2025 Volume 72 Issue 6 Pages 697-705
2025 Volume 72 Issue 6 Pages 697-705
The random C-peptide and random C-peptide index (CPI) have been shown to be useful in assessing endogenous insulin secretory capacity in adults with type 2 diabetes. This study aimed to clarify the utility of C-peptide and the CPI in early classification of long-term insulin-dependent status in pediatric diabetes patients. A total of 204 patients aged ≤15 years who received an initial diagnosis of acute-onset type 1 diabetes mellitus (T1DM), slowly progressive insulin-dependent diabetes mellitus (SPIDDM), or type 2 diabetes mellitus (T2DM) at Yokohama City University Medical Center between April 1, 2003 and March 31, 2018 were included. The acute-onset T1DM, SPIDDM, and T2DM groups included 140, 8, and 56 patients, respectively. The median random C-peptide values were 0.67, 3.18, and 4.16 ng/mL and median random CPI values were 0.19, 2.02, and 2.53 for acute-onset T1DM, SPIDDM, and T2DM cases, respectively (acute-onset T1DM vs. T2DM, p < 0.001 (C-peptide), p < 0.001 (CPI), acute-onset T1DM vs. SPIDDM, p < 0.001 (C-peptide), p < 0.001 (CPI), SPIDDM vs. T2DM, p = 0.04 (C-peptide), p = 0.19 (CPI)). Receiver operating characteristic analysis cutoff values of C-peptide levels in differentiating acute-onset T1DM from SPIDDM and acute-onset T1DM from T2DM were 1.60 ng/mL (sensitivity 87.5%, specificity 90.6%) and 1.81 ng/mL (sensitivity 91.1%, specificity 93.5%), while the respective CPI values were 0.46 (100% sensitivity, 77% specificity) and 1.05 (92.1% sensitivity, 87.5% specificity). This study indicates that the random C-peptide and random CPI at diagnosis are helpful in the early classification of childhood diabetes and determining an appropriate time to introduce insulin and predicting the subsequent clinical course.

As has been previously shown, type 1 diabetes mellitus (T1DM) has increased in some regions worldwide [1-3]. T1DM is caused by the destruction of pancreatic beta cells, and is classified into two types: type 1A, caused by autoimmunity and type 1B, the causes of which remain unidentified. Conversely, type 2 diabetes mellitus (T2DM) is caused by a deficiency of insulin action due to multiple genetic factors that result in decreased insulin secretion and the development of insulin resistance, in addition to environmental factors such as lifestyle and obesity. Due to an increase in childhood obesity levels, the incidence of T2DM is increasing worldwide [4-7]. Although a similar trend in the incidence of T2DM has been observed in Japan as in the rest in the world, obesity is absent in 10–15% of T2DM cases, making differentiation from T1DM important [8].
The pathogenesis of T1DM differs from that of T2DM. In T1DM, the rate of progression of pancreatic beta cell destruction varies, while the insulin secretory capacity may be preserved in the early stages of the disease, meaning that some cases detected by chance in urine glucose screening at school or general medical care difficult to distinguish from T2DM. Further, 80–90% of patients with T1DM test positive for islet autoantibodies such as anti-glutamic acid decarboxylase and anti-islet antigen 2 antibodies, which are considered helpful in the diagnosis of the disease type. Autoantibodies are negative in some cases, while slowly progressive insulin-dependent diabetes mellitus (SPIDDM) presents with the same clinical features as T2DM at diagnosis [9, 10]. It is often difficult to diagnose the disease type at an early stage and determine when to introduce insulin.
Serum C-peptide is an insulin precursor, or proinsulin, which is broken down into one molecule each of insulin and C-peptide in pancreatic beta cells, which are subsequently released into the blood. Because serum insulin levels are affected by insulin administration and/or the presence of insulin antibodies, C-peptide, which is free of these effects, is used as an indicator of endogenous insulin secretion [11]. It is known that the fasting serum C-peptide level at diagnosis is higher in patients with T2DM than those with T1DM [12]. However, the fasting serum C-peptide cannot always be evaluated in pediatric patients with diabetes mellitus, as the incidence of T1DM is high and the diagnosis is often made at the time of an emergency visit. There are many situations in which a decision regarding whether or not to introduce insulin must be made without waiting for islet autoantibody test results; as such, an indicator to allow differential diagnosis is required, as the introduction of insulin therapy in pediatric diabetes patients may affect their quality of life in daily life and at school.
