2019 Volume 65 Issue 3 Pages 76-98
2019 Volume 65 Issue 3 Pages 76-98
Cryptorchidism (undescended testes) is among the most common congenital diseases in male children. Although many factors have been linked to the incidence of cryptorchidism, and testicular androgen plays a key role in its pathogenesis, the cause remains unknown in most cases. Recently, a Japanese group published a speculative paper entitled, “Nationwide increase in cryptorchidism after the Fukushima nuclear accident.” Although the authors implicated radionuclides emitted from the Fukushima accident as contributing to an increased incidence of cryptorchidism, they failed to establish biological plausibility for their hypothesis, and glossed over an abundance of evidence and expert opinion to the contrary. We assessed the adequacy of their study in terms of design setting, data analysis, and its conclusion from various perspectives. Numerous factors must be considered, including genetic, environmental, maternal/fetal, and social factors associated with the reporting of cryptorchidism. Other investigators have established that the doses of external and internal radiation exposure in both Fukushima prefecture and the whole of Japan after the accident are too low to affect testicular descent during fetal periods;thus, a putative association can be theoretically and empirically rejected. Alternative explanations exist for the reported estimates of increased cryptorchidism surgeries in the years following Japan’s 2011 earthquake, tsunami, and nuclear crisis. Data from independent sources cast doubt on the extent to which cryptorchidism increased, if at all. In any case, evidence that radionuclides from the Fukushima Daiichi Nuclear Power Plant could cause cryptorchidism is lacking.
Cryptorchidism, the failure of one or both testes to descend into the scrotum, is among the most common congenital diseases in boys. In utero, testicular descent occurs in two phases: transabdominal and inguinoscrotal1). The testis finishes transabdominal descent by 10 weeks’ gestation, and passes through the inguinal canal toward the scrotum between 20 and 28 weeks’ gestation (inguinoscrotal phase). Testicular descent is usually completed before birth (around 35 weeks’ gestation)2,3). Androgen levels, especially testosterone secreted by Leydig cells, peaks between 14 and 17 weeks’ gestation2). In most cases of cryptorchidism, the testis locates between the internal inguinal ring and the scrotum, owing to some disturbance in the androgen-dependent inguinoscrotal phase. Indeed, androgen blockage during the inguinoscrotal phase induces cryptorchidism in animal studies1). Recent reports have described the association between pathogenetic mechanisms for cryptorchidism and genetic, environmental, and maternal/fetal factors3); despite this, the cause of cryptorchidism remains unknown in most cases. Environmental endocrine disrupters (EEDs), a group of compounds with potentially adverse health effects, are thought to be associated with cryptorchidism. EEDs can be found in foods, packaging, and pesticides, and some EEDs inhibit the normal function of androgen in animal studies. Although experimental studies using animal models have provided support for the hypothesis that EEDs impact on human fetal testicular descent and cryptorchidism, as yet there is insufficient evidence to conclusively implicate EEDs in cases of human cryptorchidism3).
A recent publication by Murase et al., “Nationwide increase in cryptorchidism after the Fukushima nuclear accident,” suggests that radionuclides emitted from the Fukushima Daiichi Nuclear Power Plant could be a major cause of increased cryptorchidism4). However, numerous limitations of their study impair the authors’ credibility. Herein, we first summarize global trends pertaining to cryptorchidism. We then review known effects of radiation exposure on testicular function, spermatogenesis, and steroidogenesis in order to determine if and when radiation could plausibly interfere with testicular descent. Finally, we consider whether or not cryptorchidism anywhere in Japan increased after Japan’s earthquake, tsunami, and nuclear crisis, and whether the dispersion of radionuclides could be implicated.
Various epidemiological surveys have reported changes in the prevalence of cryptorchidism. Some older surveys, before the 1980s, reported prevalences of cryptorchidism increasing year by year. Chilvers et al. reported that the annual number of hospital patient discharges including a diagnosis of cryptorchidism had risen by a factor of 2.3 in the Examination of Hospital Inpatient Enquiry data for England and Wales over the years 1962-19815). Campbell et al. studied trends in hospital discharges of patients with cryptorchidism in Scotland from 1961 to 1985 and found substantial increases in the discharge of boys with cryptorchidism in different age groups6). The John Radcliffe Hospital Cryptorchidism Study Group examined a total of 1,849 boys born to mothers residing in a defined area around Oxford and showed that the cryptorchidism rate had apparently increased by 65% over two decades, in contrast to a concurrent doubling of the national orchidopexy rate7).
Other surveys before and after the 1980s reported that the prevalence of cryptorchidism did not always increase. Paulozzi examined data from a larger number of countries participating in the International Clearinghouse for Birth Defects Monitoring Systems to address the question of whether cryptorchidism increased worldwide between the 1960s and the 1990s8). They reported clear increases in two U.S. systems and in the South American system, but not in seven other systems elsewhere8). Richiardi et al. analyzed Swedish trends in orchiopexy rates using data from the Swedish Hospital Discharge Register between 1977 and 1991 and reported that the prevalence of cryptorchidism decreased in Sweden starting from the early 1980s9). Toledano et al. examined the number of orchiopexy procedures using routine hospital admission data for England, Wales, and Scotland for the fiscal years 1992-1993 through 1998-1999 and showed that the orchiopexy rates for boys 0-14 years old fell by 33% between 1992 and 199810). Cappello et al. examined all orchiopexies performed between 1984 and 2002 using the New York State Statewide Planning and Research Cooperative System database and demonstrated that although the annual number of orchiopexies exhibited some fluctuation, there was no significant trend during the study period11). A more recent report showed year-to-year variation, but no increase in the prevalence of cryptorchidism over a 26-year period, using data obtained from the Nova Scotia ATLEE Perinatal Database of all live births in Nova Scotia, Canada since 198812). Thus, some previous studies have reported an increasing prevalence over time, whereas others have not, or have reported no significant trends of prevalence. Such variation calls attention to difficulties of design and execution in epidemiological surveys of cryptorchidism. Indeed, the use of orchiopexy rates to measure the prevalence of cryptorchidism has fundamental limitations because the number of orchiopexies does not always directly correlate with the incidence of cryptorchidism.
Murase et al. cited an incidence of cryptorchidism at 1 year of age as 1.0%-1.7% according to Japanese guideline for diagnosis and treatment of cryptorchidism published in 2005. They also estimated that 5,000-8,500 surgeries will be required annually, given that approximately 500,000 males are born in Japan every year4). However, the data do not necessarily align with the actual incidence of cryptorchidism in Japan. While citing Japanese guidelines13), the incidence range of 1.0%-1.7% is from references14-18) pertaining to Western people rather than Japanese. There are no similar reports on the incidence of cryptorchidism based on a national survey of Japanese people.
Rodent studies indicate that mammalian testes are sensitive to radiation19). Testicular function includes spermatogenesis and steroidogenesis, and the impairment of testicular function can result in male infertility and abnormality of both internal and external development, such as cryptorchidism and hypospadias. Therefore, when the effects of radiation on testicular function are discussed, spermatogenesis and steroidogenesis should be considered individually.
Although high-dose radiation exposure in animals, and radiation therapy in humans, can affect testicular function, both spermatogenesis and steroidogenesis are dose-dependent to different degrees. Although high-dose radiation exposure can impair steroidogenesis in adults, there is no evidence that it can affect testicular descent during the fetal period.
1) Radiation and spermatogenesis(1) Effects of radiation on spermatogenesis
Radiation deletes germ cells and can result in permanent azoospermia, suggesting that it can lead to male infertility20). There is also some evidence of association between radiation exposure and the impairment of spermatogenesis in rodents and humans.
