2015 年 84 巻 2 号 p. 140-147
2015 年 84 巻 2 号 p. 140-147
Several kinds of double-flowered cultivars have been produced from spontaneous mutants in Cyclamen persicum Mill. The morphology and number of organs in double flowers were investigated, and were categorized into three types: “petaloid-stamen type”, “petaloid-sepal type”, and “extra petals in whorl 2 type”. Morphological observations showed that petaloid mutations of stamen and sepal did not appear together within an individual. In addition, three types of AG-like gene were isolated from single and petaloid-stamen-type double-flower buds, and expressions in each whorl were compared. All AG-like genes were expressed in whorl 3 of single flowers, but not in that of petaloid-stamen-type double flowers. In whorl 4, although expressions of the three types of AG-like gene were observed in both single and double flowers, the expression of double flowers was lower than that of single flowers. These results suggest that naturally occurring double flowers of cyclamen can be explained by the ABC-model, and it is suspected that petaloidy of the stamen is caused by the repression of AG-like gene expression in whorl 3.
Cyclamen belongs to the Primulaceae, and species within this genus are distributed mainly in the Mediterranean region. Most of the ornamental cyclamen cultivars were established from a single species, Cyclamen persicum Mill. As this species has a long flowering period, and various kinds of flower color and shape, it is one of the most popular pot flowers in Europe and Japan. Its production scale is the largest among Japanese pot flowers, but has been decreasing in recent years (Ministry of Agriculture, Forestry and Fisheries of Japan, 2014; http://www.e-stat.go.jp/SG1/estat/List.do?bid=000001024933&cycode=0, November 17, 2014). Therefore, the production of new cultivars with remarkable characteristics has been desired for further promotion of its horticultural use.
A double-flower state is one of the most remarkable characteristics that enhance the value of ornamental flowers because it usually has a large flower size by the duplication of petals. Although there are many kinds of floral mutation including double flowers in plant species, their mechanism can generally be explained by the “ABC-model” (Coen and Meyerowitz, 1991). This model represents the relationship between MADS-box transcription factor genes and floral morphogenesis. In dicot flowers, the expression of A-class genes alone governs the sepal development in whorl 1. Co-expression of A- and B-class genes in whorl 2 determines the differentiation of petals, whereas B- and C-class genes in whorl 3 give rise to the stamens. Finally, the expression of C-class genes alone specifies carpel in whorl 4. In some monocots, such as Agapanthus, Alstroemeria, Crocus, Tulipa, and Lilium, the co-expression of A- and B-class genes was recognized in both whorl 1 and whorl 2, explaining the outer and inner perianth found in these species (Hirai et al., 2007; Kanno et al., 2003; Nakamura et al., 2005; Tsaftaris et al., 2006). This type of flower was explained by the “modified ABC-model” and the “sliding boundary model” (Kramer et al., 2003; van Tunen et al., 1993). One type of double flower in dicots was found by the petaloidy of sepal, generated by the expression of B-class genes in whorl 1, which can usually be seen in monocots. The other type of double flower occurs by the alteration from stamens to petals resulting from a decrease of C-class gene expression in whorl 3. If C-class gene expression is also decreased in whorl 4, secondary flowers are simultaneously observed, as reported in Arabidopsis, Antirrhinum, and Rosa (Coen and Meyerowitz, 1991; Mibus et al., 2011; Schwarz-Sommer et al., 1990; Weigel and Meyerowitz, 1994). Recently, from this viewpoint, new double-flowered plants were bred by the repression of C-class genes using chimeric repressor gene-silencing technology (CRES-T) (Shitaka and Ohme-Takagi, 2008) in Torenia fournieri (Narumi et al., 2008) and Pharbitis nil (Sage-Ono et al., 2011).
In several kinds of double-flowered cyclamen established from spontaneous mutants, the expression mechanism of double flower is unclear, but Tanaka et al. (2013) reported that silencing of the C-class AGAMOUS (AG)-like genes by CRES-T resulted in double flowers with petaloid-stamen in cyclamen. Thus, petaloid-stamen-type double-flowered cyclamen cultivars can arise not only from natural mutation, but also through genetic transformation by C-class gene silencing. Additionally, MADS-box genes of cyclamen have been isolated, and it was reported that two distinct AG-like genes (CpAG1, CpAG2) play important roles in stamen and pistil formation in whorls 3 and 4 during floral morphogenesis (Tanaka et al., 2011). Therefore, it is suggested that the AG-like genes participate in petaloidy of stamens even in natural mutation.