In adult diabetic patients, random serum C-peptide levels have been reported to be useful in diagnosing the disease type [13]. Further, there have been reports that random serum C-peptide levels are also useful in diagnosing disease type in pediatric cases [14]. However, there are currently no reports comparing the random serum C-peptide values in acute onset T1DM with those in SPIDDM or T2DM.
Conversely, as serum C-peptide is synthesized in a 1:1 ratio with insulin, its secretion increases at high blood glucose levels, and does not accurately reflect endogenous insulin secretory capacity. Recently, it has been reported that the C-peptide index, calculated as the serum C-peptide level / blood glucose level × 100, may be useful as an indicator of insulin secretory capacity with minimal influence of blood glucose level in patients with diabetes mellitus [15]. Both casual and fasting CPIs are correlated with insulin secretory capacity in adult T2DM, and are expected to be applied as a more accurate indicator for judging when to introduce insulin therapy in adult patients; however, there have been no reports of use in pediatric diabetes cases [16, 17]. Given this context, the present study aimed to determine whether random serum C-peptide levels and CPI at diagnosis could facilitate the early diagnosis of diabetes under random conditions at the initial diagnosis of pediatric diabetes.
The subjects were patients aged ≤15 years who developed diabetes during the 15-year period between April 1, 2003 and March 31, 2018, and who had visited our hospital for at least 3 years. The diagnostic criteria for diabetes mellitus were established according to the diagnostic criteria of the Japan Diabetes Society [18]. The final diagnosis was made by the attending physician, based on each patient’s clinical condition at the time of the initial examination, the results of islet-associated autoantibodies, while the clinical course was based on the fact that the insulin secretory capacity of T1DM declines significantly over 2 to 4 years [19-21]. Of the patients diagnosed with T1DM, those with reduced endogenous insulin secretory capacity at diagnosis and requiring early insulin therapy with ketosis or diabetic symptoms were initially classified as having acute-onset T1DM, while those who were positive for islet-related autoantibodies but remained insulin-independent for 3 months after diagnosis were classified as having SPIDDM [22]. T2DM was comprehensively diagnosed among patients who were negative for islet-associated autoantibodies, based on the presence of preserved insulin secretory capacity, insulin resistance, and obesity, and the final diagnosis of two patients was changed from type 2 diabetes to SPIDDM as they were found to be positive for islet-associated autoantibodies.
A total of 257 patients were diagnosed with diabetes at our hospital. Of these, 1 patient with onset of diabetes at <1 year of age, 19 who had attended our clinic for <3 years, 15 for whom serum C-peptide levels were not measured at the initial visit, 4 whose serum C-peptide levels were not measured 3 years after diagnosis, 8 with psychomotor retardation or hearing loss (possibly due to mitochondrial disease, genetic abnormalities, or a specific disease or syndrome), 3 with a strong family history of diabetes mellitus (due to the possibility of mitochondrial disease or Maturity Onset Diabetes of the Young), and 3 with suspected soft drink ketosis (because they had reduced initial insulin secretion, meaning that the insulin secretion capacity at diagnosis is not helpful in disease diagnosis, as insulin secretion is restored later in the disease course) were excluded from the study. Finally, a total of 204 patients were enrolled. This study was reviewed and approved by the Ethical Review Committee of Yokohama City University (F220900068).
Information such as patient age at initial diagnosis, sex, whether the disease was detected in urine glucose screening at school, BMI percentile (%ile), blood glucose level, HbA1c, random serum C-peptide level and random CPI at diagnosis, random serum C-peptide level and blood glucose level at 3 years after onset, insulin treatment, and insulin-dependent status were collected and compared for each disease type.
Statistical analysisEZR software (Saitama Medical Center, Jichi Medical University, Saitama, Japan) was used for all statistical analysis [23]. Age at diagnosis, BMI%ile, and HbA1c values for each diabetes type were compared using the Mann-Whitney U test, as a skewed distribution was indicated. The Kruskal-Wallis test was applied to compare serum C-peptide level and CPI at the time of diagnosis of each diabetes type, as these values indicated a skewed distribution. ROC curve analysis was further applied to determine the sensitivity and specificity of C-peptide level and CPI as predictors of diagnosis. Data are expressed as medians, and p < 0.05 was considered statistically significant.