Animal studies
Delic et al. reported that spermatogenic damage was observed after doses of 3-5 Gy but not after doses of 0-2 Gy to adult rat testes21). Furthermore, Shetty et al. reported that irradiation with 6 Gy caused a complete block of spermatogonia differentiation in LBNF1 rats22). Among non-human primates, macaques have the most histologically similar testes to humans, and showed a disturbance of spermatogenesis after 2 or 4 Gy that lasted 6 months until recovery, with incomplete recovery even after 18 months23-25). Foppiani et al. evaluated the effect of bilateral testicular irradiation (2 Gy) on testicular volume and sperm parameters in adult cynomolgus monkeys26) and reported a decrease in testicular volume and sperm count after irradiation.
Human studies
Testicular injury is common following radiotherapy in humans, and patients often suffer from azoospermia or male infertility. Moreover, testicular x-irradiation results in the suppression of sperm counts in humans27). Compared to the mouse, spermatogenesis in man is approximately 3.1 times more sensitive to ionizing irradiation27). A single exposure of the testis to ionizing radiation at dose levels of 6 Gy or below can cause significant disturbance of spermatogenesis28). A dose of 0.15 Gy leads to a significant decrease in semen volume, and 0.3-0.5 Gy causes temporary oligospermia29). Speiser et al. assessed ten patients who received daily testicular doses of 0.12 Gy for a total dose of 1.4-3.0 Gy;all such patients had azoospermia, but only two of them for longer than 16 months30). De Felice et al. reported that doses of irradiation > 0.35 Gy caused azoospermia, which was reversible in some cases31). The time taken for recovery increases with larger doses;complete recovery takes place within 9-18 months following radiation with < 1 Gy, but doses in excess of 2-6 Gy may result in permanent azoospermia31). According to the International Atomic Energy Agency (IAEA), a dose of 1.0 Gy leads to a temporary reduction in the number of spermatozoa, that of 1.5 Gy leads to temporary sterility, and that of 5.0 to 6.0 Gy (acute) can produce permanent sterility in males32).
Male germ cells mainly consist of spermatogonia, spermatocytes, and spermatids. Doses that cause death of spermatocytes are higher than those for spermatogonia (2-3 Gy);while spermatids are not damaged by such doses, after 4-6 Gy a noticeable decrease in the sperm count can be observed33). De Felice et al. reported that the susceptibility of spermatogonia, spermatocytes, and spermatids to a single dose irradiation was < 1 Gy, 1-3 Gy, and > 3 Gy, respectively31).
(2) Effects of radiation on germline DNA damage and minisatellite mutations
Spermatogonia are also less susceptible to DNA damage after exposure to ionizing radiation, and the minimum dose that causes detectable DNA damage in male germ cells is 30 Gy34).
Tawn et al. investigated minisatellite germline mutation rates in childhood and young adult cancer survivors who received radiotherapy and reported no significant difference between subsequent paternal mutation rates of 5.6% in exposed fathers with a mean preconception testicular dose of 1.23 Gy versus 5.8% in unexposed fathers. Furthermore, the maternal mutation rates of 1.6% in cancer-surviving mothers with a mean preconception ovarian dose of 0.58 Gy versus 2.1% in unexposed mothers were also not significantly different. These results indicate that preconception radiotherapy for childhood or early adulthood cancer does not increase the germline minisatellite mutation rate35). The same groups also investigated germline minisatellite mutation rates in male workers who were occupationally exposed to radiation at the Sellafield nuclear facility and showed no significant difference between the paternal mutation rate of 5.0% for control fathers with a mean preconception testicular dose of 9 mSv and that of 5.8% for exposed fathers with a mean preconception testicular dose of 194 mSv. These results indicate that such exposures did not destabilize the germline passed on to future generations36).
2) Radiation and steroidogenesisIn considering the association between radiation exposure and incidence of cryptorchidism, the effects of radiation exposure on steroidogenesis, especially testosterone production, is most important, because testosterone induces testicular descent in utero. Leydig cells of the testis, which secrete testosterone, are remarkably more radio-resistant than the germinal epithelium and are only injured by high therapeutic radiation doses37). Furthermore, Leydig cell function is usually preserved up to 20 Gy in prepubertal boys and 30 Gy in sexually mature men31).
Animal studies
(1) Effect of local radiation exposure to the testes on steroidogenesis
Delic et al. reported that local radiation exposure to the testes induced Leydig cell dysfunction, as indicated by increased serum luteinizing hormone (LH, to a maximum of 385% of control after 5 Gy) and decreased serum testosterone (to a minimum of 30% of the control after 10 Gy) at 8 weeks post-irradiation21). The dysfunction, with a threshold of approximately 4 to 5 Gy, was associated with a loss of Leydig cells from the testis21). This group also examined dose- and time-response relationships measured after local X irradiation of 1 to 20 Gy to pubertal rat testes and demonstrated that the threshold dose for Leydig cell dysfunction was approximately 5 Gy38). Dysfunction after higher doses was observed by 2 weeks post-irradiation as a dose-dependent decrease in serum testosterone concentrations, and the levels were undetectable after 15 or 20 Gy38). Foppiani et al. demonstrated that bilateral testicular irradiation (2 Gy) increased follicle-stimulating hormone (FSH) levels and, to a lesser degree, testosterone levels, within several weeks of irradiation in adult cynomolgus monkeys25). Laporte et al. reported that the plasma FSH, LH, prolactin, and testosterone levels of adult rats with testicles exposed to radiocobalt (0.8 Gy) did not change significantly compared to controls39).
(2) Effect of whole-body radiation exposure on steroidogenesis
Pinon-Lataillade et al. examined the effect of continuous whole-body low-dose gamma irradiation at a dose-rate of 0.07 Gy/day for 92 days on plasma LH, FSH, and testosterone concentrations and testicular histology in rats40). They demonstrated no significant changes in LH and testosterone concentration, although a significant increase in plasma FSH concentration occurred after the numbers of spermatogonia and preleptotene spermatocytes had been reduced40).
Human studies
Some previous studies have shown effects of direct radiation exposure to the testis on steroidogenesis in humans. Acute testicular irradiation to healthy normal men at doses between 0.08 and 6 Gy resulted in no statistical change in plasma testosterone level, although increased plasma LH levels and disturbance of spermatogenesis were observed after high dose radiation exposure (0.75 and 6 Gy)41). Furthermore, high dose radiation to the remaining testis after unilateral orchiectomy (27.5-30 Gy) during childhood (1-4 years) greatly increased FSH and LH levels, with a median basal testosterone level that was significantly lower than that observed in adulthood42).
Silvakumar et al. demonstrated that fractioned gamma radiation exposure (2, 4, 6, 8, and 10 Gy) caused adverse effects on cultured human Leydig cell steroidogenesis in vitro in a dose-dependent manner43). While lower doses (2 and 4 Gy) were ineffective, higher doses (6 Gy and above) drastically decreased LH receptor levels, basal and LH-stimulated cAMP generation, and basal, LH-, and cAMP-stimulated steroidogenesis;as a result, they concluded that higher doses of radiation impair Leydig cell steroidogenesis by affecting LH signal transduction at the level of both pre- and post-cAMP generation43).
Radiotherapy of rectal carcinoma causes collateral damage to testes44,45). The mean cumulative radiation exposure to testicles during a course of pelvic radiotherapy (50 Gy) is 3.56 Gy44). Mean LH and FSH levels significantly increased after therapy (350% and 185% of pre-treatment values, respectively), while testosterone levels decreased to 78%44). After radiotherapy, the absolute risk increase was 0.17-0.30 for posttreatment testosterone levels below 8 nmol/L45). Shapiro et al. examined serum LH, FSH, and testosterone levels in 27 males with soft-tissue sarcoma who were treated with high-dose radiation (from 0.01 to 24 Gy) to the tumor bed and showed a dose-dependent increase in serum FSH and LH values following irradiation, although no significant changes in total testosterone values were observed46). They concluded that subtle Leydig cell dysfunction and germ cell depletion may occur at exposures greater than 2 Gy. Littley et al. studied the endocrine sequelae of total body irradiation for hematological malignancy in 21 patients (11 male) who were treated with 10 Gy in five fractions or 12 to 13.2 Gy in six fractions over 3 days47). In their study, serum testosterone levels (12.4-35 nmol/L) were normal, although gonadotrophin-releasing hormone-stimulated gonadotrophin levels were elevated in all patients.
