2015 年 84 巻 2 号 p. 148-155
2015 年 84 巻 2 号 p. 148-155
Flowers of Eustoma grandiflorum open in the morning and close in the evening, showing diurnal rhythms. In this study, the process of flower opening and closure of E. grandiflorum ‘Azuma-no-Murasaki’ was examined under different light cycles by capturing corolla images using interval photography. At 24-hour light cycles, the flower opening rhythm synchronized with the light cycles, and the process was composed of dual steps. The first one was immediate opening and closure at the times of initiation and cessation of the light period, respectively. The second one was gradual opening and closure, which occurred 12 hours after the end of the former light period and 2–3 hours after the initiation of the current light period, respectively. The first response appeared to be a direct effect of light, while the second one appeared to be under the regulation of circadian clocks. Under constant dark, blue, or red conditions, flowers showed circadian oscillations of 25.5 ± 0.6, 25.6 ± 0.6, or 24.3 ± 0.4 hours, respectively. Under constant white light or co-irradiation of blue and red light, flowers opened and closed once, but the oscillations did not continue thereafter. The synchronization of flower opening and closure rhythms to 24- and 20-hour day cycles was observed for both blue light and red light cycles. The synchronization was not complete for 16-hour light cycles and the flower oscillation period became 24 hours under 12-hour light cycles. The direct effect of light was found to be dependent on light intensity. When blue light intensity was adjusted at 25, 40, or 100 W·m−2, flowers opened more rapidly after illumination at a stronger light intensity, but such intensity-dependent effect was not observed for red light.
Many ornamentals show a diurnal rhythm of flower opening and closing oscillation (Bünning and Zimmer, 1962; Ewusie and Quaye, 1977; van Doorn and van Meeteren, 2003). Flower opening in the daytime and closure at night have some advantages for plants, since many pollinators are active during the day, promising successful pollination, fertilization, and fruit set. However, as ornamentals, which are displayed at flower shops and appreciated by consumers not only during the day but also at night, the ability to maintain a fully open status of flowers at night or at a specific time of the day is an important trait. Therefore, the development of techniques to control the rhythms or breeding of cultivars that can maintain open flowers at any time of the day, as consumers prefer, would potentially be useful.
The rhythm of flower opening and closure has been studied for more than a century. Many flowers open in the morning and start to close before dusk (Bünning and Zimmer, 1962; Ewusie and Quaye, 1977). Therefore, fully open flowers cannot be maintained just by keeping the lights on. In most cases, flowers open and close responding to circadian clocks. Circadian clock-regulated flower opening was studied in detail for Pharbitis (Kaihara and Takimoto, 1979, 1980). The Pharbitis flower opening was found not to be regulated directly by light, but was initiated 10 hours after dusk at 24°C. Therefore, when the dark period was less than 10 hours, flowers opened during the light period, whereas, if the dark period was longer than 10 hours, flowers opened during the dark period. Flowers of Turnera ulmifolia were also shown to start to open a certain period after the end of the previous light period (Ball, 1933).
Compared with circadian clock-regulated flower opening, circadian clock-regulated flower closure has been paid less attention and thus is less understood. The duration of the floral opening period is usually studied in consideration of floral longevity, which is associated with pollination and subsequent senescence. Significant shortening of floral longevity after pollination can be observed in many species (Niu et al., 2011; Primack, 1985; van Doorn, 1997). However, many flowers show repeated flower opening and closure and the oscillation seems to be under the regulation of circadian clocks.
Eustoma grandiflorum is one of the flowers that show reciprocal oscillations of flower opening and closure. The purpose of this study was to investigate flower opening and closure rhythms synchronized with environmental light rhythms in order to establish the basis for controlling and extending the period of full-opening of the flowers. We designed different light-dark cycles to examine the control of flower opening and closure by light in E. grandiflorum.
Seeds of E. grandiflorum ‘Azuma-no-Murasaki’ (Sakata Seed, Yokohama, Japan) were sown on wetted peat moss-based medium and placed in a growth chamber controlled at 20°C with 16-hour light/8-hour dark of light cycles at a light intensity of 5 W·m−2 using fluorescent lamps. Two months after germination, the seedlings were transplanted to 15-cm plastic pots filled with a 3:1 mixture of granular soil and a peat-based soil mix, and grown in a greenhouse for two months until flower buds became visible. About one week prior to the experiments, the plants were transferred to a growth room with light cycles of a 16-hour light period at 25°C and an 8-hour dark period at 20°C, relative air humidity of 60–80%, and light intensity of 100 W·m−2 over the canopy from ceramic-metal-halide lamps (ECO-CERA II; GS-Yuasa, Kyoto, Japan).