Of the 204 pediatric patients with diabetes who had been attending our hospital for >3 years, 140, 8, and 56 had acute-onset T1DM, SPIDDM, and T2DM, respectively (Table 1). The age at onset was 9.0 (6.0–9.0) {median (IQR)} years for acute-onset T1DM, 12.0 (10.8–14.0) years for SPIDDM, and 13.0 (11.0–14.3) years for T2DM. The age of acute-onset T1DM was significantly lower than that of SPIDDM and T2DM. The BMI%ile of patients with acute-onset T1DM was 20.7 (3.8–56.8), which was significantly lower than the BMI%ile of 95.5 (70.8–96.6) for SPIDDM and 97.7 (93.5–99.3) for T2DM. Overall, 3/140 patients with T1DM, 5/8 patients with SPIDDM, and 36/56 patients with T2DM were obese, with a BMI%ile ≥95%ile. The HbA1c level at the first visit was 11.9% (9.5–13.5%) in acute-onset T1DM, significantly higher than the HbA1c of 6.6% (5.6–9.6%) in SPIDDM and 8.8% (6.7–10.9%) in T2DM.
| Type of diabetes | Type 1 DM | SPIDDM | Type 2 DM | p-value | ||
|---|---|---|---|---|---|---|
| Type 1 DM vs. SPIDDM |
Type 1 DM vs. Type 2 DM |
SPIDDM vs. Type 2 DM |
||||
| Number of patients | 140 | 8 | 56 | |||
| Sex (male/female) | 55/85 | 8/0 | 26/30 | |||
| Age at diagnosis (yr); median (IQR) | 9.0 (6.0–12.0) | 12.0 (10.8–14.0) | 13.0 (11.0–14.3) | <0.001 | <0.001 | p = 0.59 |
| Follow up duration (yr); median (IQR) | 6.0 (3.0–10.0) | 7.5 (5.8–8.5) | 7.5 (5.0–9.3) | p = 0.46 | p = 0.08 | p = 1.0 |
| Detection by urine glucose screening at school (%) | 39 (28) | 8 (100) | 46 (82) | |||
| BMI at diagnosis (%ile); median (IQR) | 20.7 (3.8–56.8) | 95.5 (70.8–96.6) | 97.7 (93.5–99.3) | <0.001 | <0.001 | p = 0.07 |
| HbA1c at diagnosis (%); median (IQR) | 11.9 (9.5–13.5) | 6.6 (5.6–9.6) | 8.8 (6.7–10.9) | <0.001 | <0.001 | p = 0.12 |
| Number of islet-associated autoantibody-positive cases (%) | 125 (89) | 8 (100) | 0 | |||
BMI: body mass index, Type1 DM: acute-onset Type1 DM
The results of quantitative variables are given as the median with interquartile range.
Two patients initially diagnosed with T2DM were reclassified as SPIDDM after later testing positive for islet-associated autoantibodies.
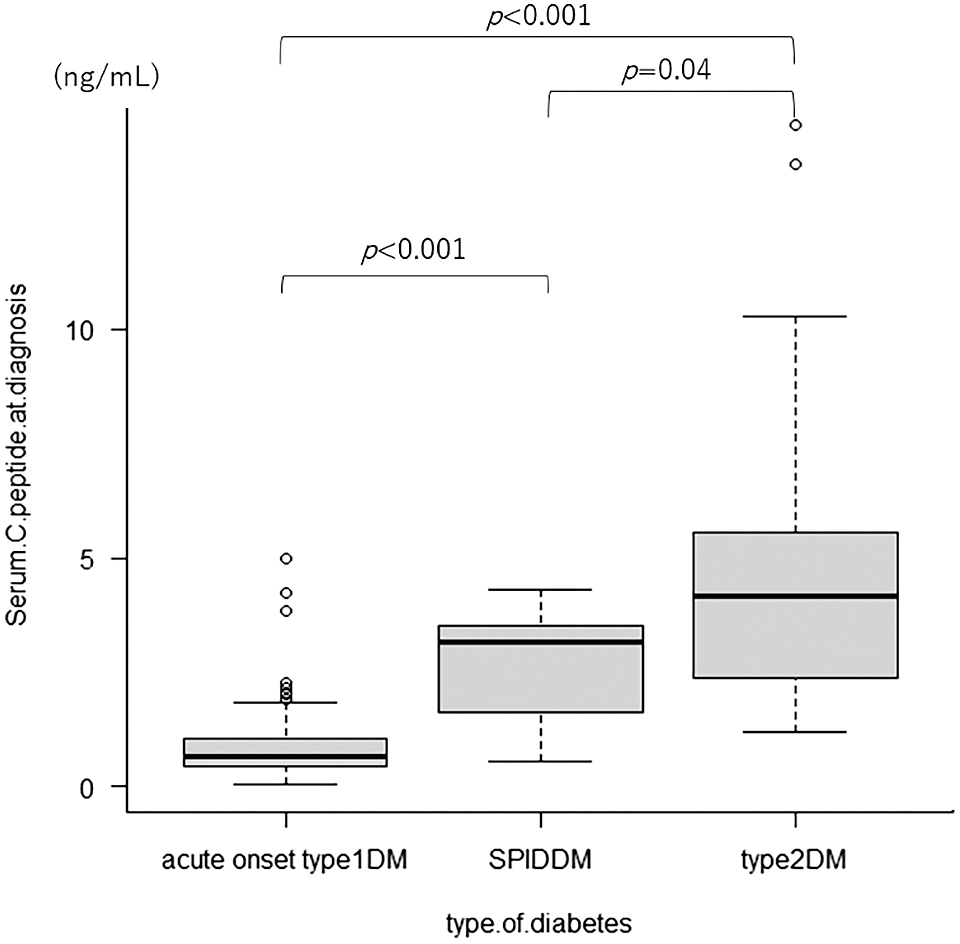