3) Effects of exposure of Cesium 137 (137Cs) on steroidogenesis:Lessons learned from the Chernobyl nuclear plant accidentThe nuclear plant accidents at Chernobyl and Fukushima caused environmental dispersion of radionuclides including noble gases, short-lived radionuclides (radioactive iodine and tellurides), and radioactive strontium and cesium. The release of 137Cs, which has a half-life of 30.1 years, may result in its widespread distribution in plants and animals. The associated radiation exposure is dominated by external gamma irradiation as 137Cs decays, and by soil-to-plant-to-human transfer of 137Cs in the food chain48). The presence of environmental 137Cs after the nuclear power plant accidents at Chernobyl and Fukushima raises many health issues for the affected populations, especially if they are chronically exposed through the food chain49).
137Cs contamination levels exceeding 1,480 kBq/m2 were observed over 3,100 km2 and 272 km2 in Chernobyl and Fukushima, respectively50). The area where 137Cs contamination exceeded 6,500 Bq/L − corresponding to the maximum concentrations found in Belarusian milk immediately after the Chernobyl accident51) − was 13,000 km2 around the Chernobyl nuclear plant, versus 600 km2 around the Fukushima nuclear power plant52-54). When the environmental impacts of the nuclear accidents of Chernobyl and Fukushima are compared, the consequences of the Chernobyl accident clearly exceed those of the Fukushima accident in the amount of radionuclides emitted and the relative size of highly contaminated and/or evacuated areas55). Food safety controls and evacuations were quickly and efficiently implemented after the Fukushima Daiichi Nuclear Power Plant accident55), so the projected health effects in Fukushima are significantly lower than those of Chernobyl55). Thus, data from areas around the Chernobyl nuclear plant cannot be arbitrarily extrapolated to areas around the Fukushima Daiichi Nuclear Power plant.
Animal studies
Several animal studies have simulated the effects of radiation exposure from the Chernobyl nuclear plant accident on testicular function. Grignard et al. examined the effects of chronic contamination with low doses of 137Cs on testicular steroidogenesis in adult rats56). In their study, rats were exposed to 137Cs doses of 6,500 Bq/L in drinking water, to mimic radiological exposure in the territories around Chernobyl. They showed that testosterone level was not affected following 137Cs ingestion, whereas the concentration of 17β-estradiol decreased threefold in adult rats exposed to 137Cs56). The same group examined the effects of chronic contamination with low doses of 137Cs (6,500 Bq/L) in utero or from birth on testicular steroidogenesis in rats57). They showed that chronic exposure of growing rats to 137Cs at doses found in the Chernobyl area exerted few effects on testicular steroidogenesis and histology despite the presence of 137Cs in the testis, and that growing organisms appeared less sensitive to 137Cs exposure than adults. They concluded that testicular steroidogenesis was not altered by chronic exposure to 137Cs Chernobyl fallout, whether in utero or post-natal57). In their study, the testis weight was identical between control and contaminated groups, and although the testicular location was not described in their paper57), this was presumably normal due to its lack of mention. Manens et al. also examined the effect of exposure by chronic ingestion of 137Cs (6,500 Bq/L) and tested for 9 months in exposed adult, neonatal, and fetal rats. In their study, the serum testosterone level was identical between control and contaminated adult, neonatal, and fetal rats49), although the blood level of 17β-estradiol only decreased in the adults49). These animal studies show that the likelihood of effects on adult testosterone production from chronic exposure to 137Cs after the Chernobyl nuclear plant accident is extremely low. Furthermore, these findings also suggest that the effect on neonatal and fetal testosterone production is low, given that the radio-sensitivity in neonates and fetuses is lower than that in adults. Therefore, chronic contamination with 137Cs (6,500 Bq/L) is not considered to affect testicular descent.
Human studies
Reproductive disorders, such as altered sperm parameters, were observed among liquidators (nuclear plant cleanup workers) in 137Cs-contaminated regions after the Chernobyl nuclear power plant accident;these workers were estimated to have received high doses of radiation exposure58-61). Fischbein et al. reported significant changes in the ultramorphology of sperm heads in Chernobyl liquidators versus controls of similar age, but no significant differences were observed between liquidators and controls with respect to sperm density, viability, morphology observed by light microscopy, semen volume, or biochemical markers59). On the other hand, Goncharov et al. examined hormone and semen parameters in liquidators exposed to 0.001-0.33 Gy60). They showed that such exposure did not cause major long-lasting disruption of endocrine status or spermatogenesis 7 ± 9 years later. Furthermore, there were no significant differences in plasma FSH levels between the liquidators and controls, and the testosterone levels in the liquidators were even significantly higher than in controls60).
On 11 March 2011, a magnitude 9.0 earthquake off the Pacific coast of Northeastern Japan provoked a tsunami that breached more than 500 km of shoreline, with run-up heights to 40 meters. The Fukushima Daiichi Nuclear Power Plant suffered major damage from the earthquake and tsunami. All operating reactors went into automatic shutdown, but a triple backup failure (grid, generator, and battery power) interrupted emergency core cooling. Meltdowns led to hydrogen gas explosions that released large volumes of radioisotopes into the environment. Thereafter, radiation exposure emerged as the most serious concern to residents living in the affected area.
According to Murase et al., “Nationwide increase in cryptorchidism after the Fukushima nuclear accident,” a 13.4% increase in cryptorchidism, based on hospital discharge rates, followed the Fukushima Daiichi Nuclear Power Plant accident4). Their title implies that a nuclear accident directly induced an increased incidence of cryptorchidism throughout Japan. Casual readers might readily believe that there was a direct relationship between radiation exposure and cryptorchidism, but as the authors admit, “A large amount of radionuclides emitted from the Fukushima was suspected to be a major cause but currently no evidence is available.4)” Thus, their provocative title does not reflect scientific reality. In 2011, other numerous disasters and events, such as the explosive eruptions of Mt. Shinmoedake and the avian influenza epidemic, happened in Japan. In spite of no evidence, why did the authors only focus on the Fukushima Daiichi Nuclear Power Plant accident? We can grant that these other disasters and events are also exceedingly unlikely to be associated with the incidence of cryptorchidism. In a subsequent paper, Murase et al. also reported a nationwide increase in complex congenital heart diseases after the Fukushima Daiichi Nuclear Power Plant accident62). The major problem in their prior paper is that a direct causal relationship between the increased incidence of cryptorchidism and the Fukushima Daiichi Nuclear Power Plant accident was not actually proven, as the authors themselves admit. We strongly doubt that cryptorchidism actually increased after the accident in Japan;as described below, we challenge the validity of their cryptorchidism analysis through six major points.
Prior to our six points, we call attention to Kobashi et al., “Unambiguous evidence is required to accurately understand the health impact of nuclear accident”63) that challenges the report by Murase et al. on a putative increase in complex congenital heart diseases after the accident62).
Androgens, especially testosterone from Leydig cells, are involved in the completion of testicular descent in utero;therefore, impaired fetal androgen action can result in cryptorchidism1). A number of reports suggest that this anomaly can be associated with genetic, environmental, and maternal/fetal factors. Many researchers have examined the association between cryptorchidism and genetic alterations of Homeobox A10 (HOXA10), insulin-like factor 3 (INSL3), INSL3 receptor (LGR8/GREAT), androgen receptor (AR), estrogen receptor α (ESR1), and Ad4BP/SF-1 genes, and have suggested that some of these genes may be implicated in cases of cryptorchidism1). In addition, several reports suggested an association between estrogen-mimicking EEDs and the incidence of cryptorchidism, because of the negative impact increased estrogen has on testicular descent in utero1). Maternal exposure to EEDs may be associated with abnormal migration of the testes in the male fetus64), although clear evidence in humans remains ambiguous3,65).
