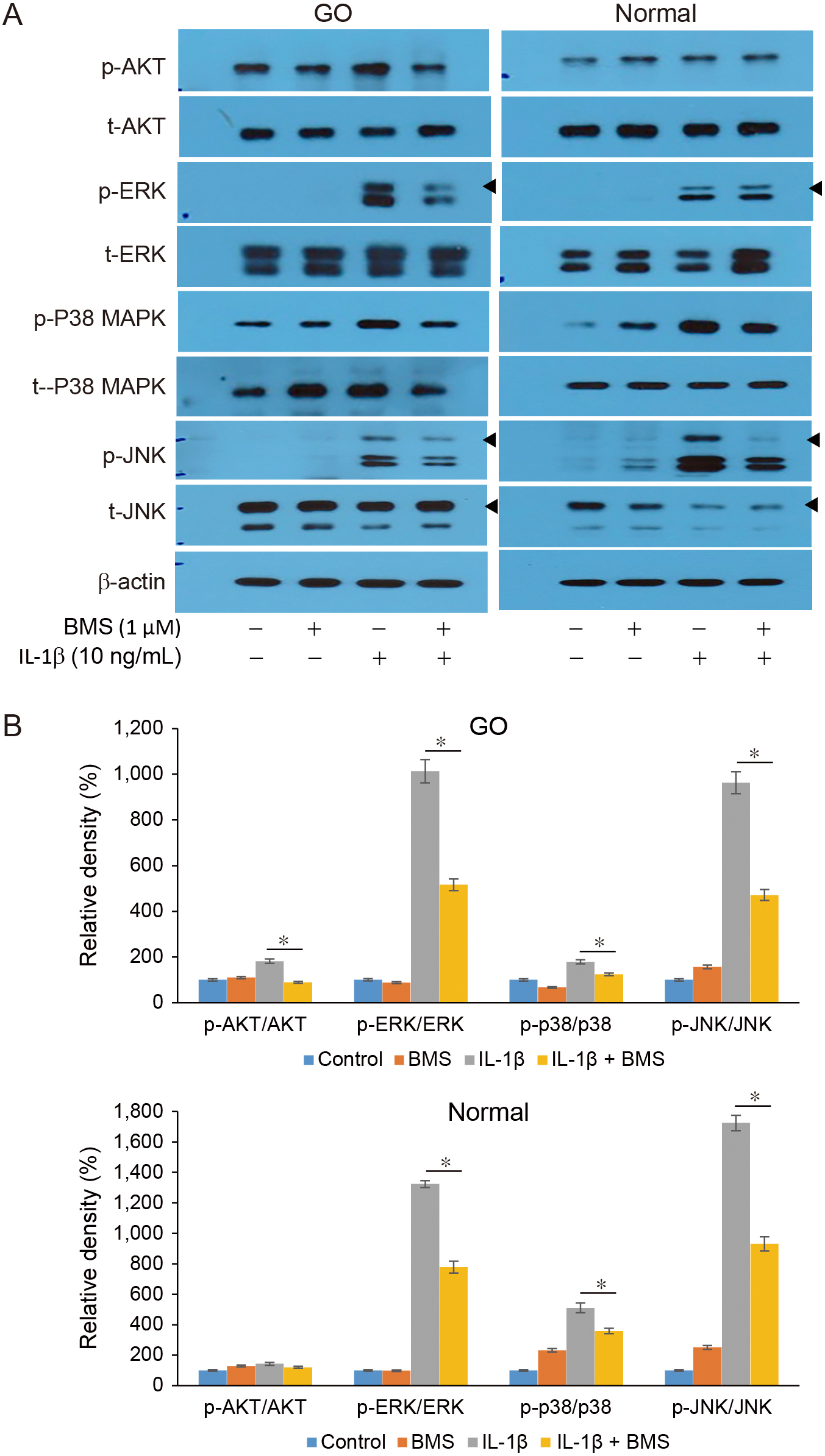
Effect of BMS on intracellular signal pathway molecules in Graves’ orbitopathy(GO) (n = 3) and normal healthy orbital (n = 3) fibroblasts. (A) The orbital fibroblasts from both GO and normal healthy control groups were pretreated with BMS (1 μM) for 1 h, followed by stimulation with IL-1β (10 ng/mL) for 1 h. Western blotting analyses were performed to evaluate phosphorylated (p) Akt, ERK, p38, and JNK protein expression in GO and normal control group. Three samples from each individual were assayed in duplicate. The representative bands are shown. (B) Relative density ratio is presented for each protein (p-level/total level), and the results are shown as means ± standard deviation (p < 0.05)
In this study, we investigated the anti-inflammatory role of the selective ITK inhibitor BMS-509744 on GO orbital fibroblasts. Primarily, ITK was overexpressed in GO orbital tissue than in normal orbital tissue. ITK phosphorylation was enhanced by IL-1β-induced inflammatory reaction. Treatment with BMS-509744 at nontoxic concentration significantly suppressed the expression of proinflammatory cytokines and transcription factors.