The random serum C-peptide levels were significantly higher in patients with SPIDDM than in those with acute-onset T1DM during the first visit (median 0.67 vs. 3.18 ng/mL p < 0.001) (Fig. 1). ROC curve analysis obtained a random serum C-peptide cutoff value of 1.60 ng/mL (sensitivity 87.5%, specificity 90.6%) (Fig. 2).

AUC 0.884 (95%CI 0.729–1.0)
Cutoff 1.60 ng/mL, Sensitivity: 87.5%, Specificity: 90.6%
(B) ROC curve analysis of C-peptide index at the time of diagnosis of acute onset Type1DM and SPIDDM
AUC 0.938 (95%CI 0.873–1.0)
Cutoff 0.463, Sensitivity: 100%, Specificity: 77%
The random CPI at diagnosis was also significantly higher in patients with SPIDDM than in those with acute-onset T1DM (median 0.19 vs. 2.02, p < 0.001) (Fig. 3), with a ROC curve cutoff value of 0.463 (sensitivity 100%, specificity 77%) (Fig. 2).

Random serum C-peptide levels were significantly higher in patients with T2DM than in those with acute-onset T1DM during the first visit (median 0.67 ng/mL vs. 4.16 ng/mL, p < 0.001) (Fig. 1). ROC curve analysis revealed a random serum C-peptide cutoff value of 1.81 ng/mL (sensitivity 91.1%, specificity 93.5%) (Fig. 4). The random CPI at diagnosis was also found to be significantly higher in patients with T2DM than in those with acute-onset T1DM (median 0.19 vs. 2.53, p < 0.001) (Fig. 3), with a ROC curve cutoff value of 1.047 (sensitivity 87.5%, specificity 92.1%) (Fig. 4).

AUC 0.974 (95%CI 0.957–0.992)
Cutoff 1.81 ng/mL, Sensitivity: 91.1%, Specificity: 93.5%
(B) ROC curve analysis of C-peptide index at the time of diagnosis of acute onset Type1DM and Type2DM
AUC 0.958 (95%CI 0.931–0.985)
Cutoff 1.047, Sensitivity: 87.5%, Specificity: 92.1%
The random serum C-peptide at diagnosis was slightly higher in T2DM compared to SPIDDM (median 3.18 vs. 4.16 ng/mL, p = 0.04) (Fig. 1). The random CPI at diagnosis was not significantly different between the SPIDDM and T2DM (median 2.53 vs. 2.02 p = 0.19) (Fig. 3).
Clinical condition 3 years after onsetThree years following disease onset, the random C-peptide level was significantly higher in patients with SPIDDM and T2DM than in those with acute-onset T1DM (0.16, 3.53, 3.69 ng/mL, acute-onset T1DM vs. SPIDDM, p < 0.001, acute-onset T1DM vs. T2DM, p < 0.001) (Fig. 5).