Other maternal and fetal factors may be associated with cryptorchidism, including family history of cryptorchidism, parameters such as birth weight, gestational age, and size for gestational age, and maternal cola consumption or smoking during pregnancy3,66,67). In spite of many such maternal and fetal risk factors, Murase et al. focused only on underweight and preterm births, which were almost constant during the study period4);however, they did not exclude the effects of other factors in their paper.
Quoting Murase et al., “...emotional stress and radioactive material would be considered as 2 main possible factors contributing to the increase. However, in human no association has been observed between cryptorchidism and severe emotional stress... although prenatal maternal stress has proved to be a risk factor for cryptorchidism in rats.”4) They continue, “In contrast, radioactive material released from the Fukushima nuclear accident may be concerned considering its amount and known toxicity4).” How such “toxicity” could induce cryptorchidism is neither explained by Murase et al. nor supported by any of their citations. To the best of our knowledge, there is no report that shows a direct association between radiation exposure and testicular descent during fetal periods.
Assuming that a large quantity of radionuclides emitted from the Fukushima Daiichi Nuclear Power Plant was suspected to be a major cause of the increased incidence of cryptorchidism, as asserted by Murase et al., a possible mechanism may be that radiation exposure disturbed steroidogenesis, especially testosterone production, during periods critical for testicular descent. As described above and below, the effects of radiation exposure to the testes are dose-dependent, and the possibility of the effects of low-dose radiation exposure on testosterone production during fetal periods is extremely low.
(1) Possibility of external radiation exposure in Fukushima Prefecture affecting the incidence of cryptorchidism
According to the IAEA report on the Fukushima Daiichi Nuclear Power Plant accident, the release of radioactive materials from the Fukushima accident were approximately one-tenth of those from Chernobyl68). In terms of cesium, the effective dose was also approximately one-tenth (Fig. 1)69). The Fukushima Health Management Survey reported results for 460,408 residents in Fukushima Prefecture during the first 4 months after the accident:66.3% received doses < 1 mSv, 94.9% received < 2 mSv, 99.7% received < 5 mSv, and the maximum dose was 25 mSv70). Fujimura et al. also reported that children between the ages of 0 and 15 years (n = 4,571) had a mean radiation dose of 1.5 mSv/year 6 months after the disaster, 1.5 mSv/year in 2012, 1.0 mSv/year in 2013, and 0.65 mSv/year in 2014, in Nihonmatsu City in Fukushima Prefecture71). Bedwell et al. assessed the doses received by members of the public in Japan following the nuclear accident at the Fukushima Daiichi Nuclear Power Plant and demonstrated that across most of Japan the estimates of the dose were very low, and were estimated to be less than the annual average dose from natural background radiation in Japan72). Even in regions closest to the Fukushima Daiichi Nuclear Power Plant, the maximum lifetime effective dose is estimated to be well below the cumulative natural background dose over the same period72).
The exposure doses resulting from the Fukushima Daiichi Nuclear Power Plant accident, as mentioned above, are shown in a unit of mSv. Since the gamma radiation exposure due to cesium was almost uniform throughout the whole body, it can be interpreted as corresponding to organ-specific exposures in units mGy. While the doses due to the Fukushima Daiichi Nuclear Power Plant accident were mostly on the order of a few mSv (mGy) or less, the doses likely to cause effects on testicular function of humans/animals are at least on the order of a few hundred or thousand mGy, as reviewed above. Therefore, the dose of radiation exposure resulting from the Fukushima Daiichi Nuclear Power Plant accident is considerably lower than that in animal studies and radiation therapy in humans that can affect steroidogenesis. According to the Guidelines for Diagnostic Imaging During Pregnancy by the American College of Obstetricians and Gynecologists, fetal risks of anomalies, growth restriction, or abortion have not been reported with radiation exposure less than 50 mGy73,74). Furthermore, the International Commission on Radiological Protection determined that 100 mGy is the threshold above which malformations may occur in newborns75), indicating that exposure of fetuses in utero to < 100 mGy should not cause teratogenicity63). According to these data, the possibility of external radiation exposure in Fukushima Prefecture inducing cryptorchidism is infinitesimally close to zero.
Murase et al. wrote that “the increase between FY (fiscal year) 2011 and FY2012 is obvious, and a gradual increase is observed from FY2012 to FY2014”4). As shown in Figure 2, the air dose of radiation in Fukushima Prefecture rapidly decreased with time76). For reference, typical air doses of radiation in major cities such as Shanghai, Seoul, Singapore, Munich, Paris, and New York are 0.59, 0.09, 0.17, 0.12, 0.10, 0.06 µSv/h, respectively77). The authors did not account for why the incidence was maintained after 2012, despite the rapid decrease in radiation levels in Fukushima Prefecture. Considering other possible mechanisms that may induce cryptorchidism after 2012, could the continued increase be due to DNA damage in germ cells by the exposure, causing abnormalities such as cryptorchidism in the next generation? In general, DNA damage in the male germline is associated with poor fertilization rates following in vivo fertilization, defective preimplantation embryonic development, and high rates of miscarriage and morbidity in the offspring, including childhood cancer78). Physical factors, such as radiation exposure, can induce DNA damage in the mammalian germline;however, to the best of our knowledge, there is no report to indicate that DNA damage in the germline induces cryptorchidism. Boice et al. showed that radiotherapy for cancer (gonadal dose:over 100 mSv) did not carry much, if any, risk for inherited genetic disease in offspring conceived after exposure79). Indeed, the rate of congenital anomalies did not increase in the children of atomic bomb survivors in Hiroshima and Nagasaki80). These data imply that radiation doses with potential transmissibility of germline damage to offspring would have to be quite high. As described above, spermatogonia are also less susceptible to the occurrence of DNA damage after exposure to ionizing radiation, and the minimum dose that causes detectable DNA damage in male germ cells is 30 Gy34). In addition, the germline minisatellite mutation rate was not affected in exposed fathers with a mean preconception testicular dose of 1.23 Gy, nor in exposed mothers with a mean preconception ovarian dose of 0.58 Gy35). As shown in Figure 2, the air dose of radiation in Fukushima City at 10:00 pm on the 15th of March, April, May, June, July, and August in 2011 was 20.7, 1.83, 1.43, 1.40, 1.24, and 1.15 µSv/h, respectively. Supposing that these mid-month doses were received 24 hours a day, each for 30 days, the cumulative radiation exposure for 6 months could be estimated as shown:
• (20.7+1.83+1.43+1.40+1.24+1.15) (µSv/h) × 24 (hours) × 30 (days) = 19.98 mSv/6 months
Judging from previous data34-36), even this degree of chronic exposure − in fact, an overestimate because rapid exponential decay was the dominant term in March76) − is considered not to induce germline DNA damage. Therefore, the hypothesis that germline DNA damage could be caused by external radiation exposure in Fukushima Prefecture and induce cryptorchidism after 2012 can be dismissed. It defies reason why “a gradual increase is observed from FY2012 to FY2014,” even if “a large amount of radionuclides emitted from the Fukushima was suspected to be a major cause” as Murase et al. assert4).
For reference, the average background dose received by the general population is around 2.4 mSv/year, which can vary depending on geology and altitude;this ranges from 1 to 10 mSv/year, but can be more than 50 mSv/year. Exposure of airline crews flying a New York-Tokyo polar route amounts to 9 mSv/year, and the effective dose from abdominal & pelvic CT scans is 10 mSv81). Table 1 summarizes the external radiation exposure levels in each situation.