Spontaneous double-flowering occurs not only by petaloidy of stamens, but also by that of other organs in cyclamen. This is a very rare case in herbaceous plants. Thus, it is considered that double-flowered cyclamens are valuable for the investigation of petaloidy and floral morphogenesis. If elucidation of the petaloidy mechanism is possible in double-flowered cyclamen, we might be able to conduct an efficient breeding program and provide various types of double-flowered cyclamen with high ornamental value based on newly obtained findings. Here, to elucidate the mechanism of double-flower formation in ornamental cyclamen, we investigated the morphological variation of flowers in naturally occurring double-flowered cyclamen. We also compared the expressions of AG-like genes between single and double flowers to understand the genetic mechanism of double-flower formation.
Single flowers of ten individuals in five lines and seventeen individuals in three seed-propagated and one clonal-propagated cultivars were used as controls (Table 1). Three types of double-flowered cyclamen were supplied. 1) Petaloid-stamen type: twenty-four individuals of one-year-old progeny obtained from two combinations of crosses between complete and incomplete petaloid plants in whorl 3 were used (Fig. 1B). 2) Petaloid-sepal type: one individual obtained from the crosses of complete petaloid-sepal plants and four spontaneous mutants from the crosses of normal plants were used, and eight individuals of three seed-propagated cultivars were also provided (Fig. 1D, F, G, H). 3) Extra petals in whorl 2 type: five individuals with extra petals in whorl 2 produced by crossing of similar mutant plants were provided (Fig. 1I). These individuals were grown in a greenhouse maintained at more than 10°C under a natural photoperiod in Kyushu University, Hakozaki, Fukuoka, Japan, and their morphological characteristics were investigated in anthesis.

Plant materials used in this study, and their profiles.

Various types of single and double flowers. A single flower (A) and a double flower with petaloid-stamens (B) within a line (H23-333). Single flowers (C, E) and double flowers with petaloid-sepals (D, F) (a Uk-8 relative and Uk-8). (E) and (F) are sepals and the base of petaloid-sepals, respectively. Different petaloid levels in petaloid-sepals (G, H) (SEM-P). Flower with extra number of petals in whorl 2 (I) (523PD). Bars = 2 cm (A–D, I), 0.5 cm (E–H).
For the isolation of AG-like genes, floral buds 5–8 mm in length were sampled from the plants with single and petaloid-stamen-type double flowers of the H23-333 line in the flowering period in May. In the AG-like gene expression analysis, each floral organ, namely, sepals, normal petals (strictly speaking, cyclamen has gamopetalous flowers; corolla fused in the base of petals and each petal is called a “lobe”; however, a “lobe” is called a “petal” for convenience in this study), petaloid-stamens and carpels, was sampled from several floral buds 5–8 mm in length of H23-333 flowers, and the samples were stored at −80°C until use.
Floral morphologyThe morphology and number of organs in each whorl were observed during the flowering period (Table 1). The morphology was categorized as “normal”, “incomplete petaloidy”, or “complete petaloidy” in each whorl. “Normal” indicated that the floral organs were identical to those of the wild type and “incomplete petaloidy” showed that the flower had an ectopic incomplete petaloid organ, except for whorl 2. “Incomplete petaloidy” was subcategorized into two subsets in terms of whether the flower had stamen or not. “Complete petaloidy” indicated that the organ was transformed to ectopic complete petal as in whorl 2.