Experiment 1—Effects of different light periods under 24-hour light cycles on flower opening rhythmsBefore flowers started to open, plants were transferred to light cycles of a 20-, 16-, 12-, 8-, or 4-hour light period under 24-hour day length (20L4D, 16L8D, 12L12D, 8L16D, and 4L20D, respectively). The light intensity and temperatures of light and dark periods were the same as the preceding conditions of 16L8D cycles, as described above. Flower images were recorded from the first cycle of flower opening and closure. Other plants were kept under 16L8D conditions until the first day of flower opening, and then subjected to continuous dark (DD) or light (LL) conditions from the end of the last 16L8D cycle. The flower images were recorded from the beginning of DD and LL conditions.
Experiment 2—Effect of blue and red light cycles on flower opening rhythmsPlants similarly prepared as in Experiment 1 and placed under the 16L8D conditions were transferred to a dark room controlled at 25°C and subjected to the following light treatments from the end of the last 16L8D condition, using blue (465–475 nm; OSB56A5111A; OptoSupply, Hong Kong, China) or red (650–670 nm; OSR7CA5111A; OptoSupply) LEDs: 1) different day-length cycles of a 16-, 12-, 8-, or 4-hour light period and an 8-hour dark period (16L8D, 12L8D, 8L8D, and 4L8D, respectively), using blue (40 W·m−2) or red (50 W·m−2) light, 2) continuous blue (40 W·m−2), red (50 W·m−2), or blue plus red lights, and 3) 16L8D light cycles using different intensities of blue (25, 40, or 100 W·m−2) or red (25 or 70 W·m−2) light.
Data collection and analysisDigital cameras (Optio WG-2; Ricoh Imaging, Tokyo, Japan) were fixed in front of the flowers such that the position of the distal tip of one petal could be seen clearly, and flower images with a side view were taken automatically at 60-minute intervals for four consecutive days or at 2-minute intervals to see the petal movements shortly after the beginning of the light and dark periods. The flower images were displayed on a computer screen and the relative position of the petal distal tip compared with the flower base (the boundary between the peduncle and receptacle) along the direction of the flower axis (H, Fig. 1) was measured in terms of the pixels using MB-ruler version 5.0 software (Markus-Bader, Iffezheim, Germany). The relative position of the distal tip was recorded for one petal from each flower for four consecutive days. Then, the normalized relative position of the petal distal tip (Y) was calculated as:
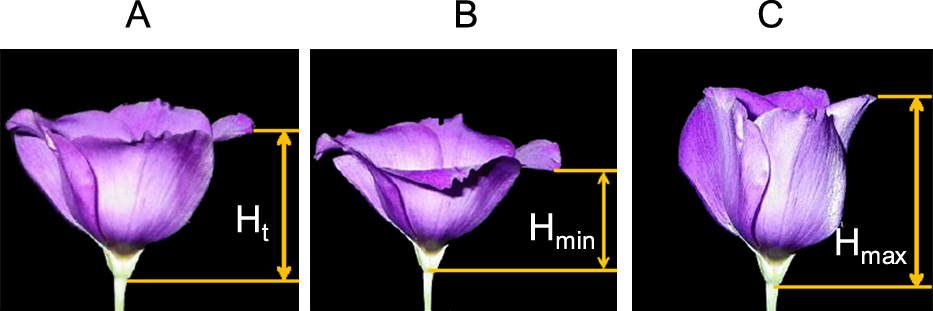
The flower opening and closure process was recorded as the movement of the position of the petal distal tip from the base of the flower. The measured position at time t (Ht) was normalized as Y = Ht/Hmax, where Hmax represents the maximum of Ht. A: Intermediate stage of flower opening and closure. B: Fully opened flower. C: Fully closed flower.
The oscillation period was estimated by manually finding the peak Y values for each flower opening and closure cycle, and calculating the average intervals between the times for these peak Y values for each flower. The estimated oscillation periods were averaged for at least three independent measurements, and are given as means ± standard deviations (SD).