(2) Possibility of internal radiation exposure in Fukushima Prefecture affecting the incidence of cryptorchidism
As described above, the maximum 137Cs concentrations measured in milk in Belarus immediately after the Chernobyl accident was 6,500 Bq/L51). On the other hand, provisional regulation values for radioiodine were exceeded for ≤ 13 days (16-28 March 2011) in Fukushima Prefecture. While the maximum radioiodine level detected in tap water was 965 Bq/kg (equal to Bq/L) on 20 March 2011, no tap water samples exceeded the provisional regulation value (200 Bq/L) for radioactive cesium (134Cs and 137Cs) after 31 March 201282).
Consumer Co-operative in Fukushima Prefecture measured radioactive cesium concentrations in the daily meals of 100 families using a gamma ray spectrometer (low detection limit < 1 Bq/kg), and showed that only 10% and 2% of families, respectively, had received detectable radio-cesium concentrations from November 2011 to March 2012 (maximum 6.7 Bq/kg of 137Cs) and from June 2012 to September 2012 (maximum 1.9 Bq/kg of 137Cs, respectively)83). Continuous consumption of such food for 1 year would result in an annual committed effective dose of 0.037 mSv70). Fukushima prefecture measured the internal exposure dose of 184,208 residents using a whole body counter between June 2011 and February 2014, and showed that 99.986% received < 1 mSv, with the maximum dose being 3 mSv70,84). Screening at Minamisoma City, which is located 23 km north of the Fukushima Daiichi Nuclear Power Plant, showed that approximately 17% of children and 38% of adults showed detectable internal exposure, with a concentration of 2.8 to 57.9 Bq/kg (median, 11.9 Bq/kg) and 2.3 to 196.5 Bq/kg (median, 11.4 Bq/kg), respectively85). The committed effective dose by cesium was < 1 mSv, with the exception of one person at 1.07 mSv85).
In the animal study outlined above, the effect of chronic contamination (6,500 Bq/L) with 137Cs following the Chernobyl nuclear plant accident on adult testosterone production is low, and that on neonatal and fetal testosterone production is estimated to be extremely low, because radio-sensitivity in the neonate and fetus is lower than that in an adult49,56,57). According to the data shown above, a dose of 137Cs found in Fukushima Prefecture after the accident is considered to be too low to affect testosterone production. Therefore, the possibility that internal radiation exposure in Fukushima prefecture induced cryptorchidism is also infinitesimally close to zero.
Ionizing radiation can arise from human activities or from natural sources81). Most radiation exposure is from natural sources;this includes radioactivity in the rocks and soil of the Earth’s crust, radon (a radioactive gas given out by many volcanic rocks and uranium ore), and cosmic radiation. For example, radioactivities of around 4,500 Bq, 1,000 Bq, 15 Bq, 3,000 Bq, and 30,000 Bq can be ascribed, respectively, to one adult human (65 Bq/kg), 1 kg of coffee, 1 banana, the air in a 100 m2 Australian home (radon), and 1 household smoke detector (containing americium)81). Table 2 summarizes internal radiation exposure levels in each situation.
(3) No evidence to support that both external and internal radiation exposure induces cryptorchidism
The 2013 United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) report on the Fukushima Daiichi Nuclear Power Plant accident stated that prenatal exposures from the accident were not expected to increase the incidence of spontaneous abortions, miscarriages, perinatal mortality, congenital effects, or cognitive impairment86). Fukushima Health Management Survey data also showed that the incidence of stillbirth, preterm birth, low birth weight, and congenital anomalies were similar to recent averages elsewhere in Japan, and concluded from a pregnancy and birth survey that no significant adverse outcomes were observed over the whole of the Fukushima Prefecture after the disaster87).
In summary, there is no evidence to support that external and/or internal radiation exposure could induce cryptorchidism by a disturbance of steroidogenesis or germline DNA damage after the Fukushima Daiichi Nuclear Power Plant accident. Furthermore, the quantity of radionuclides emitted from the power plant was too low to influence testicular descent. Thus, at the very least, Murase et al. should have detailed how such low-level radionuclides might induce cryptorchidism, theoretically or empirically, if they wanted to invoke any mechanism other than the ones we have shown to be implausible.
Murase et al. reported that a nation-wide increase in cryptorchidism was observed after the accident4). They showed increases in the rate of hospital discharge after orchiopexy in 35 prefectures of Japan4). Since there are 47 prefectures in Japan, almost three quarters of the prefectures were included in their study. The 35 prefectures included those in Southern Japan, including Fukuoka, Kagoshima, and Okinawa Prefectures, that are 1,052 km, 1,161 km, and 1,773 km from the Fukushima Daiichi Nuclear Power Plant, as shown in Figure 1, which also shows radiation exposures in 35 prefectures86). For comparison, the average doses for Chernobyl evacuees in three countries are also shown69). In general, a dose-response relationship is the effect on an organism or, more specifically, the risk of a defined outcome, produced by a given amount of an agent or a level of exposure88). A dose-response relationship exists when increasing levels of exposure are associated with either an increasing or a decreasing risk of the outcome. Demonstration of a dose-response relationship is considered strong evidence for causality88). Thus, if radiation exposure affects the incidence of cryptorchidism, the incidence in various prefectures should decrease with distance from the source of exposure, even if the decrease is not linear or uniform. However, Murase et al. failed to show any dose-response relationship in their paper. In fact, the analytical formula (2) described in their supplementary appendix S1 explicitly showed that they omitted parameters indicating a decreased difference in the hospital discharge rate with increasing distance from the Fukushima Daiichi Nuclear Power Plant4).
For reference, the whole of Japan and Fukushima Prefecture cover 378,000 km2 and 13,783 km2, respectively, whereas the area affected by evacuation orders was 370 km2, which is only 0.098% of Japan and 2.7% of Fukushima Prefecture. Thus, the majority of people in Japan and Fukushima Prefecture were not significantly affected by accident-related radiation89). Bedwell et al. reported that in the north, south, and west of Japan, all estimates of the lifetime effective dose received by members of the public following the accident are very low, and in all cases are less than 1 mSv72). Furthermore, the estimated dose received in these regions is less than the typical Japanese and worldwide average annual doses from natural background of 2.1 mSv90) and 2.4 mSv91), respectively. Across all regions of Central and Eastern Japan, including Fukushima Prefecture, they are below 10 mSv. These findings indicate that the majority of people in Japan were not significantly exposed to radiation from the Fukushima Daiichi Nuclear Power Plant, although Murase et al. concluded that “a large amount of radionuclides emitted from the Fukushima was suspected to be a major cause.”4) Such a declaration undoubtedly contributes to misunderstanding.
In addition, Murase et al. also state:“A straightforward comparison between disaster-affected area and non-affected area seemed to be difficult because many residents migrated from the affected area4).” Figure 3A shows the actual number of people who migrated from Fukushima Prefecture to other prefectures after the Fukushima Daiichi Nuclear Power Plant accident. The total number throughout Japan in fiscal year 2011 (FY2011) was 62,70092). The population of Fukushima Prefecture in 2011 was 1,988,99593). Thus, only ~2% of people living in Fukushima Prefecture migrated to other prefectures in 2011. Figure 3B shows specific migration patterns from Fukushima Prefecture following the earthquake, tsunami, and nuclear crisis. Most people migrated from Fukushima Prefecture to Japan’s capital city, Tokyo (7,627 in 2011), or neighboring prefectures such as Yamagata (12,944 in 2011) and Niigata (6,762 in 2011) 92). The number of people who migrated to further south or west was extremely low. For example, the number of people who migrated to Kyoto, Osaka, Hiroshima, and Okinawa in 2011 was 767, 838, 310, and 701, respectively92). The population in Kyoto, Osaka, Hiroshima, and Okinawa in 2011 was 2,632,496, 8,865,448, 2,855,734, and 1,401,93393), respectively, indicating that only 0.029, 0.0094, 0.0035, 0.005%, respectively, of the population in each prefecture migrated from Fukushima Prefecture in 2011. How could such a small number of people migrating from Fukushima Prefecture affect the incidence of cryptorchidism elsewhere? Such publically available data could have informed the analysis of Murase et al., but by their own admission, even a straightforward comparison was too difficult for them to undertake.