Isolation of the AG-like genes from single and double flowers, and their sequence analysisTotal RNAs were extracted from flower buds of single and double flowers of the H23-333 line by a modified CTAB method based on Chang et al. (1993). First-strand cDNA was synthesized from 11 μL of purified mRNA solution using oligonucleotide primer (5'-GACTCGAGTCGACATCGA(T)17-3') and AMV reverse transcriptase (Promega, Madison, WI, USA). The cDNA fragments of the AG-like genes were amplified by RT-PCR using the primers designed in the 5'- and 3'-UTRs of CpAG1 (forward 5'-GAGGAGAGGCCAAGTGAAGA-3' and reverse 5'-TCGAATACAAGCACGAGACG-3') and CpAG2 (forward 5'-GGTCTCATCTCCCTATCTTG-3' and reverse 5'-CTCGTCTTCAACAGTGATCC-3') based on the sequences reported by Tanaka et al. (2011). The PCR products were cloned into pGEM-T easy vector (Promega), and sequencing was performed using BigDye Terminator Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) with ABI 3130 genetic analyzer (Applied Biosystems), according to the manufacturer’s protocol. Nucleotide and amino acid sequences were aligned using Clustal W (Thompson et al., 1994) with the software MEGA ver. 5.1 (Tamura et al., 2011).
Expression analysis by real-time PCRTotal RNAs were extracted separately from each floral organ, and the cDNAs were synthesized in the same way as for the isolation of AG-like genes. The cDNAs synthesized from whorls 1 to 4 in single- or double-flowered individuals were used as templates. The gene-specific primer combinations were designed for each type of AG-like gene, CpAG1-DF (forward 5'-CGGAGTACGAGTTGATGCAG-3' and reverse 5'-GGAGAAGTTTCCGCAAGAG-3'), CpAG2-DFa (forward 5'-GTCTGGGAGTTCGGAGTATC-3' and reverse 5'-ATCGGGTGGAAGACCATTC-3') and CpAG2-DFb (forward 5'-TGTCCGTCTCTCGCTTTCTC-3' and reverse 5'-TCGTACTCCGAACTCCCAGA-3'), which were obtained in previous studies. Real-time PCR was conducted using 2-μL cDNA samples in premixture containing 7.8 μL of sDDW, 0.1 μL of each primer and 10 μL of SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA) with a MiniOpticon real-time PCR system (Bio-Rad Laboratories). The PCR reaction was performed with the following cycling parameters: one cycle of 3 min at 95°C for denaturation followed by 40 cycles of 10 s at 95°C, 1 min at 60°C and 10 s at 95°C. Each cDNA sample was analyzed in three replicates. The eEF1A gene was amplified as an internal control using the following primer pair (forward 5'-TAAGTCTGTTGAGATGCACC-3' and reverse 5'-CTGGCCAGGGTGGTTCATGAT-3') at the same time. The expression level of each AG-like gene was standardized with the eEF1A expression.
Petaloidy of either stamen or sepal was recognized; however, double mutation, that is, petaloidy of both stamen and sepal, was not identified within an individual (Fig. 2). Pistil malformation in whorl 4 (categorized as “incomplete petaloidy”) was sometimes found only in petaloid-stamen individuals, and not in petaloid-sepal plants. As for organ number, the basic number of organs in whorls 1 to 3 is five, but flowers with six or more organs were occasionally found in C. persicum (Grey-Wilson, 1988). Even in this study, most of the single flowers had five organs in whorls 1 to 3 (over 93%), while a large number of double flowers had six or more organs (the greatest number was 13 in whorl 3 of petaloid-stamen type and 15 in each whorl 2 of extra petal type). On the other hand, a decrease of organ number was observed in only a few flowers in single and double flowers. The distributions of organ number were similar in whorls 1 to 3, and it was considered that the number of organs in whorls 1 to 3 synchronized to each other within a flower; if the number of organs increased in a whorl, an increased organ number could also be observed in other whorls, except for whorl 4. The double flowers with petaloid-stamens showed variations in number and shape of petals in whorl 3. The degree of variation differed among the lines. In addition, variations were observed among flowers within an individual. In contrast, the double flowers with petaloid-sepal showed relatively stable number and size in whorl 1, although petaloidy levels at the base of petaloid-sepals in whorl 1 differed greatly among flowers (Fig. 1G, H). The number of petals ranged from 5 to 15 in the extra petal type in whorl 2, and the number of organs in whorls 1 and 3 also increased with high frequency. However, the conversion of organs in each whorl was not recognized in this type.