ITK is the predominant Tec family kinase that is primarily expressed in T cells. It plays a pivotal role in T-cell receptor (TCR) signaling by inducing T-cell differentiation and proinflammatory cytokine expression by activating helper T cells [17]. An ITK-deficient mice model verified that the ITK loss significantly affects cytokine production associated with Th2, Th9, and Th17 cells [18-20]. In contrast, a relatively mild contribution of Th1-related cytokines has been reported as the expression of Rlk partially compensate for the loss of ITK [21]. Additionally, ITK plays a critical role in activating and translocating transcription factors involved in the calcium signaling pathway such as NFAT and NFκB [22].
Based on those findings, recent studies have suggested that ITK is involved in various inflammatory and autoimmune diseases that affect diverse tissues including skin, gut, and central nervous system. In atopic dermatitis, ITK levels are elevated in peripheral blood T cells [23]. An ITK-deficient mouse model showed reduced acute contact hypersensitivity and inflammatory reactions [24]. In addition to that observed in murine models, ITK suppression consistently resulted in inhibition of IFNγ and IL-2 production in human cells [24]. Furthermore, ITK plays an important role in inflammatory bowel disease. Cho et al. reported that an ITK inhibitor (PRN 694) suppressed Th1-cell differentiation and ulcerative colitis development [25]. The mice group treated with ITK inhibitor showed decreased proportion of CD4+ cells and IFNγ level in the lamina propria and intestinal epithelium [25]. In a mouse model of experimental autoimmune encephalitis (EAE), which is a model for multiple sclerosis (MS), the depletion of ITK attenuates disease severity by diminishing the transmigration of CD4+ cells into the central nervous system and secretion of cytokines from Th1 and Th17 cells [26].
As ITK plays an essential role in regulating TCR signaling pathways, ITK inhibitors have been actively investigated as new therapeutic agents for modulating T-cell associated inflammatory diseases. BMS-509744 was one of the first ITK-specific inhibitors, which is comprised of aminothiazole [27]. Similarly, other inhibitors including aminobenzimidazoles, indoles, and pyridones that target ATP-binding sites in the kinase domain have also been developed [27]. Recently, ibrutinib, which is a B-cell homologue BTK inhibitor, has been demonstrated to inhibit both BTK and ITK [28]. Furthermore, newly designed ITK inhibitors such as PRN694, which exerts an irreversible inhibitory effect by covalent bonding, or CPI-818, which targets PLCγ1 phosphorylation, are also capable of successfully blocking TCR signaling and T-cell immunity [29].
Among various ITK inhibitor candidates, this study focused on BMS-509744, which is the first developed ITK-specific inhibitor. BMS-509744 displays a 200-fold higher selectivity to ITK than to other Tec family kinases via an ATP competitive mechanism. BMS-509744 reduced T-cell proliferation and IL-2 production in an in vitro model and significantly inhibited lung inflammation in a mouse model of ovalbumin-induced asthma [15]. Sho et al. reported that topical application of BMS-509744 showed a favorable effect on imiquimod-induced skin inflammation resembling psoriasis [30]. BMS-509744 suppresses CD3+ T-cell differentiation and Th17-related cytokine expression, resulting in attenuated skin inflammation [30].
Similarly, this study proposes that BMS-509744 exerts beneficial effect on controlling GO by suppressing TCR signaling. Although GO pathogenesis is still not fully understood, several studies have demonstrated that Graves’ disease is closely associated with T-cell differentiation and sequential expression of proinflammatory cytokines. For example, the expression of IL-8, which is a major neutrophil chemotactic factor produced by CD4+ T cells, is significantly higher in patients with GO than in healthy individuals [31]. IL-17, which is produced by Th17 cells, activates signaling cascades to induce chemoattractants to recruit immune cells, which further contributes to GO pathogenesis. Increased IL-17 serum level correlated with the clinical activity scores of GO [32]. This study demonstrates that BMS-509744 inhibits IL-8 expression and downstream intracellular signaling in GO orbital fibroblasts. Previous studies have shown that BMS-509744 suppressed diverse T-cell related cytokines, including IL-17, ultimately resulting in reduced inflammation.
Furthermore, the IGF-1 receptor pathway has been identified as a crucial mechanism in GO, and targeting the IGF-1 receptor with monoclonal antibodies has been approved by the FDA as a first-line treatment [33]. Some reports have indicated that the IL-8 level increases because of the stimulation of the IGF-1 receptor pathway, which is inhibited by IGF-1 antibodies [33]. In our study, we additionally stimulated orbital fibroblasts with IGF-1, and the increased IL-8 gene and protein levels were both reduced by the ITK inhibitor. This suggests the potential of the ITK inhibitor to block proinflammatory cytokine production through the IGF-1 receptor pathway in GO.
In conclusion, we have demonstrated that ITK suppression exerts an anti-inflammatory effect on primary cultured orbital fibroblasts in the in vitro model. BMS-509744, which is a selective IL-2-induced tyrosine kinase inhibitor, attenuates the activation of proinflammatory signaling pathways and production of proinflammatory cytokines in in vitro setting of GO. However, drawing a conclusion that the anti-inflammatory effects of ITK inhibition are specific to the GO mechanism rather than a generalized anti-inflammatory effect is premature, as our results do have limitations. Therefore, further clinical studies in in vivo models and practical settings are required to build up the clinical certification of ITK inhibitor.
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT, Ministry of Science and ICT; grant number RS-2023-00208570). This study was also supported by the Soonchunhyang University Research Fund.
We have no financial conflicts of interest to disclose.