As described above, radiation exposure is not only external exposure but also internal via the food chain48). Murase et al. described it as follows: “After the nuclear accident, radionuclides may have been distributed through the ecosystem through food contamination.”4) That may have been true after Chernobyl, but Japan has the world’s strictest standards for managing radioactive contamination of food, and anything exceeding those standards cannot be distributed. According to the Japan Food Sanitation Act, derived intervention levels for radioactive cesium, which are the upper limits allowed for food to be distributed in the supply chain, are 10, 50, 50, and 100 Bq/kg (~Bq/L) in drinking water, milk, infant foods, and general foods, respectively89). EU Council Regulations (Euratom) are more lenient:1,000, 1,000, 400, and 1,250 Bq/kg in drinking water, milk, infant food, and other food, respectively. American Guidance Levels for Radionuclides in Domestic and Imported Foods allow 1,200 Bq/kg in general89). Results of Japan’s thorough monitoring of agriculture, forestry, and fishery products prior to shipment are announced, and very few foods have exceeded the standard limit (100 Bq/kg). Necessary measures are in place to ensure that foods are not distributed in the market if they are found to have exceeded the standard limit. Because of intense and immediate monitoring, and the rapid decrease of initially high contamination levels94), foods in Fukushima Prefecture were very safe and remain so, making the possibility of internal exposure from food extremely low in Fukushima and throughout Japan.
Murase et al. cited two papers94,95) in their description as follows:“After the nuclear accident, radionuclides may have been distributed through the ecosystem through food contamination4).” However, these two papers dispute what Murase et al. sought to imply. Mert et al. wrote as follows:“It seems very unlikely that more than very few members of the public in Japan exceeded the maximum permissible internal exposure of 1 mSv/year. This observation is in agreement with the results of previous studies94).” Furthermore, Shozugawa et al. wrote as follows:“we can conclude that 137Cs detected in remote areas 300 km or more from Fukushima nuclear power plant contained activity from Pre-Fukushima events such as Chernobyl accident (1986) and atmospheric nuclear explosions (from 1945).” 95) Therefore, Murase et al. seem to have misrepresented the two papers to suit their own ideas. In addition, Murase et al. referred to another two papers in the context of “...incineration of debris as part of the treatment of disaster waste.”96,97) In fact, per Iwami and Sasai97), if a dose of 1.4 Bq/m3(N) of 137Cs was emitted into the air by the incineration of debris, and people inhaled the air every day, 24 hours a day, 365 days a year, the dose of radiation exposure from the air by the incineration of debris in a year is calculated as below. The intake air volumes per day are 20 m3 and 5 m3 for an adult and a 1-year-old child, respectively, and the inhalation dose coefficients of the radionuclides (effective dose per unit intake) are 4.6×10-9 and 5.4×10-9 Sv/Bq, respectively98).
• Adult:1.4 Bq/m3×20 m3×4.6×10-9 (Sv/Bq)×365=0.047 mSv/year
• 1-year-old child:1.4 Bq/m3×5 m3×5.4×10-9 (Sv/Bq)×365=0.014 mSv/year
These data indicate that the dose of radiation exposure emitted into the air by the incineration of debris is extremely low and is unlikely to affect the incidence of cryptorchidism.
In summary, for the reasons described above, the hypothesis that radiation provoked an increase of cryptorchidism throughout Japan has no support.
Murase et al. counted the number of orchiopexies using the Diagnosis Procedure Combination (DPC) database, which is a national administrative claims and discharge database covering acute-care inpatients in Japan. All 82 academic hospitals in Japan are obliged to adopt this system, while community hospitals participate on a voluntary basis. The DPC database contains discharge abstract and administrative reimbursement claims data for inpatient episodes collected from participating hospitals. This database system started in Japan in 2003, and its use has recently spread to acute care hospitals and is in transition. The ratio of hospitals involved in the DPC survey per total hospitals is very low (Figure 4). This system is unsuitable to assess year-on-year comparisons in the number of surgeries performed all over Japan, and thus represents a serious flaw in the design setting of the research by Murase et al..
In the event that we assess the number of surgeries using the DPC system, as Murase et al. did, the majority of hospitals in Japan are excluded. Murase et al. obtained cryptorchidism discharge data collected over a 6 year period from the DPC survey database in Japan in order to estimate the discharge rate after cryptorchidism surgery before and after the accident4). Only 94 hospitals in Japan that participated in the DPC system and reported 10 or more discharges after cryptorchidism surgery within 6 years covering before and after the accident were involved in their survey. However, there are many more hospitals (7,528 as of 2011)4) in Japan, some of which are considered to have performed orchiopexy, even though their number of orchiopexies per year is under 10. In 2008, the Ministry of Health, Labour, and Welfare started to construct a database of all electronic health insurance claim data, the so-called National Database of Health Insurance Claims, and Specific Health Checkups of Japan (NDB), which currently covers approximately 98% of the healthcare services provided by health insurance99-101). The NDB has grown to become one of the largest medical databases in the world100,102,103). The NDB can be a powerful tool to survey the status of Japanese medical care in the future100). According to NDB open data104), the total number of orchiopexies (open orchiopexy [segment number:K836] + laparoscopic orchiopexy [segment number:K836-2]) was 9,810 (9,658 + 152) and 9,497 (9,368 + 129) in 2014 and 2015, respectively, in the whole of Japan. Murase et al. showed, in Supplementary Table S3 of their paper, that the number of orchiopexies was 6,404 and 6,042 in 2014 and 2015, respectively, in the DPC hospitals. Therefore, because the NDB has recently covered approximately 98% of healthcare services provided by health insurance, the highest estimate of the number of orchiopexies in DPC hospitals per those of all hospitals in Japan in 2014 and 2015 was only 65.3% (6,404/9,810) and 63.6% (6,042/9,497), respectively. The authors claim as follows:“The discharge number in the DPC data reflects the actual number of surgeries in Japan well4)”, but their description is incorrect, because over 35% of orchiopexies were performed in non-DPC hospitals in Japan, a point that was ignored in their study. Although the NDB has been used in several recent clinical epidemiological studies105-107), it is also unsuitable to assess the prevalence of orchiopexy between 2011 and 2012, much like the DPC database, because the number of claims submitted electronically from medical institutions had increased year-by-year (1.217, 1.511, 1.619, 1.681, 1,728, 1.808, and 1.892 billion in 2009, 2010, 2011, 2012, 2013, 2014, and 2015, respectively), and this system was still in transition between 2011 and 2012108). In addition, the Ministry of Health, Labour and Welfare has opened aggregate data to the public since 2014, after the disaster, with the spread of the on-line systematization of claims, indicating that we have only recently been able to utilize the NDB data officially. Murase et al. estimated in their paper that the total number of cryptorchidism surgeries in the DPC hospitals was 5,000 to 6,0004). As described previously, the authors estimated the number of surgeries based on past reports about Western people before 2003. According to the NDB data, the number of orchiopexies per year was estimated to be over 9,000, well above the estimates of Murase et al..
Another problem is that the registered DPC hospitals in their study during the observation period are not always same every year, and just because hospitals are categorized as performing 10 or more orchiopexies does not always mean that they perform 10 or more every year.