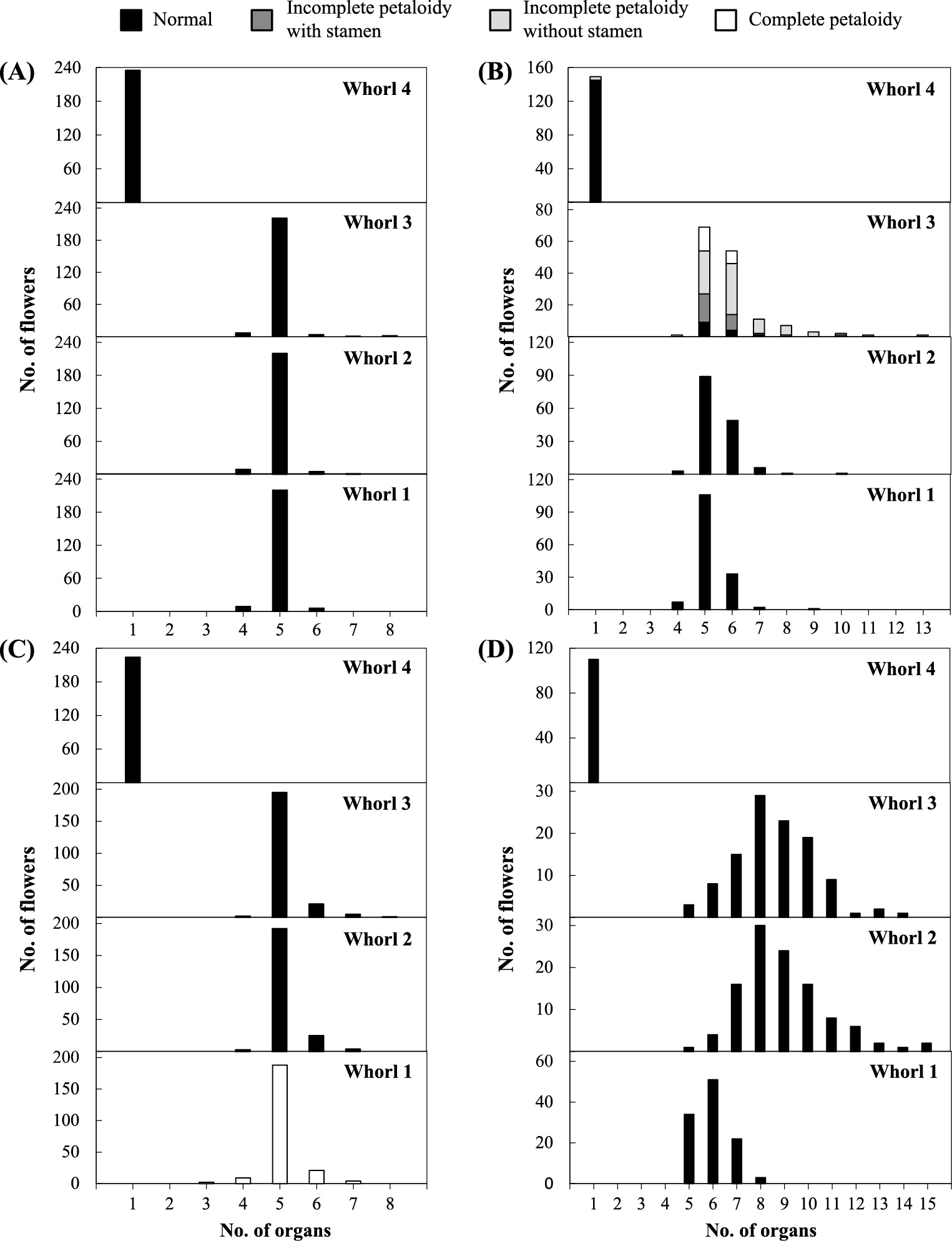
Variation of organ numbers in whorls 1 to 4 in the single flower (A), and double flowers with petaloid-stamens (B), petaloid-sepals (C), and extra petals in whorl 2 (D).