When the flowers opened for the first time at 16L8D, petal unfolding of the tightly closed buds proceeded very slowly during the light period, but once the flowers had fully opened, they always started to open within 15 minutes after the onset of the light period (referred to as “dawn”), and maintained rapid opening for 2–4 hours (Figs. 2A and 3B). After this rapid opening, flowers gradually closed until the end of the light period (referred to as “dusk”), and started to close rapidly within 5 minutes after dusk (Figs. 2B and 3B). Since the flower-opening pattern differed between the day of the first flower opening and the following days, as described above, the first flower-opening day was regarded as the “preceding day” (Fig. 2) and excluded from the measurement of diurnal flower rhythms.
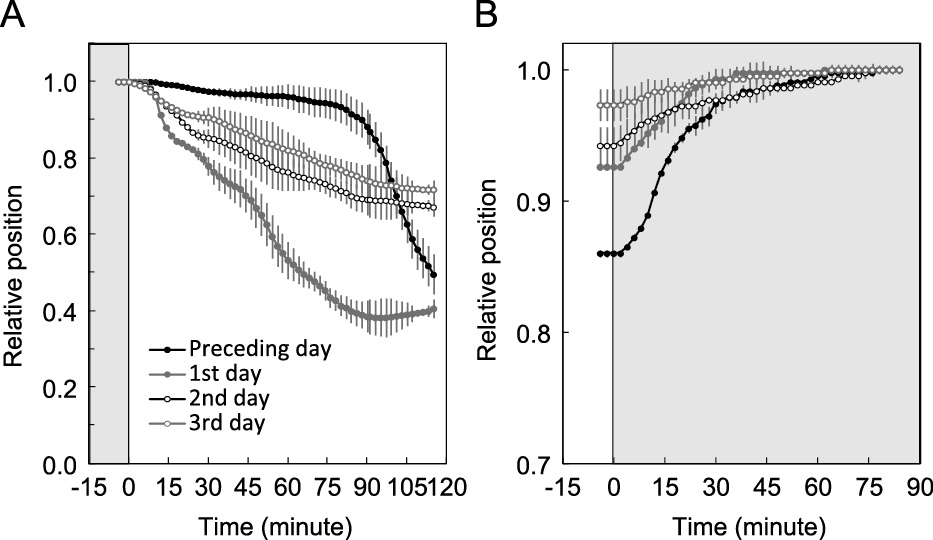
The movement of petals after the transition of the light conditions from dark to light (A) and from light to dark (B). The petal movement was recorded during the day of first flower opening (“preceding day”) and subsequent three consecutive days. Gray and white areas of each figure indicate dark and light periods, respectively. Bars indicate the standard deviations (n = 3).

Effects of the length of light periods under 24-hour light cycles on flower opening and closure rhythms. White and black bars above the figures indicate light and dark periods. For the periods indicated by gray bars in LL (continuous light), the corresponding time of the day during the pre-experimental light conditions was dark. A–E: Flower opening and closing oscillation rhythm in 24-hour light periods (20L4D, 16L8D, 12L12D, 8L16D, and 4L20D respectively). F–G: Flower opening and closing oscillation rhythm in constant dark and light periods, respectively. Each value is the mean of at least three replicates. Bars indicate the standard deviations (n ≥ 3).
At 12L12D light cycles (Fig. 3C), flowers also started to open immediately after dusk, reaching the maximum in 2 to 4 hours, closed gradually until the end of the light period, and then closed rapidly immediately after dusk.
At shorter light period cycles (8L16D, 4L20D), flower opening started during the dark period approximately 12 hours after dusk (Fig. 3D, E). Flowers also exhibited rapid opening after dawn and rapid closure after dusk, similarly to the cycles with shorter dark periods.
In the longest light period cycles (20L4D), flowers opened only slightly after dawn (Fig. 3A). The extent of maximum opening became less as flowers repeated daily opening and closure cycles at longer light period cycles (20L4D, 16L8D, 12L12D), so that the Y value drifted upward toward the end of the measurement (Fig. 3A, B, C).
Under continuous dark conditions (Fig. 3F), flowers exhibited opening and closing rhythms with an oscillation period of 25.5 ± 0.6 hours. Under continuous light (Fig. 3G), flowers opened and closed once as observed under the light period of light/dark cycles, and opened again around 24 hours after dawn, but they did not show any more opening and closure thereafter.