Three years following disease onset, the random CPI was significantly higher in patients with SPIDDM and T2DM than in those with acute-onset T1DM (0.08, 2.52, 2.55, acute-onset T1DM vs. SPIDDM, p < 0.001, acute-onset T1DM vs. T2DM, p < 0.001) (Fig. 5).
Comparison of the random serum C-peptide levels and random CPI at diagnosis of acute-onset T1DM and after 3 years revealed a significant decrease in both parameters (median 0.67 vs. 0.16 ng/mL, p < 0.001 median 0.19 vs. 0.08, p < 0.001) (Fig. 5). After 3 years, all patients with acute-onset T1DM were on basal-bolus therapy (MDI: multiple daily injection or CSII). At 3 years post-diagnosis, intensive insulin therapy to correct hyperglycemia was initiated in two patients with SPIDDM during the disease course; however, both patients had high serum C-peptide levels of 3.14 and 2.71 ng/mL, as well as low total insulin doses of 0.5 and 0.3 U/kg/day, indicating a lack of insulin-dependence. Four patients with SPIDDM were treated with dietary therapy alone, one patient was treated with Metformin as an oral hypoglycemic drug, and one was treated with a combination of Metformin and long-acting insulin. All patients with SPIDDM were insulin-independent after 3 years, whereas all patients with acute-onset T1DM were insulin-dependent (Table 2). Among patients with T2DM, 10 were treated only with diet and exercise therapy, 36 were treated with oral hypoglycemic drugs, and 8 were treated with oral hypoglycemic drugs and insulin.
| Type of diabetes | Type 1 DM | SPIDDM | Type 2 DM | p-value | ||
|---|---|---|---|---|---|---|
| Type 1 DM vs. SPIDDM | Type 1 DM vs. Type 2 DM | SPIDDM vs. Type 2 DM | ||||
| Serum C-peptide at diagnosis (ng/mL); median (IQR) | 0.67 (0.45–1.04) | 3.18 (1.63–3.52) | 4.16 (2.44–5.56) | <0.001 | <0.001 | p = 0.04 |
| Serum C-peptide after 3 years (ng/mL); median (IQR) | 0.16 (0.05–0.41) | 3.53 (2.74–4.20) | 3.69 (2.56–8.13) | <0.001 | <0.001 | p = 0.65 |
| C-peptide index at diagnosis; median (IQR) | 0.19 (0.10–0.44) | 2.02 (1.04–3.03) | 2.53 (1.34–4.57) | <0.001 | <0.001 | p = 0.19 |
| C-peptide index after 3 years; median (IQR) | 0.08 (0.03–0.23) | 2.52 (1.94–2.84) | 2.55 (1.30–5.64) | <0.001 | <0.001 | p = 0.75 |
| Number of patients dependent on insulin after 3 years | 140 | 0 | 0 | |||
| Number of patients on insulin therapy after 3 years (%) | 140 (100) | 3 (38) | 8 (14) | |||
Type 1 DM: acute-onset Type 1 DM. The results of quantitative variables are given as the median with interquartile range.
Two patients with SPIDDM who were undergoing intensive insulin therapy 3 years after diagnosis were classified as insulin independent because of high serum C-peptide levels of 3.14 ng/mL and 2.71 ng/mL and low total insulin doses of 0.5 U/kg/day and 0.3 U/kg/day.
Two patients with T2DM who were undergoing intensive insulin therapy after 3 years from diagnosis were classified as insulin independent because of high serum C-peptide levels of 2.44 ng/mL and 2.60 ng/mL and low total insulin doses of 0.3 U/kg/day and 0.5 U/kg/day.
In this study, we examined whether random C-peptide levels at diagnosis, which ignore the influence of diet and random CPI at diagnosis, which minimizes the influence of blood glucose levels, are useful for the diagnosis of early disease type in pediatric diabetes patients, many of whom have difficulty obtaining fasting blood samples at diagnosis. We further examined whether random C-peptide and random CPI at diagnosis is useful in predicting clinical course, including the presence or absence of insulin therapy and intensity of therapy, 3 years after diagnosis. The results indicated that random C-peptide and random CPI at diagnosis is useful for disease type diagnosis and prediction of insulin-dependent status.