Three types of AG-like gene, CpAG1-DF, CpAG2-DFa, and CpAG2-DFb, were isolated (Fig. 3). CpAG1-DF and CpAG2-DFa isolated in both single and double flowers shared 99% identity of nucleotide sequence with CpAG1 and CpAG2, respectively, isolated in a previous study (Tanaka et al., 2011). CpAG2-DFb was isolated only from double-flowered cyclamen, and was very similar to CpAG2-DFa in the nucleotide and amino acid sequences (Figs. 3 and 4). Nevertheless, CpAG2-DFb was distinguished from CpAG2-DFa because CpAG2-DFb had a deletion from 23 to 38 bp and 11 nucleotide differences in the coding region, which led to 4 amino acid differences in the coded protein. In addition, an insertion was located from 553 to 631 bp in CpAG2-DFb. However, this insertion had GT and AG sequences at both ends, suggesting an intron. CpAG2-DFb shared 96% identity of nucleotide sequence with CpAG2-DFa. The deduced amino acid sequences of MADS-domain, K-domain, and AG motifs I and II encoding CpAG1, CpAG2-DFa, and CpAG2-DFb were highly conserved among their homologous genes (Fig. 4).

The alignments of nucleotide sequences of AG homologous genes in H23-333. Length of the alignment is shown on the right, and homologous sequences are marked with an asterisk. Initiating and stop codons are indicated by a box. Deletion site and deduced intron region in CpAG2-DFb are indicated by a dashed arrow and a solid arrow, respectively.

Comparison of deduced amino acid sequences encoded by AG homologous genes in H23-333. Length of the alignment is shown on the right, and homologous sequences are marked with an asterisk. MADS domain, K domain, and AG motifs I and II are indicated by a box and a line above the sequence.
The expression of the AG-like genes, CpAG1-DF, CpAG2-DFa, and CpAG2-DFb, was not detected in whorls 1 and 2 of single and double flowers (Fig. 5). In whorl 3, AG-like genes were expressed in single flowers, while only slight expression was found in double flowers. Although the expressions of the three types of AG-like gene were detected in whorl 4 of both single and double flowers, the level of expression of double flowers was less than that of single flowers. The expression levels of genes in whorl 4 of double flowers were reduced to approximately 60% compared with those of single flowers.

Expression analysis of AG-like genes in single and double flowers with petaloid-stamens in H23-333. W1–W4: whorls 1–4. eEF1A was used as an internal control. Expression data were standardized in respective genes independently. The data represent the mean and standard errors obtained from three replicates.
In the current study, petal formation in whorls 1 and 3 was treated as petaloid-sepal and petaloid-stamen for each mutant. Individuals with petaloid-stamens and petaloid-sepals had normal sepals and stamens, respectively (Fig. 2). Real-time PCR showed AG-like genes being expressed in whorl 3 of single flowers, whereas this occurred at a very low level in that of double flowers with petaloid-stamen (Fig. 5). The gene expression in whorl 4 was recognized in both single and double flowers. These results of morphological and gene expression analyses were in good agreement with the ABC-model. In addition, the following results imply that a single factor causes petaloidy of stamen in cyclamen. In most petaloid-stamen-type double-flowered lines and cultivars, transformation occurred in whorl 3 only, whereas a pistil formed normally in whorl 4 (Fig. 2). In petaloid-stamen-type double flower, H23-333 plants showed reductions of AG-like gene expressions in whorls 3 and 4 (Fig. 5). In a previous study, Tanaka et al. (2013) produced petaloid-stamen-type double-flowered cyclamen by AGAMOUS chimeric repressor expression (CRES-T). Similarly, petaloid-stamen-type double flowers in other ornamental flowers were also produced by AG-like gene silencing (Narumi et al., 2008; Sage-Ono et al., 2011). Therefore, it is suspected that the petaloidy of stamens even in other naturally occurring double-flowered cyclamens might be caused by the reduced expression of AG-like genes in whorl 3.
As for double-flowered cyclamen with petaloid-sepal, they were explained by the ABC-model in floral morphology observation (Fig. 2). If petaloidy of sepal in double-flowered cyclamen follows the ABC-model in terms of the molecular mechanism involved, it may be generated by ectopic expression of B-class genes in whorl 1.