Experiment 2—Effect of blue and red light cycles on flower opening rhythmsUnder 16L8D and 12L8D light cycles using blue or red light (Fig. 4A, B), the flowers also exhibited opening and closure oscillations synchronized to the light cycles. The periods of oscillation were 24.2 ± 0.3 hours and 20.3 ± 0.3 hours in 16L8D and 12L8D blue light cycles, respectively. In red cycles of 16L8D and 12L8D, they were 23.9 ± 0.1 hours and 20.1 ± 0.2 hours, respectively. However, the synchronization was not complete for 16-hour light cycles (8L8D) (Fig. 4C), where flowers exhibited around 24-hour oscillation for three cycles by red light or irregular movements by blue light. Under 4L8D light cycles, flower-opening rhythm was synchronized to 24-hour cycles both for the blue light and for the red light; the periods were 24.3 ± 0.4 hours and 23.8 ± 0.6 hours in the blue and red 4L8D light cycles, respectively (Fig. 4D).

Effects of day length on flower opening and closure under red (upper figures) or blue (lower figures) light cycles. The length of the dark period was fixed at 8 hours. White and black bars above the figures indicate light and dark periods. A–D: Flower opening and closing oscillation rhythm in different red or blue light cycles (16L8D, 12L8D, 8L8D, and 4L8D respectively). Each value is the mean of at least three replicates. Bars indicate the standard deviations (n ≥ 3).
Under constant blue or red light (Fig. 5), flower opening and closure showed free running oscillations; the period of the cycle was 25.6 ± 0.6 hours for blue and 24.3 ± 0.4 hours for red. Under constant co-irradiation of blue and red light (Red + Blue), flower movements were similar to those under constant white light; flowers exhibited opening and closure once, but did not show any more rhythms thereafter.
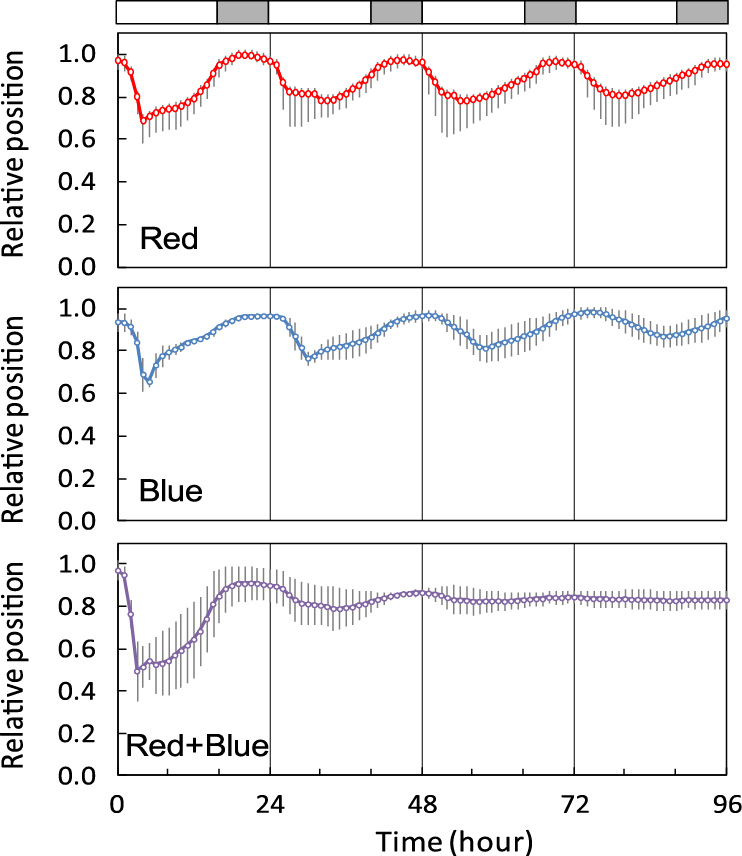
Effect of continuous irradiation of red (upper) or blue (middle), or co-irradiation of red and blue (bottom) on flower opening and closure. For the periods indicated by gray bars, the corresponding time of the day during the pre-experimental light conditions was dark. Each value is the mean of more than three replicates. Bars indicate the standard deviations (n ≥ 3).
To see the light intensity response of flower opening and closure, flowers were subjected to a 16L8D rhythm of blue or red light at different light intensities (Fig. 6). The flowers exhibited the most rapid response after dawn to the strongest blue light, and the flowers closed slowly at lower blue light intensities. By contrast, such rapid changes after dawn were not observed for red at both weak and strong intensities. At weak red light cycles, petals opened more widely, as represented by the lower values of Y at full opening, and did not close fully, resulting in a downward shift of Y.