The treatment of diabetes depends on the exact disease type, with insulin therapy being particularly burdensome for patients. This is important, as there are concerns regarding the impact on quality of life due to the introduction of insulin therapy in daily life and school life [24]. Early diagnosis is important to determine whether insulin therapy is necessary or not; however, islet-associated autoantibody testing is often time-consuming, and there are cases in which early diagnosis is difficult. Conversely, there have been reports that the early introduction of insulin therapy is desirable in T1DM to preserve beta-cell function [25], and there have also been reports in Japan that the early initiation of insulin therapy in SPIDDM preserves residual insulin secretory capacity [26]. Further, residual endogenous insulin secretory capacity has been shown to be associated with a decreased incidence of subsequent complications [27], making it important to maintain residual insulin secretory capacity.
Another type of diabetes mellitus, termed SPIDDM or latent autoimmune diabetes, presents an intermediate course between T1DM and T2DM at the onset of diabetes and thereafter [28]. In this study, we observed one case in which the patient was positive for islet-associated autoantibodies, but clinically presented with symptoms of T2DM, such as obesity and hyperinsulinemia, making it difficult to determine whether immediate insulin therapy was necessary. In addition, T2DM varies from typical cases of obesity and insulin resistance to emaciation and deficient insulin secretion. In the present study, deciding whether or not to initiate insulin therapy in non-obese patients with T2DM was difficult, until their disease type could be determined. Ludvigsson et al. reported that 3% of the patients were initially unaware of their disease type [14]. Therefore, a method for early and appropriate diagnosis of the disease type is needed.
In the present study, serum C-peptide levels declined over time in those with acute-onset T1DM; however, all patients with SPIDDM retained their insulin secretory capacity for more than 3 years, and did not become insulin dependent (Fig. 5). Although it is necessary to monitor the duration of the loss of insulin secretory capacity in the future, it is highly important to determine when to introduce insulin therapy at an early stage, because of the difficulty in making a decision to discontinue therapy once it has been introduced.
In one prior Swedish multicenter pediatric diabetes study, Ludvigsson et al. found that only one in 1,037 patients with random serum C-peptide levels <0.6 ng/mL at diagnosis was not eventually diagnosed with T1DM [14]. Min et al. also found that a fasting serum C-peptide level ≤0.6 ng/mL at diagnosis could exclude T2DM in a study of 223 patients at five centers in Korea [12].
In the present study, only 55 patients with acute-onset T1DM had levels of 0.6 ng/mL or less, while 85 patients had levels of 0.6 ng/mL or greater. In addition, there were several emergency or referral cases of each disease type for which patients were not fasting at the initial examination. Serum C-peptide levels are influenced by diet, and fluctuate with the passage of time after eating. Uehara et al. previously suggested that casual CPI is useful as an indicator of the need for insulin therapy in adult patients with T2DM; we examine its usefulness in pediatric diabetes as well. Therefore, in this study, the cutoff value of random CPI at diagnosis was detected along with the cutoff value of random serum C-peptide level at diagnosis among each diabetes type. A random serum C-peptide level below 1.60 ng/mL at diagnosis indicates a high probability of acute-onset T1DM. A random CPI below 0.46 at diagnosis indicates a high probability of acute-onset T1DM, meaning that the early introduction of insulin therapy could be considered necessary. Among patients who test positive for autoantibodies, a random C-peptide level of 1.60 ng/mL or random CPI of 0.46 or higher at diagnosis may indicate SPIDDM, requiring careful judgment regarding the introduction of insulin.
This study has some limitations. First, the information obtained was limited because the study was conducted retrospectively using data extracted from the medical records. Second, highly sensitive serum C-peptide values have made it possible to measure trace amounts of serum C-peptide [29]. In the present study, the low sensitivity of serum C-peptide measurements may not have accurately reflected the serum C-peptide levels. Finally, this was a single-center study with a limited number of participants. Future studies should be conducted in multiple institutions with a larger number of cases.
The diagnosis of pediatric diabetes mellitus is expected to become more complex with the recent increase in the number of pediatric T2DM cases and the existence of SPIDDM, in which the insulin secretory capacity is maintained for several years, even after islet-autoantibody positivity develops. In such a situation, it has been suggested that random serum C-peptide levels and random CPI may be useful in determining an appropriate time introduce insulin therapy, which affects the quality of life in childhood (Graphical Abstract).

None of the authors has any potential conflict of interest associated with this research.
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.