In double flowers with petaloid-stamens, there were variations in petaloidy level between the lines (Fig. 2). It was suspected that petaloidy level generally depended on the genetic background. However, we also found variations of petal morphology and number in whorl 3 within an individual in both H23-332 and H23-333 (data not shown). Since the degree of morphological alteration in flowers may result from the expression level of MADS-box genes, it is estimated that their expression level is affected by exogenous factors. Double-flowered lily cultivar ‘Elodie’ altered petal morphology in whorl 3 depending on the AG-like gene expression level (Akita et al., 2011). Although it was not clear what kinds of exogenous factors affect gene expression level, the expression level of the AG-like gene LelAG1 in whorl 3 was highly correlated with the degree of petaloidy of stamens. In Arabidopsis, it has been reported that a MADS box gene involved in stamen identification, APETALA3, was affected by temperature-dependent epigenetic regulation in its splicing process (Sablowski and Meyerowitz, 1998). In addition, deficiens mutant in Antirrhinum formed sepaloid petals and carpelloid stamens under lower-temperature conditions, but it was similar to the wild-type morphology at a higher temperature (Schwarz-Sommer et al., 1992). These results suggest that floral formation can be governed by genetic factors as well as exogenous factors such as environmental and physiological factors.
An increased petal number in whorl 2 is also categorized as double flower. We also found an increase in organ number in whorls 1 to 3 of petaloid-stamen and petaloid-sepal mutants, and a mutant with extra petals in whorl 2 without alteration of floral morphology (there was variation of organ number not only in double-flowered mutants but also in clones of plants with a single flower) (Fig. 2). In many plant families, floral organ number is a relatively stable characteristic, but there are many reports about exceptional cases (Running and Meyerowitz, 1996; Zhao et al., 2004). In the model plant Arabidopsis thaliana, there is typically a stable organ number, but there are several reports showing the variation in organ number. For example, stem cell maintenance in Arabidopsis floral meristems is terminated by interactions between WUSCHEL (WUS) and AG (Lenhard et al., 2001; Lohmann et al., 2001; Sun et al., 2009). The homeobox gene WUS was shown to regulate the specification of stem cell maintenance in shoot apical meristems (Laux et al., 1996; Mayer et al., 1998). Although plants could produce organs continuously under WUS regulation, this gene expression was repressed by negative feedback of AG expression induced by WUS expression. In A. thaliana and A. majus, floral homeotic mutants containing ag formed additional whorls interior to the fourth whorl (Bowman et al., 1991; Coen and Meyerowitz, 1991). In addition, repression of C-class genes by CRES-T also caused organ (sepal and petal) increase in transgenic plants in A. thaliana (Mitsuda et al., 2006), P. nil (Sage-Ono et al., 2011), and C. persicum (Tanaka et al., 2013). Therefore, there was no contradiction that cyclamen with petaloid-stamen and with decreased AG-like gene expression showed an increase of floral organs. However, on this issue, it is not currently clear whether this hypothesis can be confirmed in the case of cyclamen because the increase of organ number in two types of double flower, namely, petaloid-sepals and extra number of petals in whorl 2, in the current study could not be explained sufficiently by previous studies. In terms of floral organ number, it has been reported that environmental factors cause fluctuation of this variable. Lateral stamen number of Cardamine hirsuta (Brassicaceae) flower was unstable even within individual inflorescences, and developments of their lateral stamens were temperature-dependent (Matsuhashi et al., 2012). For the reasons mentioned above, more detailed analysis will be needed to obtain a complete understanding of this issue.
In conclusion, this study revealed that the mechanism of double-flowered cyclamen could be explained by the ABC-model, and it is suspected that double-flowered cyclamen with petaloid stamens is caused by repression of AG-like gene expression in whorl 3. However, variation of floral morphology, such as petal number and size in whorl 3 within an individual, remains to be clarified. Therefore, the relationship between floral morphology and gene expression level, the effect of environmental and physiological factors on the floral organ morphology and the number of double flowers in C. persicum should be investigated.
We are grateful to Mr. Tetsuro Kage for providing valuable plant materials.