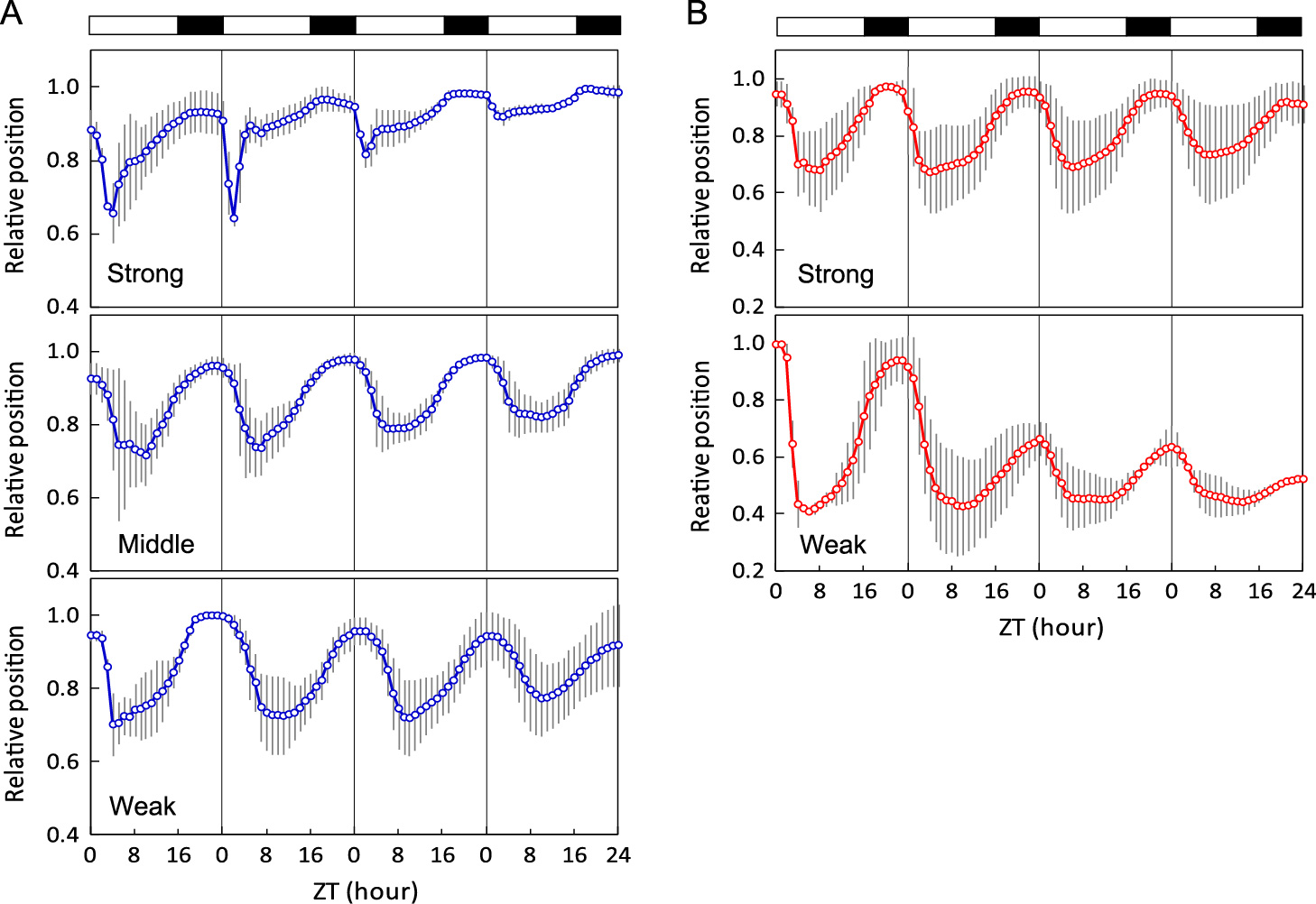
Flower opening and closing oscillation rhythm of different blue light intensities (A) and different red light intensities (B). Light intensities of blue were 100 (Strong), 40 (Middle), and 25 (Weak) W·m−2, and those of red were 75 (Strong) and 25 (Weak) W·m−2. Each value is the mean of more than three replicates. Bars indicate the standard deviations (n ≥ 3).