The basic policy for the revision of medical treatment fees for FY2012 announced by the Ministry of Health, Labor, and Welfare in Japan stated that steadily implementing the promotion of clinical specialization and collaboration and home medical care was necessary109). To this end, the Japanese government aspires to establish a system to ensure the sharing of functions between medical institutions, the coordination of operations, and the centralization of surgery in high-volume hospitals110). After the introduction of the DPC system, surgeries tended to be aggregated into high-volume hospitals adopting this system. Figure 5 shows the rate of patients referred to DPC hospitals from other hospitals to receive cryptorchidism surgery per total number of patients receiving cryptorchidism surgery in DPC hospitals from 2010 to 2017. The rate of patients referred to DPC hospitals from other hospitals rapidly increased, suggesting that the concentration of orchiopexies in DPC hospitals has increased (57.6% in 2011 to 62.0% in 2012). One of the reasons why orchiopexies seemed to increase during this period may be simply that orchiopexies were aggregated into DPC hospitals, most of which are high-volume referral centers. Murase et al. described it as follows: “Even though health-care consolidation may have occurred in the hospitals included in the DPC because of the reform of local public hospitals, the sudden increase between FY2011 and FY2012 cannot be explained by such issues related to diagnosis and treatment or health-care consolidation.”4) This is incorrect because health-care consolidation and the reform of local public hospitals accelerate the centralization of surgery to high-volume hospitals and result in an increased number of surgeries in DPC hospitals. Such policies of the Ministry of Health, Labor, and Welfare have taken root all over Japan during this period.
Murase et al. declare as follows:“The number of discharges was multiplied by 4/3 because only 9 months of data were available for that year in FY2010, 1 year before the accident.”4) This extrapola-tion may lead to incorrect results owing to the possibility of seasonal variability in the number of orchiopexies. For example, parents likely want to schedule elective surgery at a convenient time, such as summer or spring vacation. Furthermore, the monthly number of surgeries may vary according to a doctor’s schedule, which can be affected by vacations and medical meetings. Moreover, some reports have proposed that cryptorchidism occurs in cycles according to the calendar period of birth, with seasonal peaks that occur between September to November, and again sometime between January and May111-113), although there is limited evidence for this association. Therefore, multiplying the number of discharges by 4/3 is likely to lead to an unreliable calculation that may underestimate the number of orchiopexies during the period;thus, this approximation has no place in a proper epidemiological study.
From DPC data, we created Figure 6 to show the change in the number of discharges after orchiopexy in each of 94 hospitals between FY2011 and FY2012. The number increased in some DPC hospitals (n = 54), while it decreased in other DPC hospitals (n = 34). Changes in the number of orchiopexies where such surgeries increased between FY2011 and FY2012 amounted to +623, while changes in the number of orchiopexies where such surgeries decreased amounted to −219, indicating that in total, +404 (+623−219) more orchiopexies occurred between FY2011 and FY2012. Murase et al. present data in their Sup-plementary Appendix S4 to show that the aggregate number of discharges after cryptorchidism surgery increased from 2,837 in FY2011 to 3,241 in FY2012 (+404) in 94 DPC hospitals. A notable point is that the number of surgeries in 4 hospitals rapidly increased by 237 from FY2011 to FY2012:+92 at Juntendo University Hospital in Tokyo, +55 at Fukuoka Children’s Hospital in Fukuoka, +45 at Dokkyo Medical University Koshigaya Hospital in Saitama, and +45 in Saga-ken Medical Centre Koseikan in Saga. These data indicate that surgeries in just 4 of 94 hospitals accounted for a large percentage (+237/+404 = 59%) of the total increase between FY 2011 and FY2012. In other words, the increased number in four major hospitals strongly influenced the overall difference between FY2011 and FY2012, thus casting doubt on a “nationwide increase in cryptorchidism after the Fukushima nuclear accident.” Furthermore, since these four hospitals are far removed from the nuclear plant (Fig. 1:227 km, 1,052 km, 213 km, and 1,080 km, respectively), where exposure to accidental radiation has been minimal, a causal relationship between the accident and any increase in the number of orchiopexies in these hospitals is quite unlikely. While the numbers attributed to these four hospitals warrant further analysis, in the meantime the data imply a concentration of orchiopexy cases in DPC hospitals during the period of study.
In summary, for the above-described reasons, in order to assess the incidence of cryptorchidism before and after the accident, the design setting, analyses, and interpretation by Murase et al. using the DPC system falls short of the necessary rigor.
Murase et al. showed age distributions of patients discharged after cryptorchidism surgery in DPC-participating hospitals between FY2010 and FY2015 in their Supplementary Table S34). From this data, we created Figure 7, which shows the change in the number of discharges after cryptorchidism surgery in each age class (0-2, 3-5, and 6-15 years old). As they described, the number of patients age 0-2 and age 3-5 increased from FY2011 to FY2012 by 27% and 34%, respectively. The patients of age 3-5 in FY2012 were born in FY2007 to FY2009, which is before the 2011 accident. As described above, since testicular descent is normally completed before birth2,3), the increase in orchiopexy for patients of age 3-5 in FY2012 would imply an increase in cryptorchidism in FY2007 to FY2009, before the Fukushima Daiichi Nuclear Power Plant accident. Therefore, the authors should have shown that patients of age 3-5 in FY2012 did not increase in their study, if they wanted readers to believe that the incidence of cryptorchidism increased after the accident. In the same manner, patients of age of 2 in FY2012 were born in 2010, which also precedes the accident. Thus, the authors should have considered only patients of age of 0-1 in their study. This is also one of several major limitations of their using the DPC system. Moreover, Murase et al. did not address this contradiction at all in their paper4).
Murase et al. reported the number of orchiopexies as well as pediatric inguinal hernia sur-geries from the DPC data in order to predict the number of tapped testes cases in their Supplementary Table S54). This table shows a change in the number in orchiopexies per 100 hospitals, as well as the number of pediatric inguinal hernia surgeries between FY2010 and FY2015. It is noteworthy that cryptor-chidism and inguinal hernia surgeries both increased in FY2012, but it is quite curious that the authors did not mention the increase in inguinal hernia surgeries in their paper. It is extremely difficult to suppose that both of these conditions increased as a result of radiation exposure.
Figure 8 shows the change in the number of surgeries in each field in the 94 DPC hospitals that are re-ported in the paper by Murase et al. The total number of surgeries in these hospitals increased be-tween 2011 and 2012, and the number of surgeries in each field increased as well. These results indicate that not only orchiopexy, but also a number of other surgeries, increased between 2011 and 2012 in these 94 DPC hospitals. The increased number of these surgeries is consistent with progress in the centralization of sur-geries to these DPC hospitals, rather than any environmental effect such as radiation exposure.
As described above, the use of orchiopexy rates to measure the prevalence rate of cryptorchidism has limitations. If the orchiopexy rate is used as a surrogate for the cryptorchidism rate, changes in social circumstances should not be ignored as they may influence the number of surgeries. Murase et al. demonstrated one social factor, the consultation rate of infant health checkups, which did not change. However, there were at least two more changes of social circumstances in FY2012 − a medical fee revision and a change in board certification practice by the Japanese Urological Association (JUA) − which may also have influenced the number of orchiopexies. The Ministry of Health, Labor, and Welfare revises medical treatment fees every 2 years. In general, the medical fee revision can influence the number of surgeries from the point of view of hospital management. FY2012 was a year of medical fee revision114);in this year, medical remuneration points (medical treatment fees) for both open (segment number:K836) and laparoscopic orchiopexy (segment number:K836-2) were raised from 8,260 points (82,600 yen) and 24,440 points (244,400 yen) in FY 2011 to 8,470 points (84,700 yen) and 31,770 points (317,700 yen) in FY2012, respectively.
In April 2012, the JUA decided to revise the board certification program from FY2013. Prior to FY2012, pediatric urological surgery, including orchiopexy, was compulsory training for subjects to gain board certifica-tion by the Japanese Board of Urology115). Residents were required to experience not only general urological surgery but also pediatric urological surgery in order to gain certification; however, it was decided in FY2012 by the JUA that pediatric surgery would be optional from FY2013. In Japan, not only pediatric urolo-gists but also general urologists usually perform orchiopexy. Before FY2012, residents in general hospitals were required to experience pediatric urological surgery in order to gain board certification. Since there is no established educational system specifically for pediatric urology in Japan, some general urologists were hesitant to educate residents in pediatric surgery. However, due to the change in board certification requirements, there is a possibility that general urologists may more actively refer children with urological diseases, including cryptorchidism, to specialists of pediatric urology in high-volume hospitals, such as children’s hospitals and university hospitals. This is partly due to the fact that after the JUA announcement in FY2012, they were not necessarily required to give their residents experience in pediatric urological surgery in order for them to gain certification.