Flowers of many species show reciprocal opening and closure synchronized with environmental light rhythms. This process is usually slow and takes hours for full opening and closure (Tanaka et al., 1989). In this study, the petal movements during flower opening and closure were recorded by interval photography using digital cameras.
At the standard cycle of 16L8D, flowers opened shortly after dawn and started to close 2 to 4 hours after dawn, showing 24-hour period oscillations (Fig. 3B). Careful observation of the flower opening process revealed that flower opening was composed of dual steps: one started at dawn and the other started 12 hours after dusk. When the dark period was longer than 12 hours, flowers partially opened during the dark period and rapidly open immediately after dawn. Similarly, flower closure started during the light period and flowers immediately closed after dusk. The dark period opening and light period closure can be considered as circadian clock-regulated movement and the latter one would be a direct and immediate effect of lights. Such a dual system was proposed for single flower opening of Asiatic lily, in which petals opened to approximately 40° in a “dark phase”, and opened further for full opening in a subsequent “light phase” (Bieleski et al., 2000b).
Circadian clock-regulated flower openingAs described above, the flower opening started during the dark period before dawn when the dark period was longer than 12 hours (8L16D and 4L20D), and flower closure started during the light period when the light period was longer than 4 hours (Fig. 3D, E). The dark period flower opening and light period flower closure suggested the involvement of circadian clocks in the regulation, since these changes required substantial numbers of hours after the actual changes in light conditions. To confirm this, the persistence of flower opening rhythms under constant dark (DD) or light (LL) conditions was tested (Fig. 3F, G). The flowers showed an approximately 25-hour rhythm for at least three days in DD (Fig. 3F), indicating the involvement of the circadian clock in the regulation of flower opening and closure in Eustoma flowers.
In contrast to the continuous dark conditions, upon being placed in the continuous light conditions, flowers opened and closed once, and showed no more oscillation of flower opening and closure thereafter (Fig. 3F, G). Thus, more than a minimum dark period seems to be required for the flowers to open. The requirement of extension of the dark period for flower opening was reported for other plants. The minimum time requirement was 7 hours for Asiatic lily flower (Bieleski et al., 2000a, b) and a few hours for Turnea ulmifolia (Ball, 1933). In Eustoma flowers, flowers showed only slight opening and closure at 20L4D cycles (Fig. 3E). Thus, the minimum requirement of the dark period would be around 4 hours in Eustoma flowers.
Direct and immediate effects of light on flower opening and closureThe Eustoma flowers exhibited rapid opening within less than 15 min after the initiation of the light period (Fig. 2A). Rapid flower opening after dawn has been reported for other plants. It took less than one hour for Portulaca (Ichimura and Suto, 1998), less than 20 min for Oenothera biennis (Sigmond, 1930), and only 5 min for Hedera helix (Sigmond, 1929) to reach full flower opening after the initiation of illumination. Rapid flower closure was also observed at the beginning of dark periods (Fig. 2B). These immediate responses to the light phase transition were observed regardless of different light/dark cycles and even in shorter day length cycles (Fig. 4B, C, D). Therefore, it is likely that the immediate response is the direct effect of the transition of light conditions.
The immediate flower opening after dawn appears to be dependent on both light quality and light intensity. Under the blue/dark cycles with the strongest blue light, flowers exhibited the sharpest changes at the points of lights on and off (Fig. 6A), while the light intensity-dependent response was not clear under the red light cycles (Fig. 6B). In excised leaves of Oxalis corymbosa, leaf movement, which was directly induced by light, was found to be mediated by blue light, but not by red light (Nakanishi et al., 2005). It is generally found that the extent of flower opening depends on environmental light intensities. On cloudy days at low sunlight intensities, flowers often show insufficient flower opening. On the basis of our results, blue light may play an important role in these light-intensity-dependent flower openings. Although many studies showed the association of flower opening with light intensity, as reviewed previously (van Doorn and van Meeteren, 2003), the physiological basis for this response remains unknown.