There is no proof that these changes in social circumstances influenced the number of orchiopexies; however, we wish to emphasize that, since social circumstances can influence the number of surgeries, it is important to collect and organize such information from various fields, to analyze and assess its impact.

Effective radiation doses after the Fukushima Daiichi Nuclear Power Plant accident in 35 prefectures of Japan, compared to the dose for evacuees in three countries due to the Chernobyl accident.
*35 prefectures are those that Murase et al. showed in Figure 3 of their paper4). Distance between each prefectural office and Fukushima Daiichi Nuclear Power Plant is also shown. First-year effective dose (averaged over each prefecture) after the accident86).
#Effective dose for evacuees in 1986 (for ~8 months after the accident)69).

Changes in air doses of radiation in six cities of Fukushima Prefecture from 2011 to 201576). Fukushima City, Koriyama City, Shirakawa City, Minami Soma City, and Iwaki City are located approximately 64 km northwest, 58 km east, 81 km southeast, 98 km east, 24 km north, and 43 km southeast, respectively, of the Fukushima Daiichi Nuclear Power Plant. Reference values of radiation levels in these cities are 0.04, 0.04-0.06, 0.04-0.05, 0.04-0.05, 0.05, and 0.05-0.06 μSv/h, respectively.

External radiation exposure levels in various situations

Internal radiation exposure levels in various situations

Changes in the number of emigrants from Fukushima Prefecture after the Fukushima Daiichi Nuclear Power Plant accident. A. Total number of Japanese emigrants. B. The number of emigrants in each prefecture.

Changes in the number of all Japanese hospitals and those involved in the DPC survey (DPC hospitals).

The percentage of patients referred to DPC hospitals from other hospitals to receive cryptorchidism surgery with respect to the total number of patients who received cryptorchidism surgery in DPC hospitals.

Changes in the number of discharges after cryptorchidism surgery in 94 DPC hospitals between FY2011 and FY20124).
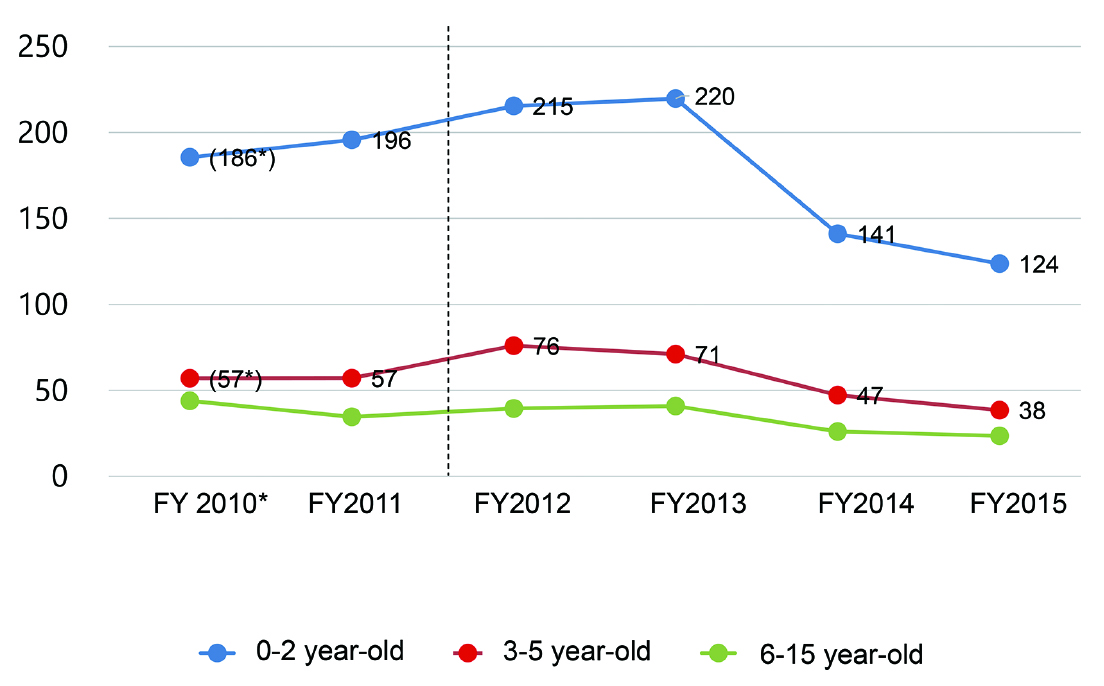
Changes in the number of discharges after cryptorchidism surgery per 100 DPC hospitals in each age group (0-2, 3-5, and 6-15 years)4).

Changes in the number of surgeries by field in 94 DPC hospitals*
*94 DPC hospitals were shown in the paper by Murase et al.4)
It is difficult clarify the prevalence of cryptorchidism because of complexities in design settings for epidemiological surveys of this disease. We have to take into account numerous factors, including genetic, environmental, maternal/fetal, and social factors. Judging from previous reports, although high dose exposures of several Gy (Sv) to several tens of Gy (Sv) can damage testicular steroidogenesis and germline DNA, the possi-bility that single-digit mGy (mSv) external exposures, or dozens of Bq/L internal exposures, could affect testicular steroidogenesis during fetal testicular descent or damage germline DNA is negligible. Actual radiation exposures arising from Japan’s earthquake, tsunami, and nuclear crisis were such that speculations about it causing cryptorchidism can be theoretically and empirically denied. Rather than “fishing” a single accident from among various events, including other accidents, disasters, and changes to the healthcare system that occurred contemporaneously, critical thinking requires a more comprehensive assessment. We therefore reject the hypothesis that cryptorchidism increased anywhere in Japan because of the Fukushima Daiichi Nuclear Power Plant accident. Scientists, medical doctors, and other health care professionals, not only in Fukushima Prefecture but from around the world, are earnestly investigating the consequences of our compound disaster. Concurrently, we are making sustained efforts to communicate what is known, what is not known, and what is believed, while clearly distinguishing among these three. Our duty of responsible disclosure is for future generations to better understand and make use of what we experienced and subsequently learned. After the accident, reports on health-related outcomes emerged in public media and scientific literature. Some, unfortunately, made radical errors in terms of their design setting, interpretation, and conclusions, and occasionally even lacked any scientific basis. Indeed, polls conducted by the National Institute of Science and Technology Policy showed that public trust in science in Japan was greatly damaged after the Fukushima Daiichi Nuclear Power Plant accident116).
Science is a human undertaking that requires great effort, and, therefore, great motivation, but motivation arising from personal preconceptions or ideologies should not be allowed to compromise the accurate reporting of scientific investigations. Errors of judgement, misinterpretation, and incorrect data can mislead the public, induce pseudoscience-based policy-making, and send society into a state of confusion. We strongly believe that the responsibility of scientists is to analyze and interpret facts correctly;this, in due course, may advance societal prosperity and happiness. Mindful of this, we should consciously bear responsibility for the impact of our actions on others.
Following Japan’s 2011 earthquake, tsunami, and nuclear crisis, words and deeds of support have come to us from around the world. We would like to thank everyone for their efforts toward the revitalization of Fukushima, and urge all speakers and authors to carefully consider the impact of their words on people living in Fukushima, who have endured groundless rumors that add to the emotional and economic damage of our multiple disasters. We are now taking positive steps to revitalize our prefecture and make it a comfortable place for all who live in Fukushima Prefecture, and all who visit. We welcome skeptics as well as supporters. We greatly thank Professor Kenneth E. Nollet, MD, PhD, FCA. Department of Blood Transfusion and Transplantation Immunology and Vice Director. Global Exchange Center Fukushima Medical University, for English editing of our manuscript.
The authors declare no conflicts of interest pertaining to the content of this article.