Flower opening and closing rhythm controlled by multiple photoreceptorsOur data showed that red light or blue light was sufficient for the synchronization of flower opening and closure rhythms (Fig. 4). The flower opening oscillation synchronized with both red/dark and blue/dark cycles of different day lengths. The circadian rhythm was also observed under continuous blue and continuous red as in DD (Figs. 3F and 5). However, when both red and blue were irradiated continuously, the circadian rhythm disappeared, as observed for LL (Figs. 3G and 5). Therefore, signals of multiple photoreceptors would be integrated, and the integrated signals may control the flower opening and closure rhythms. Red/far-red-light photoreceptor phytochromes and blue-light photoreceptor cryptochromes were found to play a key role in the synchronization of circadian oscillations to light/dark cycles (Yanovsky and Kay, 2001; Mas and Yanovsky, 2009; Somers et al., 1998). In Pharbitis, the time of flower opening was delayed by red irradiation during the dark period, and the effect could be cancelled by subsequent far-red irradiation (Kaihara and Takimono, 1979, 1980), suggesting the involvement of red/far-red reversible phyB in the regulation. From our data, both cryptochromes and phytochromes seem to be involved, but the involvement of other photoreceptors for blue light would be possible.
Molecular mechanisms for circadian clock regulation of flower opening and closureThe circadian free running under DD but not under LL was similar to the flower opening rhythm of roses (Horibe and Yamada, 2014), but different from those of some other plants. Bellis perennis flowers showed a circadian rhythm under LL, while Kalanchoe blossfeldiana showed a circadian rhythm under both LL and DD (Karvé et al., 1961). In Arabidopsis, leaf movement and hypocotyl elongation oscillated in LL after LD pretreatment, but this oscillation was not clear under DD.
Our understanding of circadian clock regulation is too limited to speculate on why different species showed different responses to LL and DD, but these variations in the response to continuous light conditions should be related to the entrainment mechanism of circadian clocks. The absence of oscillation under LL suggests that the oscillation of an endogenous clock was disturbed or the output of clock signals was interrupted by the continuous light signal. In Arabidopsis, even though the oscillation disappeared under DD, the transcript levels of LATE ELONGATED HYPOCOTYL (LHY), CIRCADIAN CLOCK ASSOCIATED 1 (CCA1), GIGANTEA (GI), and EARLY FLOWERING 4 (ELF4) genes, which were discovered to control internal rhythms, continued to exhibit circadian rhythms under both LL and DD following the light/dark cycle (Doyl et al., 2002; Higuchi et al., 2011; Park et al., 1999; Schaffer et al., 1998; Wang and Tobin, 1998), while the expression of TIMING OF CAB EXPRESSION 1 (TOC1) showed oscillation in LL, but not in DD (Strayer et al., 2000). These data imply that circadian oscillators were active under LL and DD in Arabidopsis, but the irregular output signals under DD caused the arrhythmic behavior of the leaves. Accordingly, the loss of oscillation of Eustoma flowers under continuous LL would result from defects in the output pathway of oscillator genes, rather than arrhythmicity of oscillators (Fig. 3G). To clarify these relationships, it would be necessary to analyze the oscillations of clock genes in E. grandiflorum.
The observed immediate response upon the transition of light conditions appears to be the “acute response”, which represents the “gating” of clock output signals (Millar and Kay, 1996). The morning genes are usually induced by light exposure, and this entrains circadian clocks to the environmental light rhythms. However, the extent of the response is not stable throughout the day, but shows circadian rhythms. When the response is small, the “gate” is regarded as being closed. Therefore, the entrainment of the rhythm can be completed effectively only during specific periods of the day.
In this regard, the immediate response upon the transition of light conditions should be influenced by the manner of the light/dark cycles. In fact, the immediate response was not clear for 20L4D (Fig. 3E), and in this condition, the flower opening and closure rhythm was disturbed. Similarly, at 4L8D cycles, no immediate response was observed and the period of flower opening and closure was not 12 hours, but 24 hours (Fig. 4D). These examples may imply that the gate was closed at dawn in these conditions so that the rhythm could not be entrained to the light cycles.
In conclusion, Eustoma grandiflorum flowers showed 24-hour cycles of flower opening and closure, and these cycles were precisely synchronized to environmental light/dark cycles. The rhythms could be synchronized both by blue and by red light, indicating that both red photoreceptors such as phytochromes and blue light photoreceptors such as cryptochromes were involved in entrainment of the rhythm. The data also showed that the flower opening and closure were controlled by a dual system: one was controlled by a circadian clock and the other functioned immediately after the lights were turned on and off. Flower opening gradually started approximately 12 hours after the start of the dark period, and rapidly after the light-dark phase changes. Blue light seemed to be especially important for the light-intensity-dependent regulation of flower opening and closure.